Affiliation:
Interventional Ultrasound Unit, Pineta Grande Hospital, 81030 Castel Volturno, Italy
Email: giampierofrancica@gmail.com
ORCID: https://orcid.org/0000-0002-9821-7966
Explor Drug Sci. 2024;2:555–566 DOI: https://doi.org/10.37349/eds.2024.00061
Received: April 04, 2024 Accepted: July 18, 2024 Published: September 13, 2024
Academic Editor: Fernando Albericio, Universities of KwaZulu-Natal, South Africa, Universidad de Barcelona, Spain
The article belongs to the special issue Innovative Therapeutics in Hepato-Gastroenterology
Aim: This study aimed to evaluate the utility of the combined use of contrast-enhanced ultrasound (CEUS) and intracavitary CEUS (IC-CEUS) with diluted SonoVue in the management of percutaneous treatment for pyogenic liver abscess (PLA).
Methods: 36 patients (23 males, 13 females; mean age 64 ± 13.9 years) with 39 PLAs (mean size 7.6 ± 3.4 cm) were selected for percutaneous catheter drainage (PCD) and/or percutaneous needle aspiration (PNA). CEUS and IC-CEUS were employed during the interventional maneuver and follow-up during hospital stay in all cases.
Results: 33 patients with 24 PLAs underwent PCD, 8 patients with 10 PLAs were treated with single or multiple PNA, and the combination of PCD and PNA was used in the remaining 5 cases. During the treatment planning phase, the combined use of CEUS and IC-CEUS affected therapeutic choices (e.g., drainage technique, additional therapeutic measures) in comparison with pre-operative imaging in 66.7% of patients. Throughout the follow-up period, CEUS and IC-CEUS facilitated monitoring of PLA evolution, providing crucial information, especially with IC-CEUS, on the optimal timing of catheter removal. No adverse events occurred after CEUS and IC-CEUS.
Conclusions: The combination of CEUS and IC-CEUS proved to be a powerful and safe tool for tailoring US-guided percutaneous treatments to patients with PLA.
Over the last two decades, contrast-enhanced ultrasound (CEUS) has gained popularity as a potent diagnostic tool for detecting and characterizing liver focal lesions. Its primary advantages include real-time examination, safety, and the ability to be performed in patients with renal impairment [1, 2]. More recently, attention has been directed towards CEUS applications in percutaneous interventional procedures such as liver biopsy, liver tumor ablation [3–7], and the intracavitary use of contrast agent (IC-CEUS) [6–12]. A significant benefit of CEUS-guided liver biopsy is the enhancement of diagnostic accuracy by selecting viable areas in complex focal liver lesions (FLL) with necrotic areas and targeting poorly visualized or invisible FLL [13]. In liver ablation, in addition to its well-known role in immediate post-ablation assessment [14], CEUS allows for the targeting of poorly visible FLL on B-Mode US [15]. CEUS may also play a role in detecting complications after interventional liver procedures (biopsy and ablation) [16].
IC-CEUS involves the injection of a diluted contrast solution directly into the catheter or needle to visualize the exact location of the cavity or tissue around the catheter or needle tip. This relatively new CEUS application has primarily been used in the management of abscess drainage, percutaneous nephrostomy, and biliary interventions [6–12].
Pyogenic liver abscess (PLA) is an uncommon but potentially life-threatening disease, with its incidence rapidly increasing in recent decades [17–19]. Causative agents include bacteria, fungi, and parasites, and the etiology is extremely variable, with the most frequent causes being biliary stone disease, post-surgical or post-interventional radiologic maneuvers, underlying malignancy, and hematogenous spread from intestinal infections [17–19]. Percutaneous drainage (PD) under US or computed tomography (CT) guidance combined with targeted antimicrobial therapy has long been recognized as the first-line therapy for PLA [20–22]. Surgery is generally considered when PLA fails to respond to PD, or the patient has concomitant abdominal diseases [23].
Two types of PD are available for PLA treatment: percutaneous catheter drainage (PCD) and percutaneous needle aspiration (PNA), both demonstrating overlapping results in terms of technical and clinical success rates [24, 25].
The aim of this study is to assess the role of the combined use of CEUS and IC-CEUS in the management of percutaneous treatment of PLA, with special reference to the decision-making process by a single operator caring for patients with PLA.
52 patients with 64 PLAs were treated at our Interventional Ultrasound Unit between January 2019 and December 2023. From this population, 36 patients (23 males and 13 females; mean age 64 ± 13.9 years) with 39 PLAs were selected for inclusion in the study if CEUS and IC-CEUS were used in combination to manage the clinical case.
This study was approved by the institutional review board and conducted in accordance with the ethical standards outlined in the Helsinki Declaration of 1975 and its later amendment. Signed informed consent was obtained from all patients before US-guided interventional procedures and the administration of US contrast media.
Table 1 presents the main characteristics of the PLAs, which were mostly polymicrobial, primarily attributed to E. Coli, Streptococcus, and Klebsiella pathogens. The differentiation between single and multiple abscesses was based on the presence of normal intervening liver parenchyma separating multiple abscesses and/or evidence that the abscesses were in different segments of the liver. Unilocular and multilocular abscesses were defined based on CEUS findings, considering the presence or absence of enhancing internal septations.
Main characteristics of the patients with pyogenic liver abscesses (PLAs)
| Characteristics | Cases |
|---|---|
| No. patients | 36 |
| No. PLAs | 39 |
| Mean size (cm, ± SD) | 7.6 ± 3.4 |
| Site right/left | 27/12 |
| Single/multiple* | 30/6 |
| Etiology | |
| Neoplasia colon/pancreas/biliary tract | 2/4/6 |
| Choledocholithiasis | 6 |
| Post-surgery | 7 |
| Idiopathic | 5 |
| Diabetes | 3 |
| Diverticulitis | 2 |
| Foreign body | 1 |
* Per patient
B-Mode US, CEUS, and contrast-enhanced examinations were performed before interventional procedures in all patients. Contrast-enhanced CT and/or contrast-enhanced magnetic resonance imaging (MRI) were available in 19 cases before US-guided procedures, while the remaining 17 patients were evaluated in an emergency setting, and PD was promptly performed based on B-Mode US and CEUS results.
CEUS was conducted following a standardized protocol: SonoVue (sulfur hexafluoride with a phospholipid shell; Bracco SpA, Milan, Italy) was administered intravenously as 2.4 mL boluses through the antecubital vein in 2–3 seconds, followed by a 5 mL flush of normal saline (0.9%).
State-of-the-art US machines (Resona 7 system, Mindray Bio-Medical Electronic Co, Shenzhen, People’s Republic of China; GE Logiq E10, GE Healthcare) were used in all cases. For IC-CEUS images, diluted SonoVue (0.1–0.5 mL of medium contrast in 20 mL of saline) was injected through needles during the interventional procedures, including needle aspiration and before passing the guidewire during the Seldinger maneuver. This method highlighted the internal abscess structure, distinguishing single vs multiple cavities and revealing communication either between liquified cavities in multilocular PLA or between PLA and adjacent anatomic structures (e.g., biliary ducts, hollow viscera, thorax). IC-CEUS was also performed just after catheter deployment to confirm correct positioning in all cases of PCD.
In addition to clinical and laboratory findings, CEUS and IC-CEUS were repeated every 2–3 days in all patients with the catheter left in place for continuous drainage to monitor PLA evolution in terms of morphology, providing useful information on the proper timing of catheter removal and patient discharge. All patients with uneventful outcomes were followed with imaging (prevalently US) up to three months after discharge to verify complete healing or relapse.
To assess the clinical impact of contrast-based techniques in the therapeutic management of PLA, CEUS and IC-CEUS results were considered clinically relevant findings (CRF) if they modified one of the following therapeutic choices in comparison with pre-operative imaging (B-Mode US, CT/MRI) results: 1) PCD vs PNA; 2) a combination of PNA and PCD; 3) the use of single vs multiple catheters according to the presence or absence of communication between compartments of complex abscess cavities; 4) additional therapeutic measures required (e.g., biliary stenting).
All US-guided interventional maneuvers were performed through transhepatic route access and with a biopsy guiding system. For continuous drainage, 8–12 French pigtail catheters with thread locks were placed into PLAs using the Seldinger technique, followed by vigorous irrigation with multiple boluses of 10–20 mL of saline until the returning material was clean. Multiple catheters were always deployed in the same session. PNA was carried out with 18-gauge Chiba needles in single or multiple sessions as needed. PLAs equal to or smaller than 3 cm did not undergo PD.
Aspirated pus was immediately sent to the laboratory for culture. Broad-spectrum antibiotics were usually administered to all patients before PD until the results of the laboratory tests were available, and the treatment was modified according to the causative organisms.
A single physician with more than 30 years of experience performed all interventional maneuvers, CEUS, and IC-CEUS and followed up on the clinical and imaging evolution of all patients. Adverse events after CEUS and IC-CEUS were recorded.
In total, 23 patients with 24 PLAs (mean size 8.5 ± 2.7) underwent PCD using 8–10F catheters in size, except for one case where a 12F catheter was utilized. PNA was associated with PCD in 5 patients to drain small cavities not in communication with major ones in the context of multilocular PLAs. In the remaining 8 patients with 10 PLAs (mean size 6.4 ± 2.3 cm) underwent single or multiple PNA using 18-gauge Chiba needles.
Out of 36 patients, 24 (66.7%) with 26 PLAs had an uneventful outcome after percutaneous treatment and complete resolution of the PLA and without relapses as assessed during follow-up after discharge. 11 patients did not benefit from interventional procedures due to the progression of their underlying neoplastic disease. In one patient, a large pseudo-tumoral mass remaining after PCD of the initial multilocular PLA was resected along with a metachronous right-sided colon cancer. Histology revealed only inflammatory features without neoplastic foci in the excised liver mass.
CEUS and IC-CEUS did not influence therapeutic decisions in 12 patients (33.3%) with 14 PLAs amenable to PD. In all cases but one (with a two-compartment PLA), the liquified cavity was clearly single on pre-operative imaging findings (Figure 1). PCD was carried out in 7 patients (using a single catheter in 6 cases), and single or multiple PNAs were performed in the remaining 7 patients.
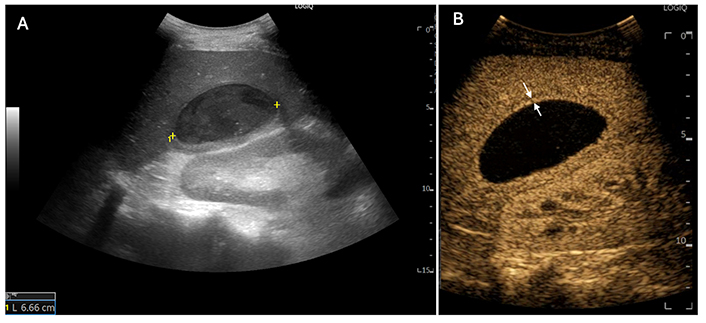
A 56-year-old man reported a high-grade fever one month after undergoing laparoscopic cholecystectomy for acute calculous cholecystitis. (A) Gray-scale ultrasound (US) reveals a well-marginated 6-cm lesion (between markers) with dense, necrotic content in the 6th segment; (B) contrast-enhanced ultrasound (CEUS): The abscess appears as a non-enhancing mass with a hyperenhancing peripheral rim (between white arrows) in the arterial phase. In this case, CEUS did not contribute to the treatment plan (deployment of a single catheter)
CEUS provided CRF in 21 out of 36 patients (58.4%) with 22 PLAs, leading to tailored PD due to the improved definition of PLA echostructure. In these patients, CEUS determined that multiple compartments existed in 18 PLAs, and a single necrotic and liquified cavity was present in 4 PLAs with complex echostructure at B-Mode US.
Consequently, a single catheter was confidently placed in 14 PLAs (4 unilocular and 10 with communicating loculi). In 8 PLAs with multiple non-communicating compartments, multiple PNAs (3 cases) and PNA combined with PCD (5 cases) were carried out (Figures 2, 3).
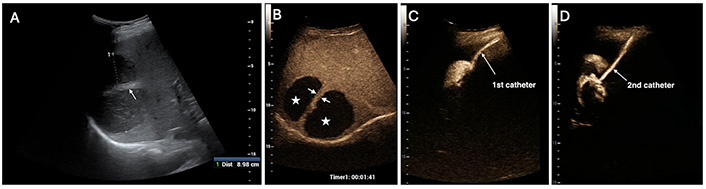
A 56-year-old woman presents with a right-sided pyogenic liver abscess (PLA) abscess measuring 9 cm. (A) B-Mode ultrasound (US) reveals a thick septum that separates two loculi (white arrowheads); (B) the two cavities (white stars) are not communicating, as the septum (white arrows) shows no disruption on contrast-enhanced US (CEUS); (C) and (D) two catheters (white arrows) are required for draining the PLA. It is noteworthy that the two cavities are visualized separately after the injection of a diluted contrast agent into their respective catheters
A 65-year-old man is diagnosed with a postoperative pyogenic abscess in the right lobe measuring 8.3 cm. (A) B-Mode ultrasound (US) depicts a thick septum (yellow arrowhead) similar to that in Figure 2A; (B) contrast-enhanced US (CEUS) reveals a “hole” (white arrow) in the septum, guiding the therapeutic choice as a single catheter deployment is considered the optimal treatment; (C) the two cavities (white stars) are shown to be communicating, as intracavitary CEUS (IC-CEUS) clearly indicates. White arrows point to the diluted contrast agent present in both loculi after injection through the catheter placed in the left-sided cavity
In 3 patients (8.3%), IC-CEUS was the only way to demonstrate communication between cavities in large PLAs with either a solid-like aspect on B-Mode (1 case) and “honeycomb” features on CEUS, CT, and MRI (2 cases), allowing the operator to place a single catheter in the major liquified cavity (Figures 4, 5, 6).
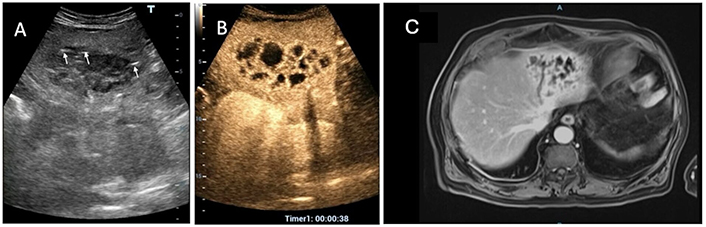
A 73-year-old man, was referred to emergency owing to high-grade fevere and a liver mass. Conventional ultrasound (US) reveals an 8.0 cm hypoechoic, heterogeneous, solid-like focal lesion in the left liver lobe with hyperechoic linear spots suggesting presence of air bubbles (white arrows) (A). The arterial phase of both contrast-enhanced US (CEUS) (B) and magnetic resonance imaging (MRI) (C) displays the characteristic “honeycomb” appearance indicative of a pyogenic liver abscess
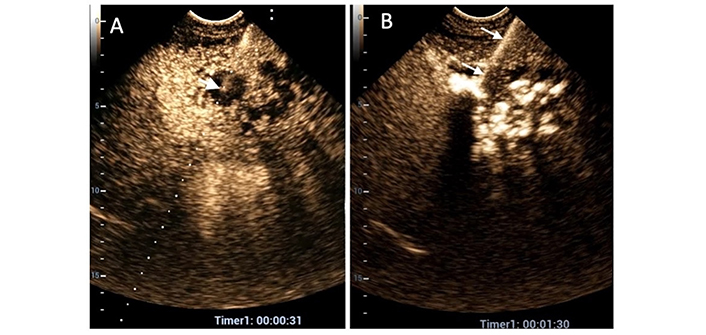
The same patient as in Figure 4. (A) Due to poor visibility on conventional ultrasound (US), contrast-enhanced US (CEUS) is employed to guide an 18-gauge Chiba needle into the largest liquified area (white arrow indicating the needle tip); (B) diluted SonoVue is promptly administered through the needle (white arrows), and the contrast agent diffuses into all liquified areas. In this case, the placement of a single catheter in the largest cavity is considered the optimal therapeutic choice
The same patient as in Figure 4. (A) A follow-up contrast-enhanced ultrasound (CEUS) scan on the 5th day reveals a clear reduction in the size of the abscess (between white arrows) with tiny residual liquified areas; (B) on the 7th day, it was not possible to enhance the abscess cavity (white star) with intracavitary CEUS (IC-CEUS) due to catheter occlusion. Since the daily output in the preceding two days was less than 20 mL, along with improvement in clinical and laboratory findings, the catheter was removed, and the patient was discharged. Forty-five days after discharge, magnetic resonance imaging (MRI) (C) and B-Mode US (D) reveal that no liver lesion is seen in the left liver parenchyma (black stars) where pyogenic liver abscess (PLA) was located
IC-CEUS demonstrated fistulous tracts between PLA and the biliary tree in 4 patients, prompting biliary stenting in three of them (Figure 7). In one more patient, IC-CEUS showed communication with the pleural cavity (Figure 8); thoracic PD was carried out.
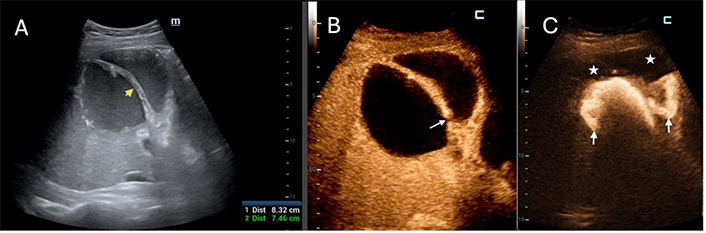
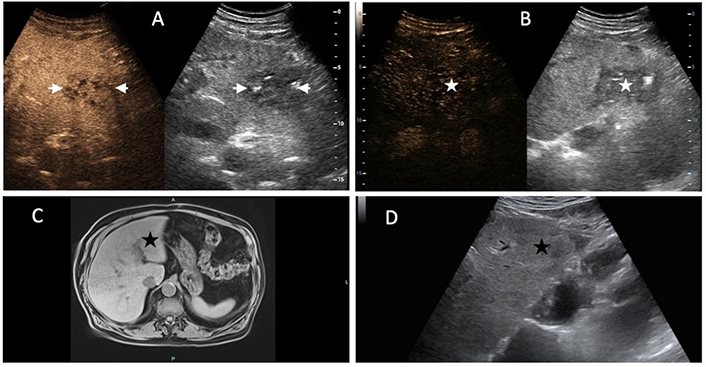
An 80-year-old woman with common bile duct cancer and recent palliative endoscopic stenting experienced an abrupt onset of high fever and malaise, prompting the clinical investigation. A 7 cm pyogenic liver abscess (PLA) was identified in the 7th segment on B-Mode ultrasound (US) (not shown). (A) contrast-enhanced US (CEUS) revealed an almost entirely non-enhancing abscessed mass with an incomplete septum (white arrow); (B) a single catheter was deployed, and intracavitary CEUS (IC-CEUS) displayed contrast agent filling the abscessed cavity without extravasation into the liver (L); (C) after three days of continuous drainage, B-Mode US showed a reduction in the size of PLA (black arrowheads) with the pig-tail catheter in place (black arrow). Note the absence of dilation of intrahepatic bile ducts; (D) diluted SonoVue was injected: The contrast agent filled up the residual cavity (white arrow), but a fistulous tract was visible (white arrowheads); (E) the contrast agent moved from the abscess (A) to the right bile ducts (white arrows) through the fistula (between arrowheads). The biliary stent was then replaced
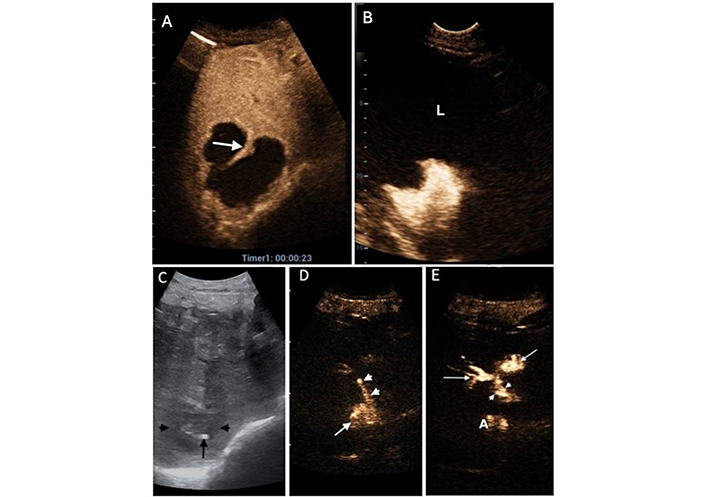
A 75-year-old woman with pancreatic cancer, biliary obstruction, and multiple liver abscesses had a pyogenic liver abscess (PLA) located at the hepatic dome, which underwent percutaneous catheter drainage. (A) Immediately after catheter deployment, intracavitary contrast-enhanced ultrasound (IC-CEUS) was performed: The contrast agent collected not only in the subdiaphragmatic liver abscess (LA) but also in the thorax (T) (between white arrows). Arrowheads indicate the catheter; L= Liver (B) a computed tomography (CT) scan confirmed the presence of PLA (black arrows) and an abscess above the diaphragm (white star) in the thorax (Tx). The patient underwent percutaneous drainage of the purulent collection in the chest (the yellow “R” and “L” represent “Right” and “Left”)
Moreover, CEUS was employed as a guidance system to reach the optimal area for needle puncture not clearly visible on conventional US in 4 patients (11.1%) (Figure 5A).
At the time of catheter deployment, IC-CEUS immediately confirmed the correct positioning of the catheter inside the target PLA in all 23 patients treated with PCD. No patient required catheter repositioning, yielding a 100% success rate for the interventional procedure.
To monitor the evolution of PLA morphology, CEUS and IC-CEUS were used in combination to provide a “from outside” and “from inside” view, respectively. This information, along with the daily catheter output and the clinical and laboratory data, made the operator more confident regarding the timing of catheter removal and patient discharge. During the hospital stay, IC-CEUS provided information on catheter patency and position: In 2 cases, the lack of passage of ultrasound contrast agent (UCA) inside the PLA cavities signaled catheter occlusion (Figure 6B), whereas in 2 more patients, diffusion of UCA outside of the PLA cavity suggested catheter dislodgment (Figure 9). The catheter was immediately removed in all these 4 patients. The average duration of the catheter in place was 7.3 ± 3.3 days.
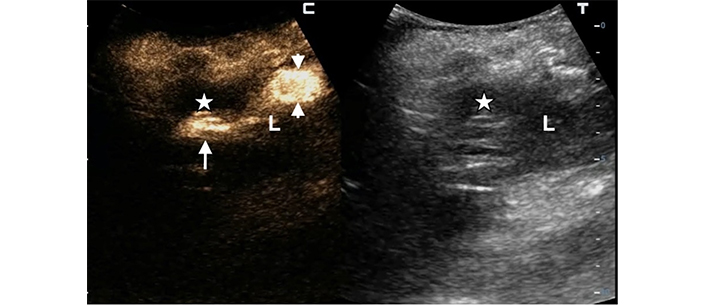
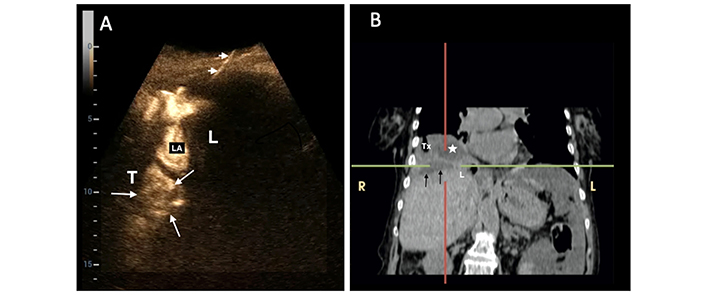
A 68-year-old woman underwent percutaneous catheter drainage (PCD) for a 6 cm subcapsular pyogenic liver abscess (PLA) in the left lobe. In the split-screen image of the follow-up intracavitary contrast-enhanced ultrasound (IC-CEUS) on the 4th day, the contrast agent collected only partially in the abscessed cavity (white arrow in the left split screen); most of the diluted SonoVue was observed in the perihepatic peritoneal space (between white arrowheads), indicating partial catheter dislodgment (white stars = residual abscessed cavity; L = liver)
No adverse effects were registered after intravenous and intercavitary injection of the contrast medium. Major complications and death were never recorded after PCD and PNA, whereas a minority of patients (20%) experienced one or more minor complications (intense pain at the site of puncture, abdominal pain, small pleural effusion).
In this study, we aimed to assess the clinical utility of the combined use of CEUS and IC-CEUS in the daily practice of a single operator managing patients with PLA amenable to percutaneous treatment. The therapeutic decisions were modified and better tailored to individual patients in 58.4% of cases after CEUS. The demonstration of real PLA echostructure, accurate measurement of liquified areas, and determination of the presence or absence of communication between abscessed loculi assisted the operator in the decision-making process. Specifically:
PNA was favored as the sole therapy when a small (< 4 cm) liquified area, even in large abscesses, was observed.
Not-communicating multiple cavities within complex multilocular PLAs prompted either multiple needle aspirations or the combination of PNA and PCD.
A single catheter positioned in the largest cavity was deemed sufficient for drainage in cases of large multilocular PLAs with communicating loculi.
In a minority of cases (11.1%), CEUS was also crucial for guiding needles in the largest liquified compartment of multilocular PLAs that appeared poorly defined on B-Mode US.
During the initial phase of the therapeutic plan decision-making process, IC-CEUS provided a limited contribution. It helped in tailoring the therapeutic approach in 8.3% of cases by demonstrating, contrary to all pre-operative imaging techniques, real communication between the largest necrotic area and multiple tiny cavities inside PLA.
Overall, the combination of CEUS and IC-CEUS guided PD options in 66.7% of our patients. The added value of IC-CEUS was particularly evident in cases where fistulous communication with adjacent anatomic structures (the biliary tree/pleural cavity) was demonstrated, prompting additional therapeutic measures (biliary stenting/thoracic drainage). Throughout patients’ follow-up during hospital stay, the combination of CEUS and IC-CEUS facilitated the monitoring of morphology and size (of both the entire PLA and the liquified component) and optimized the timing of catheter removal in cases of continuous drainage, along with clinical and laboratory findings. Moreover, IC-CEUS revealed occlusion or dislodgement of catheters, necessitating immediate catheter withdrawal.
It is noteworthy that, despite a relatively short drainage duration (one week on average), none of our patients with an uneventful outcome experienced a PLA relapse, confirming that catheters had been removed at the right time. This is crucial, as premature catheter removal may worsen prognosis and increase the risk of treatment failure [23, 26].
In our series, clinical success was independent of the multilocular structure and size (> 7 cm) of PLA, contrary to some reports associating these characteristics with PD failure [27]. This aligns with the majority of findings reported by other authors [28–30].
The absence of adverse events after intravenous and intracavitary injection confirms the high tolerability profile of the US contrast agent [2, 6, 7, 10]. Due to its safety and non-invasiveness, CEUS and IC-CEUS can be liberally used to follow-up patients according to operator’s judgment even at the bedside. Only conventional US could be an alternative to monitor PLA evolution but limits of B-Mode US in recognizing catheter tip inside or outside (in case of dislocation) drained cavities are well known. CT and/or MRI expose patients to radiations, are more expensive, and are not invariably available in all geographical areas.
Limitations of our study include: 1) the lack of randomization to a control group (managed by either CT or CEUS and IC-CEUS), which is challenging to implement in real-life scenarios; 2) the absence of radiological verification in some cases, but exhaustive imaging studies might not have necessarily added value to diagnosis and treatment; 3) the potential non-reproducibility of a single operator’s experience elsewhere.
In conclusion, the combined use of CEUS and IC-CEUS proved to be a powerful and safe tool for a single operator managing patients with PLA. It enabled the tailoring of treatment approaches to individual cases, facilitated the monitoring of PLA evolution over time, and provided decisive information, especially with IC-CEUS, on the optimal timing of catheter removal.
CEUS: contrast-enhanced ultrasound
CT: computed tomography
FLL: focal liver lesions
IC-CEUS: intracavitary contrast-enhanced ultrasound
MRI: magnetic resonance imaging
PCD: percutaneous catheter drainage
PD: percutaneous drainage
PLA: pyogenic liver abscess
PNA: percutaneous needle aspiration
GF: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Validation. The author read and approved the submitted version.
The author declares that there are no conflicts of interest.
The present study “Clinical utility of combined contrast-enhanced ultrasound and intracavitary contrast-enhanced ultrasound in the percutaneous treatment of pyogenic liver abscess” was approved by the Pineta Grande Ethics Committee, protocol number 123/2022.
Informed consent to participate in the study was obtained from all participants.
Patients gave their consent to publication of images of their examinations.
The datasets [clinical records and imaging] for this study can be requested to archivio@pinetagrande.it.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3168
Download: 20
Times Cited: 0
Davide Scalabrini ... Pietro Andreone
Elena Franchi ... Pietro Andreone
