Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Affiliation:
Molecular Oncology Laboratory, Department of Zoology, University of Delhi (North Campus), Delhi 110007, India
Email: alokchandrab@yahoo.com
ORCID: https://orcid.org/0000-0002-5996-2832
Explor Drug Sci. 2024;2:583–613 DOI: https://doi.org/10.37349/eds.2024.00063
Received: March 01, 2024 Accepted: June 28, 2024 Published: September 24, 2024
Academic Editor: Noah Isakov, Ben Gurion University of the Negev, Israel
The article belongs to the special issue Remedial benefits of natural products in inflammation and cancer
Cervical cancer (CaCx) is the fourth most prevalent cancer in women contributing to 341,831 annual deaths globally in 2020. Owing to its high mortality rate, the identification of novel inhibitors preventing CaCx progression is of utmost importance. Recent studies have emphasized the use of phytochemicals for cancer prevention due to their low toxicity. Psoralidin, a bioactive compound extracted from the seeds of the medicinal plant Psoralea corylifolia, showcases the potential for promoting health benefits. A range of studies showing anti-inflammatory, anti-oxidant, estrogenic, neuroprotective, anti-diabetic, anti-depressant, antimicrobial, and anti-tumor activities substantiate its promising biological effects. The anti-tumor potential of psoralidin has been well-documented. Its capacity to effectively target cancer stem cells (CSCs) in general adds to its therapeutic potential. Psoralidin carries out its anti-cancer activity by inducing oxidative stress, autophagy, and apoptosis. This unique characteristic suggests its potential to be used as an adjunct molecule in combination with existing treatment to enhance the efficacy of chemo/radiotherapy for treating CaCx. However, low bioavailability and intestinal efflux limit the use of psoralidin in clinical applications. Therefore, further investigation is needed in area of drug delivery and mechanism of action to fully harness the beneficial effects of psoralidin. The present study examines the current understanding of the molecular properties of this coumestan, as well as its various molecular targets with a particular emphasis on its anti-cancer activity. The study will help in designing effective and novel therapeutic interventions for targeting signaling pathways and other regulators involved in mediating CaCx progression, which will eventually help in effective management of CaCx.
Cervical cancer (CaCx) manifests as slow-growing malignancy in the cervix region [1] and ranks as the fourth most prevalent cancer in women [2]. Over the past decade, various intervention strategies, including screening and vaccination, have successfully transformed CaCx into a preventable disease in developed nations. The United States has witnessed a notable and consistent decrease of 54% in CaCx cases from 1973 to 2007 [3]. Likewise, estimates suggest that by the end of the current decade, Australia will achieve the complete eradication of CaCx cases [4]. Although there has been significant advancement in preventing cases of CaCx using frequent screening procedures globally, the rate of mortality associated with CaCx has stayed relatively constant in developing nations. The global stagnation in cancer mortality rate is an outcome of a concomitant increase in incidence and mortality rates in underdeveloped countries that nullify the intense preventive measures being undertaken in the developed nations [5]. In many low resource regions, the incidence and mortality rates of CaCx exceed the thresholds established by the World Health Organization (WHO). India alone accounted for approximately 18.3% of the total CaCx cases occurring at the global level in 2020. According to the Indian Council of Medical Research (ICMR), about 96,922 new cases of CaCx were reported in India with mortality of about 60,078 per year during 2012–2018 [6]. Indian women like many others from underdeveloped regions face similar challenges of lack of screening and detection of malignancy at relatively advanced stages leaving them with limited therapeutic options other than conventional anti-cancer therapies having several debilitating effects.
In this context, WHO launched the “CaCx Elimination Initiative” in 2020 as a global strategy to expedite the eradication of CaCx. This initiative was introduced with the objective of reducing the incidence rate of CaCx to fewer than 4 cases per 100,000 patients by the year 2030, relying on the implementation of specific targets, commonly referred to as the “90-70-90” plan. This plan involves ensuring that 90% of girls under the age of 15 years are vaccinated, 70% of women undergo high-performance screenings at least twice before reaching the age of 45 years, and 90% of women identified with CaCx receive appropriate treatment [7]. To achieve the first two targets, preventive strategies like vaccination and screening procedures are already in place and have only operational challenges. However, the third target specifically requires discovery and development of novel therapeutics for effective CaCx management.
Recent studies have emphasized the use of phytochemicals as novel therapeutics for cancer prevention due to their low toxicity. In this regard, a phytochemical psoralidin obtained from the seeds of Psoralea corylifolia has gained the attention of scientific community for its multifaceted biological effects. This comprehensive review aims to consolidate the evidence on the health benefits of psoralidin with particular emphasis on its anti-tumor effects. Additionally, the review delineates the specific target proteins and signaling pathways modulated by psoralidin, shedding light on its mechanism of action.
CaCx arises in the cervix region of the uterus and ranks as the 4th most common cancer among women. According to GLOBOCAN 2020 data, 604,127 new cases of CaCx occur every year with 341,831 deaths reported annually worldwide [2]. However, the statistic changes considerably across countries as well as within each country [5]. Less developed countries with poor economic development and health infrastructure harbor over 85% of CaCx cases [8].
The primary etiological agent of CaCx is persistent infection with high-risk human papillomaviruses (HPV) [9]. Over 99% of CaCx cases are attributed directly to HPV infection [10]. However, HPV infection alone is not sufficient to cause CaCx. Impaired immune system, prolonged use of oral contraceptives, smoking habits, multiple sexual partners, early sexual activity, and co-infection with other sexually transmitted infections, can increase the risk of CaCx in individuals infected with high-risk HPV types [11]. HPV is a non-enveloped epitheliotropic double-stranded DNA virus belonging to the Papillomaviridae family. More than 200 different HPV genotypes, mainly infecting the squamous and cutaneous epithelium containing body parts, have been recognized till date [12]. Depending upon their association with genital tract cancer, these HPVs are divided into high- and low-risk types. The high-risk group includes types 16, 18, 31, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 70 and are associated with cervical carcinoma [13]. The most common high-risk types of HPV associated with CaCx are HPV16 followed by HPV18 that are collectively contributing to 70–90% of CaCx at the global level, however, in India HPV16 is the predominating strain causing nearly 90% of CaCx cases alone [14].
CaCx originates in the cervix region of the uterus and progresses through well-defined stages. Initially, HPV infects the basal cells residing in the squamo-columnar junction of the cervix commonly described as “transformation zone”. Following HPV infection, these basal cells undergo dysplastic changes to form precursor lesions. On the basis of severity, the degree of dysplastic changes is graded as: cervical intraepithelial neoplasia grade 1 (CIN1), CIN2 and CIN3 [15]. In the majority of cases, HPV infection is clear within a year due to high immune surveillance, however, in 10–20% of cases infection persists for years and leads to the development of CaCx [16]. CIN1 stage is characterized by mild dysplasia affecting only 1/3 of the epithelium, whereas CIN2 and CIN3 are characterized by severe dysplasia affecting the entire epithelium [17]. Till CIN1 stage, HPV remains in the episomal stage, however, at CIN2 stage there is a likelihood of double-stranded breaks in both viral and host DNA due to inflammation induced reactive oxygen species (ROS) generation. This break facilitates the integration of HPV genome into host DNA and activation of viral oncoproteins E6 and E7 required for maintenance of CaCx phenotype [18]. E6 mediates cervical carcinogenesis by degrading tumor suppressor protein p53 [19], whereas E7 inhibits the repressive function of retinoblastoma proteins [20]. The degradation of tumor suppressor proteins in CIN3 stage leads to invasive cervical carcinomas.
Surgery, chemotherapy, radiation, and concurrent chemoradiotherapy are among the therapeutic modalities being used in clinical management for CaCx treatment, depending on the stage of the disease [21]. Surgery is a commonly used and successful technique in combatting early-stage cancers as it involves the physical removal of cancerous tissue [22]. Cisplatin is the most common therapeutic agent used for chemotherapy [23]. Studies have reported that combining cisplatin with other agents is potentially more effective than single drug treatment. Currently, topotecan, paclitaxel, and other non-platinum-based chemotherapeutics such as 5-fluorouracil and bleomycin, are commonly used in combination with cisplatin for treating CaCx [21]. Radiotherapy uses high-energy X-rays and is a major treatment in the management of CaCx [24]. Although these approaches are effective therapeutic strategies, they have been associated with severe side effects, some of which can be life-threatening [25]. Despite notable progress in combining therapies to enhance the effectiveness of individual treatments, there is still a pressing need for novel and improved therapies. Various alternative approaches have been investigated for treating CaCx, including immunotherapy [26, 27], targeted therapy [28], and genetic interventions like CRISPR/Cas9 [29] and RNAi [30, 31]. Although these therapies exhibit growing potential in improving treatment results, a significant portion of them are still in the investigational stage and costly alternatives.
In the last two decades, our group has extensively worked on therapeutic targeting of CaCx with different plant derivatives and plant-based bioactive compounds. Berberine derived from Berberis aquifolium showed anti-CaCx activity by targeting activator protein 1 (AP-1) and HPV oncoproteins E6 and E7 [32, 33]. Likewise, the Bryophyllum pinnata leaf extracts [34] and Phyllanthus emblica fruit extracts [35] showed significant anti-cancer and anti-HPV activity in CaCx by inhibiting the expression of fos proto-oncogene (c-fos), AP-1 and HPV gene expression [32, 34]. The plant-derived homeopathic preparations of B. aquifolium and B. vulgaris also showed significant anti-CaCx activity [36]. However, none of the formulations have been taken further for clinical use. Another major hurdle in the existing CaCx treatment regimen is therapy resistance and tumor relapse/recurrence. After employing chemo/radio therapy, recurrence is reported in 20–70% of cases depending upon the clinical stages [37]. The frequent recurrence of CaCx can be attributed to the presence of therapy-resistant cells or CSCs within the tumor microenvironment, which significantly hampers the efficacy of treatment [38]. Therefore, new therapeutic leads that can target the different hallmark features of CaCx progression and cervical CSCs (CCSCs) are urgently needed for its complete elimination.
There are various molecular markers that mediate cervical carcinogenesis that could serve as potential therapeutic targets for emerging therapeutic approaches against CaCx. Tumor necrosis factor alpha (TNF-α), an inflammatory cytokine promoted CaCx initiation, progression angiogenesis, and migration via vascular endothelial growth factor C (VEGFC)-mediated Akt and extracellular signal-regulated kinase (ERK) signaling pathways [39]. The other crucial target for preventing cervical carcinogenesis is p53. HPV E6 oncoprotein relies on the p53-dependent pathway [40]. By targeting p53 for degradation via ubiquitination with the help of E6AP (angelman syndrome-associated protein), HPV E6 disrupts the normal function of p53 [41]. This interference prevents p53 from inducing cell cycle arrest or apoptosis in response to cellular stress, facilitating uncontrolled cell proliferation and further leading to the development of CaCx [42]. Therefore, targeting E6 could be an effective therapeutic strategy. The epidermal growth factor receptor (EGFR)-AS1/ACTN4 axis also plays a significant role in activating the Wnt signaling pathway in CaCx progression. EGFR-AS1 enhanced CaCx cell growth by up-regulating ACTN4, which in turn promoted the nuclear translocation of CTNNB1 (β-catenin), further leading to the activation of downstream Wnt pathway genes such as cyclin D1 and c-myc [43]. This activation promotes cell proliferation, migration, and invasion, thus contributing to CaCx progression. Transcriptome analysis studies have also shown the pivotal role of PI3K in initiation and progression of CaCx [44]. Receptor tyrosine kinases (RTKs) activation triggered PI3K, leading to conversion of phosphatidylinositol (4,5)-bisphosphate (PIP2) to PIP3 and subsequent Akt activation [45]. PTEN (phosphatase and tensin homolog) could reverse Akt activation by dephosphorylating PIP3, but its absence reported in CaCx allows Akt to activate mechanistic target of rapamycin (mTOR), promoting cell growth, proliferation, and survival. This dysregulation is usually induced by HPV oncoproteins E6 and E7 [46].
The NF-кB signaling pathway, activated by HPV infection, plays a dual role in CaCx progression. In early stages, it aids immune response against HPV [47], while in persistent infection, it facilitates viral replication [48]. E6 and E7 HPV protein activated NF-кB, which further upregulated the expression of other downstream factors in the signaling cascade such as cIAP2 [49], CD47 [50], LPTS [51], and STAT3 [51, 52]. This cascade ultimately helped in HPV-infected cells to proliferate, survive, and evade apoptosis highlighting NF-κB as a key player in CaCx development [53].
The Notch signaling pathway also plays an important role in the progression of CaCx. By binding of ligands such as Delta and Jagged, the Notch receptor undergoes cleavage and generates activated intracellular Notch [54]. This activation triggers transcriptional activation of Notch target genes like HES1 and cyclin D1, and activation of pathways like PI3K-Akt in CaCx [55]. CaCx progression is also facilitated by NOX (NADPH oxidase) which plays a vital role in different cellular processes like epithelial-mesenchymal transition (EMT), angiogenesis, apoptosis evasion, and metastatic potential [56]. NOX increases ROS, proteins like Src (a non-RTK), and focal adhesion kinase (FAK; a cytoplasmic tyrosine kinase). This activation of Src and FAK helps in driving certain cellular processes related to cancer spread. NOX isoforms, such as NOX2 and NOX4, are induced in response to transforming growth factor β1 (TGF-β1) stimulation and impact cell proliferation, migration, and cytoskeletal remodeling [57]. NOX2 and NOX4, triggered by a molecule called TGF-β1, have different effects on the growth of CaCx cells [57]. Therefore, considering the valuable contribution of these markers in CaCx progression, they can serve as the key molecular target of different therapeutic agents for elimination of CaCx cells.
Medicinal plants have been utilized for treating various ailments since ancient times. In rural regions of developing nations, these plants remain the primary medicinal resource. A significant number of medicinal plants have been explored for their therapeutic value till date. Among them, P. corylifolia, a member of the Leguminosae family, holds considerable medicinal importance with a history of millennia in clinical practice. This plant has been extensively utilized in various traditional Chinese and ayurvedic medicine formulations to address conditions such as leucoderma and skin disorders. Moreover, the extract obtained from different parts of the plants such as fruits, leaves, or the whole plant extracts are being extensively employed as a remedy for a diverse range of ailments, including hypertension, nephritis, skin diseases, cardiovascular abnormalities, and reviewed recently [58]. There has been a renewed vigor in phytochemical research for their medicinal use due to their lesser toxicity and fewer side effects. Till now, over 165 bioactive compounds have been identified in the extracts of these plants belonging to different groups of phytochemicals such as coumarins, monoterpene phenols, flavonoids, isoflavanoids, and benzofurans [59–65]. Among these constituents, psoralidin has recently gained greater attention of the scientific community due to its multi-factorial health promoting benefits. Psoralidin can also be derived from other plant sources such as Cullen corylifolium, Dolichos trilobus, and Phaseolus lunatus. The first study about the structure of psoralidin was reported in 1961 wherein, Khastgir et al. [66], reported the chemical structure of psoralidin isolated from the phenolic extracts of seeds of P. corylifolia. Thereafter in 1996, the cytotoxicity of psoralidin was evaluated in gastric carcinoma (SNU-1 and SNU-16) [67], colon (HT-29), and breast cancer (MCF-7) cell lines [68]. Initially, the studies were limited to cytotoxic properties against cancer cells grown in vitro, later on, other health benefits of psoralidin such as anti-bacterial [69] and anti-diabetic [70] were evaluated thus opening the area for further exploration of health benefits of psoralidin.
Psoralidin, a 3,9-dihydroxy-2-(3-methylbut-2-enyl)-[1]benzofuro[3,2-c]chromen-6-one, is a prenylated coumestrol belonging to polyphenols group of phytochemicals. This coumestan has been substituted by hydroxyl groups at 3 and 9 positions with a prenyl group at 2nd position (Figure 1).
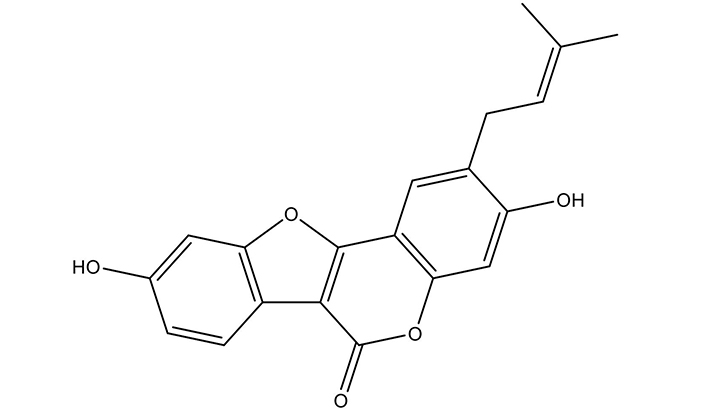
Chemical structure of Psoralidin. The 2-dimensional structure was taken from PubChem and redrawn using ChemDraw
The prenyl modification of compounds has shown to improve its binding affinity with the cell membrane resulting in better absorption and bioactivity [71]. Although it is a natural compound extracted from plants, Pahari et al. [72, 73], chemically-synthesized psoralidin in laboratory conditions by condensation, intramolecular cyclization, and cross metathesis reaction of acid chloride and phenyl actetate yielding 73% yield.
The molecular weight of psoralidin is 336.3 Da with an empirical formula of C20H16O5. It is mostly present in solid form with melting point ranging from 290°C to 292°C. A detailed description of the physio-chemical properties of psoralidin is given in Table 1. In a biological system and as a potential drug entity, psoralidin complies with Lipinski’s rule of five without any violations, suggesting its suitability for drug development. Additionally, its inability to permeate the blood brain barrier (BBB) suggests its safety for medicinal use and protection of brain tissue from any undesirable drug toxicity. With a bioavailability score of 0.55 with 1 showing maximum bioavailability, it demonstrates moderate potential for absorption (unpublished data) as calculated by freely-available webserver tools pkCSM and SwisADME [74, 75].
Physio-chemical properties of psoralidin
| Descriptor | Value |
|---|---|
| Empirical formula | C20H16O5 |
| Chemical name | 3,9-dihydroxy-2-prenylcoumestan |
| Molecular weight | 336.3 g/mol |
| Polar surface area | 79.9 Å2 |
| Physical form | Solid |
| Melting point | 290–292°C |
| LogP | 4.6123 |
| Rotable bonds | 2 |
| Hydrogen acceptors | 5 |
| Hydrogen donors | 2 |
| Solubility in water | Poor |
| Bioavailability score | 0.55 |
| BBB permeant | No |
| Lipinski’s violation | 0 |
Psoralidin has been studied for its therapeutic potential in diverse pathological conditions (Figure 2). The health benefits ranging from bolstering immune function to supporting cardiovascular health are summarized in Table 2.
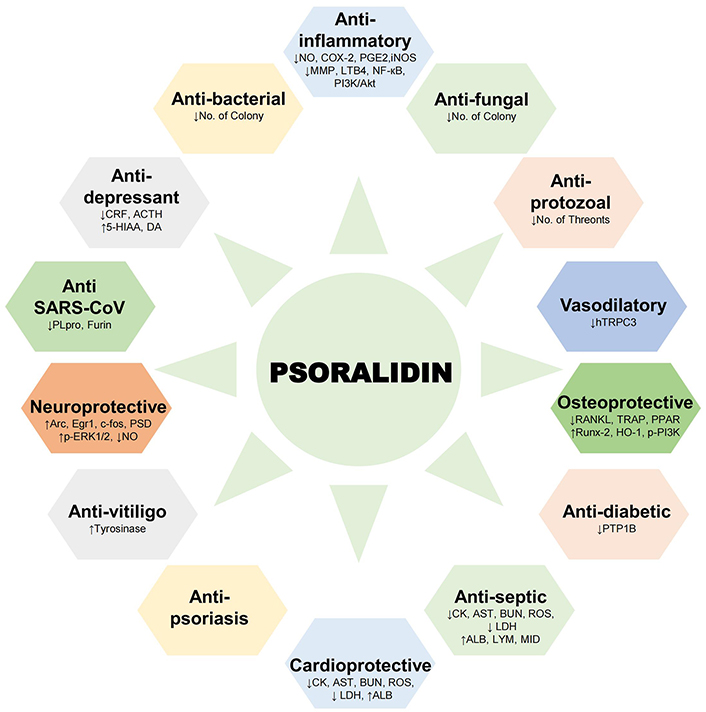
Health benefits of psoralidin. Schematic representation of pharmacological activities demonstrated by psoralidin in different physiological and pathological conditions. ACTH: Adrenocorticotropic hormone; ALB: albumin; Arc: activity-dependent cytoskeleton associated protein; AST: aspartate aminotransferase; BUN: blood urea nitrogen; c-fos: fos proto-oncogene; CK: creatine kinase; COX-2: cyclooxygenase-2; CRF: corticotropin releasing factor; DA: dopamine; Egr1: early growth response protein 1; 5-HIAA: 5-hydroxy indoleacetic acid; HO-1: heme oxygenase-1; LDH: lactate dehydrogenase; LYM: lymphocyte; NO: nitric oxide; p-ERK1/2: phosphorylated extracellular signal-regulated kinase 1/2; PI3K/Akt: phosphatidylinositol 3-kinase/protein kinase B; PLpro: papain-like protease; PPAR: peroxisome proliferator-activated receptor; PSD: post-synaptic density; PTP1B: protein tyrosine phosphatase 1B; RANKL: receptor activator of nuclear factor kappa-B ligand; ROS: reactive oxygen species; Runx-2: runt-related transcription factor 2; TRAP: tartrate-resistant acid phosphatase
Health benefits of psoralidin and key molecular targets
| Biological effect | Psoralidin source | In vitro/in vivo | Study model | Drug concentration | Test/Assay | Molecular targets | References |
|---|---|---|---|---|---|---|---|
| Anti-bacterial | Seed extract | In vivo | Shigella sonnei, Shigella flexneri | 200 µg/disc | Disc diffusion method | NA | [69] |
| Hexane extract from seeds | In vivo | Escherichia coli, Staphylococcus aureus | 90–150 µg/mL | Inhibition assay | NA | [76] | |
| Anti-diabetic | Ethanolic extract from seeds | In vitro | NA | 10 µM | PTP1B, VHR, PPase1 | ↓Tyrosine phosphatase 1B | [70] |
| Anti-inflammatory | Methanolic extract from seeds | In vitro | RBL-2H3 | 30–100 µM | β-hexosaminidase bioassay | ↓Hexosaminidase release | [77] |
| Methanolic extract from seeds | In vitro | Mice peritoneal exudate cells | 29 µM | NO production Assay | ↓NO | [78] | |
| Commercial | In vitro, In vivo | HFL-1, MRC-5, BALB/c mice | 50–100 µM | Luciferase reporter gene assay, ITc assay | ↓COX-2, PGE2↓PI3K/Akt, NF-кB↓LTB4↑TNF-α, TGF-β, IL-6, IL-1α/β | [79] | |
| Methanolic extract from seeds | In vitro | RAW 264.7 | 1–30 µM | - | ↓PI3K/Akt↓iNOS | [80] | |
| Commercial | In vivo | Osteoarthritic chondrocytic pellet cultures | 1–50 µM | Biochemical, Immunological, Histological analysis | ↑Cartilaginous matrix↑GAG/DNA ratio↓MMP13, MMP1, MMP3 | [81] | |
| Ethanolic extract from roots | In vitro | RAW 264.7 | 20 µM | NO production assay, α-glucosidase inhibitor assay | ↓α-glucosidase | [82] | |
| Anti-depressant | Seed | In vivo | ICR mice | 20–60 mg/kg | Forced Swimming Test, Open Field Test | ↑Swimming behaviour↑5-HIAA, DA↓CRF, ACTH, corticosterone | [83] |
| Seed | In vitro | N2A | 160 µM | CRF promoter activity assay | ↓CRF promoter acitivity↓ CRF transcription | [84] | |
| SARS-CoV | Ethanolic extract from seeds | In vivo | E. coli | NA | PLpro inhibition assay | ↓PLpro | [85] |
| NA | In silico | NA | NA | Molecular Docking | ↓Furin | [86] | |
| Anti-protozoal | Methanolic extract from fruits | In vivo | Ichthyophthirius multifiliis | 1 mg/L | Efficacy assay | ↓Theronts number | [87] |
| Neuroprotective | Ethanolic extract from seeds | In vitro | BV-2, HT22 | 1.25–5 µM | CCK assay | ↓NO | [88] |
| Commercial | In vivo | Cerebral cortices and hippocampi from ICR mice | 1–20 µM | PSD 95/VGLUT1-positive clusters quantification | ↑Arc, Egr1, c-fos↑p-ERK1/2↑PSD 95/VGLUT1-cluster | [89] | |
| Vasodilatory | Ethanolic extract from seeds | In vivo | Sprague Dawley rats, HEK293 | NA | Electrophysiology | Aortic ring relaxation↓hTRPC3 current | [90] |
| Osteoprotective | Commercial | In vitro | Rat calvarial osteoblasts | 1 µM | ALP and TRAP activity | ↑Osterix, Runx-2, IGF-1, β-cateninOPG↓RANKL↓TRAP | [91] |
| Commercial | In vitro, In vivo | Bone marrow macrophages, ICR mice | 1–50 µM5–50 mg/Kg | TRAP assay, Bone absorption assay, F-actin labelling | ↓TRAP-positive cells↓F-actin ring formation↓p-P38, p-JNK, p-ERK1/2, RANKL | [92] | |
| Ethyl-acetate extract from seeds | In vitro, In vivo | BMSCs, Sprague Dawley rats | 10 mg/kg | DEXA, MicroCT, TRAP, ORO staining | ↓TRAP↑p-PI3K, p-Akt↑HO-1 | [93] | |
| Commercial | In vitro | Rat chondrocytes | 1–20 µM | Caspase-3 and Caspase-9 activity assay, NO assay | ↑Bcl-2↓Bax↓NF-кB nuclear localization | [94] | |
| Commercial | In vitro | BMSCs, MC3T3-E1, 3T3-L1 | 0.001–20 µM | ALP, ORO staining | ↑ALP↑BSP, OCN↓PPAR δ, CEBPα, LPL, Fabp4 | [95] | |
| Commercial | In vitro, In vivo | BMSCs, C57BL/6J mice | 1–10 µM10 mg/kg | ARS, ALP, and ORO staining, MicroCT | ↑Runx-2, Osterix, OCN↓PPAR γ, Fabp4, LPL | [96] | |
| Anti-vitiligo | Ethanolic extract from whole plant | In vitro | NA | NA | HPLC, tyrosinase activity | ↑Tyrosinase | [64] |
| Psoriasis | Ethanolic extract from seeds | In vivo | Pigs and mice | NA | H&E staining | No effect | [97] |
| Cardioprotective | Commercial | In vivo, In vitro | BALB/c mice, HL-1 | 25 mg/kg20–100 µM | Echocardiography evaluation, LDH release assessment | ↓CK, AST, BUN↑ALB↓ROS, LDH | [98] |
| Anti-fungal | Hexane extract from seeds | In vivo | Candida albicans, Trichophyton rubrum | 160–180 µg/mL | Inhibition assay | NA | [76] |
| Anti-septic | Ethanolic extract | In vivo | CLP-injured mice | 50 mg/kg | Sepsis score analysis | ↓Sepsis score↓LDH, AST, BUN, CK ↑ALB↑WBC, LYM, MID, GRA, PLT | [99] |
ACTH: adrenocorticotropic hormone; ALB: albumin; ALP: alkaline phosphatase; Arc: activity-dependent cytoskeleton associated protein; AST: aspartate aminotransferase; Bax: B-cell leukemia/lymphoma 2 protein associated X protein; Bcl-2: B-cell leukemia/lymphoma 2 protein; BMSCs: bone marrow mesenchymal stem cells; BSP: bone sialoprotein; BUN: blood urea nitrogen; CCK: cholecystokinin; CEBPα: CAAT/enhancer binding protein alpha; c-fos: fos proto-oncogene; CK: creatine kinase; CLP: cecal ligation and puncture; COX-2: cyclooxygenase-2; CRF: corticotropin releasing factor; DA: dopamine; Egr1: early growth response protein 1; Fabp4: fatty acid-binding protein 4; GRA: glucocorticoid receptor agonists; HEK: human embryonic kidney; 5-HIAA: 5-hydroxy indoleacetic acid; HO-1: heme oxygenase-1; IGF-1: insulin-like growth factor 1; iNOS: inducible nitric oxide synthase; p-JNK: phosphorylated c-Jun N-terminal kinase; LDH: lactate dehydrogenase; LPL: lipoprotein lipase; LYM: lymphocyte; NO: nitric oxide; OCN: osteocalcin; OPG: osteoprotegerin; p-Akt: phosphorylated protein kinase B; p-ERK1/2: phosphorylated extracellular signal-regulated kinase 1/2; p-PI3K: phosphorylated-phosphatidylinositol 3-kinase; PLpro: papain-like protease; PLT: platelet count; PPAR δ: peroxisome proliferator activated receptor delta; PSD 95: post-synaptic density 95; PTP1B: protein tyrosine phosphatase 1B; RANKL: receptor activator of nuclear factor kappa-B ligand; ROS: reactive oxygen species; Runx-2: runt-related transcription factor 2; SARS-CoV: severe acute respiratory syndrome coronavirus; TNF-α: tumor necrosis factor alpha; TRAP: tartrate-resistant acid phosphatase; VGLUT1: vesicular glutamate transporter 1; WBC: white blood cell; NA: not available
Psoralidin has been shown to exhibit anti-bacterial activity. Psoralidin obtained from the seeds of P. corylifolia inhibited the bacterial colony of S. sonnei and S. flexneri, with an inhibitory concentration of 200 µg/disc as estimated by the disc diffusion method [69]. S. sonnei and S. flexneri are gram-negative bacteria causing intestinal infections. Similarly, psoralidin identified in hexane extracts of seeds demonstrated anti-bacterial activity against E. coli and S. aureus, with inhibitory concentrations ranging from 90 µg/mL to 150 µg/mL [76]. E. coli is Gram-negative bacteria causing urinary tract infections whereas S. aureus is Gram-positive bacteria causing skin infections. However, the molecular targets behind the anti-microbial activity of Psoralidin have not been explored yet. These findings suggest that psoralidin could have broad-spectrum anti-bacterial properties, making them promising candidates for the development of novel anti-microbial agents in the era of developing anti-microbial resistance.
The existing literature has also investigated the anti-diabetic potential of psoralidin. Psoralidin present in the ethanolic extract obtained from seeds of P. corylifolia showed anti-diabetic activity by inhibiting protein tyrosine phosphatase 1B (PTP1B) in an in vitro study [70]. PTP1B has been reported to be the negative regulator of the insulin signaling pathway by removing the phosphate group from the tyrosine residue on the insulin receptor. Hence it serves as an effective therapeutic target for combating diabetes type II [100]. The efficacy of psoralidin in targeting PTPB1 highlights its potential in the management of diabetes.
Later, the anti-inflammatory nature of psoralidin was also explored. The methanolic extract of seeds containing psoralidin showed the potent anti-inflammatory response in RBL-2H3 cells by inhibiting antigen-induced degranulation from mast cells and in turn the release of hexosaminidase [77]. β-Hexosaminidase, an enzyme released from mast cells through antigen-induced degranulation serves as a marker for anaphylactic reactions [101]. Further, psoralidin inhibited nitric oxide (NO) production in murine peritoneal exudate cells indicating its ability to attenuate inflammatory responses in in vitro model system also [78]. The study conducted on mice macrophage RAW 264.7 demonstrated that psoralidin inhibited PI3K/Akt pathway in the concentration ranging from 1–30 µM and demonstrated anti-inflammatory response [80] by inhibiting α-glucosidase and NO production [82]. Another study showed that psoralidin prevented ionizing radiation-induced inflammation by downregulating the expression of inflammatory molecules cyclooxygenase-2 (COX-2) and PGE2 [79]. Moreover, psoralidin treatment in the range from 50–100 µM reduced the production of ionizing radiation-induced pro-inflammatory cytokines TNF-α, TGF-β, IL-6, and IL-1α/β to reduce pulmonary inflammation in mouse lung. Similarly, psoralidin treatment prevented osteoarthritis by inducing anti-catabolic and anti-inflammatory responses in osteoarthritic chondrocytic pellet cultures obtained from patient samples [81].
The anti-depressant activity of psoralidin has been well documented. An in vivo study utilizing ICR mice, administration of psoralidin at doses ranging from 20 mg/kg to 60 mg/kg led to increased swimming behavior in the Forced Swimming Test and Open Field Test. This behavioral improvement was associated with elevated levels of 5-hydroxy indoleacetic acid (5-HIAA) and dopamine, neurotransmitters implicated in mood regulation [83]. Additionally, psoralidin administration resulted in decreased levels of corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and corticosterone, suggesting modulation of the hypothalamic-pituitary-adrenal (HPA) axis, a key player in stress response. In an in vitro study using N2A cells, investigators revealed that psoralidin reduced CRF promoter activity and transcription [84]. The hyperactive state of CRF is observed in depression, as the level normalizes after effective anti-depressant treatment [102]. The findings further support the strong and targeted anti-depressant potential of psoralidin.
Psoralidin showed promising potential in inhibiting the activity of severe acute respiratory syndrome coronavirus (SARS-CoV). Psoralidin found in ethanolic extracts obtained from the seeds of P. corylifolia dose-dependently inhibited the expression of SARS-CoV papain-like protease (PLpro) [85], a pivotal enzyme involved in viral replication [103]. An in silico molecular docking studies revealed that psoralidin potentially reduced the activity of furin [86], an enzyme involved in the proteolytic processing of viral proteins essential for viral entry and replication [104]. The above finding suggests that psoralidin may have therapeutic potential against SARS-CoV and other related viruses.
Psoralidin demonstrated anti-protozoal activity, particularly against Ichthyophthirius multifiliis, a protozoan parasite that affects fish. In vivo and in vitro assays showed that methanolic extracts from fruits containing psoralidin effectively reduced the number of theronts, the infective stage of the parasite, at a concentration of 1 mg/L [87]. These findings suggest that psoralidin-rich extracts may have therapeutic potential in the treatment of protozoal infections, offering a natural alternative to conventional anti-parasitic agents. However, a detailed study of pure compounds and human protozoal parasites is yet to be conducted.
Psoralidin also showed neuroprotective effects. The ethanolic extracts obtained from psoralidin-rich seeds at concentrations ranging from 1.25 µM to 5 µM resulted in a decrease in NO production in BV-2 microglial cells and HT22 hippocampal neurons, indicating attenuation of neuroinflammation, a common feature of neurodegenerative conditions [88]. An in vivo study conducted with ICR mice further supported its neuroprotective effects. Administration of psoralidin at concentrations ranging from 1 µM to 20 µM led to an increase in the expression of neuronal plasticity markers such as activity-dependent cytoskeleton associated protein (Arc), early growth response protein 1 (Egr1), and c-fos, indicating enhanced synaptic activity and neuronal survival [89]. Furthermore, psoralidin treatment resulted in an upregulation of phosphorylated ERK1/2 (p-ERK1/2) and post-synaptic density 95 (PSD 95)/vesicular glutamate transporter 1 (VGLUT1)-positive clusters, suggesting enhanced neuronal connectivity and synaptic function. Psoralidin present in the fruit extract of C. corylifolium inhibited the activity of ferroptosis in mouse hippocampal cells HT22 pre-exposed with erastin with the IC50 5.21 µM [105]. Moreover, the molecular docking analysis showed that psoralidin also inhibited the activity of ferroptosis target genes 5-lipoxygenase (5-LOX), and Keap-Nrf2 dimers. Additionally, treatment with psoralidin ameliorated the injury caused by cerebral hypoxia/reoxygenation in neuroblastoma by activating the growth arrest-specific 6 (Gas6)/AXL receptor tyrosine kinase (GAS/AXL) signaling pathway [106]. These findings highlight the potential of psoralidin as a therapeutic agent for neurodegenerative diseases by targeting multiple pathways involved in neuroprotection and synaptic plasticity.
Psoralidin exhibited vasodilatory effects. An in vivo study utilizing psoralidin-rich ethanolic extracts from seeds showed relaxation of aortic rings, suggesting vasodilation [90]. Additionally, it reduced the current through hTRPC3 channels, which are implicated in regulating vascular tone. These findings suggest that psoralidin-rich extracts may have therapeutic potential in conditions such as hypertension and coronary artery disease, where impaired vasodilation contributes to pathogenesis, by promoting relaxation effect.
The available literature also suggests the osteoprotective activity of psoralidin. In an in vitro study utilizing rat calvarial osteoblasts, psoralidin at a concentration of 1 µM increased alkaline phosphatase (ALP) and decreased tartrate-resistant acid phosphatase (TRAP) activity. Moreover, it upregulated key osteogenic markers including Osterix, runt-related transcription factor 2 (Runx-2), insulin-like growth factor 1 (IGF-1), and β-catenin, while concurrently downregulating osteoprotegerin (OPG) and receptor activator of nuclear factor kappa-Β ligand (RANKL), further enhancing its osteoprotective effects [91]. Another study employing both in vitro and in vivo models revealed that psoralidin decreased TRAP-positive cells and inhibited F-actin ring formation in bone marrow macrophages and ICR mice, along with suppressing phosphorylation of p38, c-Jun N-terminal kinase (JNK), and ERK, as well as RANKL expression [92]. Additionally, an ethyl-acetate extract from psoralidin seeds demonstrated osteoprotective effects by reducing TRAP activity, increasing phosphorylation of PI3K and Akt, and upregulating heme oxygenase-1 (HO-1) in bone marrow mesenchymal stem cells (BMSCs) and rats [93]. Psoralidin also exhibited anti-apoptotic effects in rat chondrocytes, reducing caspase-3 and caspase-9 activity, and inhibiting NF-κB nuclear localization [94]. Furthermore, psoralidin promoted osteoblast differentiation in BMSCs and MC3T3-E1 cells while inhibiting adipogenesis in 3T3-L1 cells by enhancing ALP activity, increasing bone sialoprotein (BSP) and osteocalcin (OCN) expression, and downregulating peroxisome proliferator-activated receptor gamma (PPAR γ), fatty acid-binding protein 4 (Fabp4), and lipoprotein lipase (LPL) expression [95]. These findings collectively underscore psoralidin’s potential as a promising therapeutic agent for osteoporosis and similar bone-related disorders.
Psoralidin-rich extracts demonstrated anti-vitiligo activity. An in vitro study utilizing ethanolic extracts from whole P. coylifolia plant with psoralidin component showed an increase in tyrosinase activity [64], a key enzyme involved in melanin production [107]. The above finding suggests that psoralidin-rich extracts may stimulate melanogenesis, offering potential therapeutic benefits in the treatment of vitiligo by promoting repigmentation of the skin.
Psoralidin demonstrated cardioprotective effects. An in vivo study conducted with commercial psoralidin preparations on BALB/c mice at a dose of 25 mg/kg resulted in decreased levels of creatine kinase (CK), aspartate transaminase (AST), and blood urea nitrogen (BUN), indicating reduced myocardial damage. Additionally, psoralidin treatment led to an increase in serum albumin (ALB), suggesting improved cardiac function and tissue repair mechanisms [98]. Furthermore, psoralidin administration resulted in a decrease in ROS levels and lactate dehydrogenase (LDH) release, indicating reduced oxidative stress and cellular damage in the heart. In vitro assays using HL-1 cells further supported the cardioprotective effects of psoralidin, with concentrations ranging from 20 µM to 100 µM leading to improvements in cardiac function and decreased LDH release, indicating its potential as a therapeutic agent for cardiovascular disorders. Psoralidin also showed anti-septic effects during myocardial injury, characterized by systemic inflammation and organ dysfunction. An in vivo study utilizing psoralidin-rich extracts showed a reduction in sepsis scores in cecal ligation and puncture (CLP)-injured mice, along with improvements in biochemical markers of organ function such as LDH, AST, BUN, and CK, increased ALB levels, and modulated immune cell counts [99]. Psoralidin treatment reversed the sepsis condition in CLP-injured BALB/c mice and LPS-induced cardiomyocytes by activating phosphoinositide 3-kinase regulatory subunit 5 (PI3KR5) [108]. The above findings suggest a potential role of psoralidin in restoring homeostasis during sepsis.
Psoralidin exhibited potent anti-fungal activity, which may be beneficial in the treatment of fungal infections. Psoralidin present in hexane extracts from seeds demonstrated significant inhibition of fungal colonies of C. albicans and T. rubrum grown in agar medium, with inhibitory concentrations ranging from 160 µg/mL to 180 µg/mL [76]. C. albicans is a pathogenic yeast causing infections whereas T. rubrum causes infection of hands and feet. These findings suggest that Psoralidin-rich extracts have the potential to combat fungal pathogens effectively, highlighting their utility as natural anti-fungal agents.
In addition to the above-mentioned activities, psoralidin and psoralidin-rich extracts also demonstrated a significant role in various other physiological and pathological conditions. Psoralidin acted as an agonist of estrogen receptor signaling and in turn activated ER-signaling pathways [109]. The extract obtained from the seeds of P. corylifolia contained psoralidin as one of the active ingredients and inhibited the activity of human carboxylexterase1 (hCSE1) [110]. Another study showed that psoralidin potentially triggered the differentiation of adipose-derived stem cells into nucleus pulposus cells via the TGF-β/Smad signaling pathway which can serve as promising prospects for psoralidin in addressing intervertebral disc degeneration [111]. Psoralidin in a theranostic nanomicelle (MRC-PPL@PSO) exerted an anti-arthritis effect by downregulating the PI3K/Akt, mitogen-activated protein kinase (MAPK), and NF-кB signaling pathways [112]. Therefore, psoralidin emerges as a versatile therapeutic agent suggesting its broad applicability in addressing a variety of health challenges.
In addition to various other health benefits described above, the anti-tumor properties of psoralidin have also gained significant attention in the past decade (Table 3).
Anti-cancer activity of psoralidin in different malignancies
| Cancer type | Model | Inhibitory concentrations | Anti-cancer activity | Mechanism of action | References |
|---|---|---|---|---|---|
| Prostate | PC-3, DU-145, PzHPV-7, xenograft | 45–60 µM | Induction of apoptosis↑Death receptors↓Tumor growth | ↓TNF-α mediated NF-кB signaling | [113] |
| PC-3, DU-145, LNCaP, C4-2B, xenograft | 45–60 µM | ↓Cell proliferation↓Tumor growthInduction of apoptosis | ↓EGFR/MAPK signaling | [114] | |
| LNCaP | 20–50 µM | Induction of apoptosis↓Cell proliferation | ↑TRAIL | [115] | |
| PC-3, PzHPV-7, C4-2B | 5–20 µM | ↓Cell proliferation↓Migration, Invasion | ROS generation | [116] | |
| RWPE-1, xenograft mice | 4 µM | ↓Cell proliferationInduction of apoptosisAutophagy inductionEMT Inhibition | ↓NF-кB signaling | [117] | |
| Cervical | HeLa | 50 µM | Induction of apoptosisDepolarization of mitochondrial membrane potential | ↑TRAIL induced R2 death receptor | [118] |
| Lung | A549 | 10–20 µM | ↓Cell proliferationAutophagy induction | ROS generation | [119] |
| Breast | MCF-7, MDA-MB-231 | 10–20 µM | ↓Cell proliferationDNA damageAutophagy induction | ROS generation↑Nitrogen oxides | [120] |
| Esophageal | Eca9706 | 10 µM | ↓Cell proliferationInduction of apoptosis | ↓NF-кB signaling↓PI3K/Akt signaling | [121] |
| Colon | SW480 | 5–20 µM | ↓Cell proliferationInduction of apoptosis | ↓NF-кB signaling | [122] |
| HT-29, HCT-116 | 0–20 µM | Induction of apoptosisdepolarization of mitochondrial membrane potential | ROS generation | [123] | |
| Liver | HepG2 | 64 µM | Induction of apoptosis | ↑p53 | [124] |
| HepG2, xenograft | 9 µM | ↓Cell proliferationG2/M cell cycle arrestInduction of apoptosisAutophagy induction↓Tumor growth | - | [125] | |
| Osteosarcoma | 143B, MG63, orthotopic mice | 30 µM | ↓Cell proliferation↓Migration, InvasionInduction of apoptosisCell cycle arrest | ↓PI3K/Akt signaling ↓FAK | [126] |
EGFR: epidermal growth factor receptor; EMT: epithelial-mesenchymal transition; FAK: focal adhesion kinase; MAPK: mitogen-activated protein kinase; NF-кB: nuclear factor kappa B; PI3K/Akt: phosphatidylinositol 3-kinase/protein kinase B; p53: tumor protein; ROS: reactive oxygen species; TNF-α: tumor necrosis factor alpha; TRAIL: tumor necrosis factor related apoptosis inducing ligand; TRAIL-R2: TRAIL-receptor 2; -: blank cell
Initially, the anti-tumor efficacy of psoralidin was demonstrated in prostate cancer, followed by its application in cervical and lung cancer in pre-clinical studies. Prostate cancer remained the most extensively investigated cancer in association with psoralidin activity till date. The inhibitory range of psoralidin in different malignancies is summarized in Figure 3.
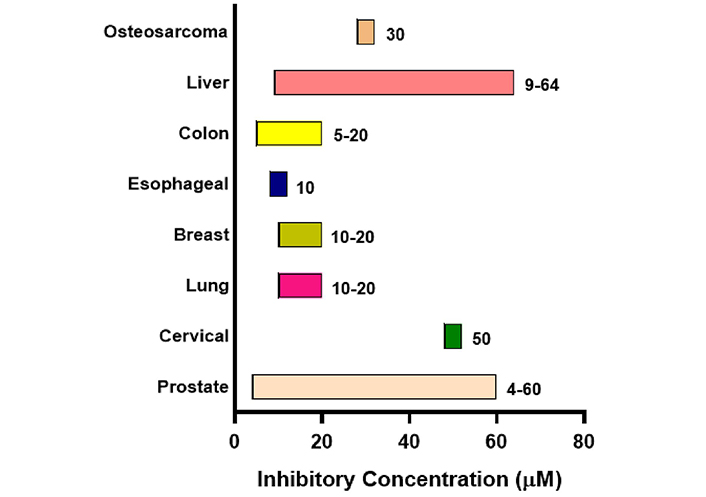
Effective inhibitory concentration range of psoralidin in different malignancies. The chart shows the effective inhibitory concentration of psoralidin in µM. Bars are only indicative and not to scale
The inhibitory concentration of psoralidin ranged from 5–60 µM in different malignancies. Psoralidin showed strong inhibition at a concentration of 4 µM in RWPE-1 cell lines of prostate cancer, whereas liver cancer HepG2 cells showed the highest concentration IC50 of 64 µM. Psoralidin carries out its anti-cancer activity by targeting different molecular targets such as NF-кB, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), Nitrogen oxides, p53, and FAK in different malignancies (Figure 4). Psoralidin’s ability to combat cancer is attributed to its ability to induce oxidative stress, apoptosis, and autophagy, and by targeting CSCs and neo-angiogenesis.
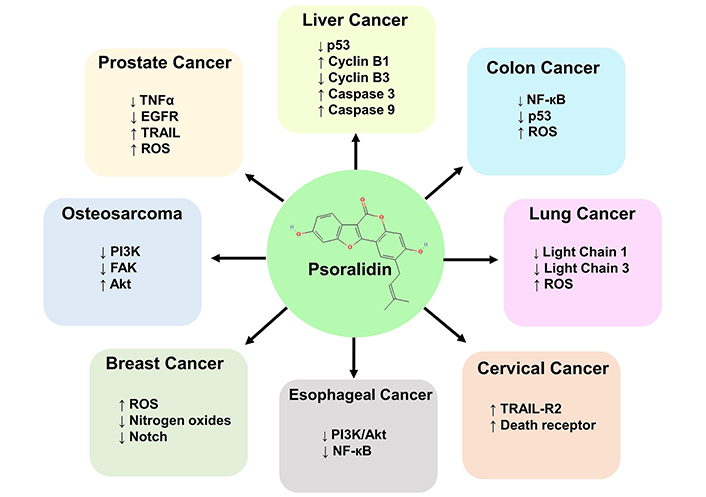
Molecular targets of psoralidin in different malignancies. Psoralidin targets cancer by inducing oxidative stress, apoptosis, autophagy, and by targeting CSCs. The different molecular taregts include TNF-α, EGFR, p53, Notch and Light chains 1 and 3. The signaling pathways targeted by psoralidin are PI3K/Akt, NF-кB and Notch pathway. Psoralidin also targets cancer cells by inducing the expression of death receptors. Akt: protein kinase B; CSCs: cancer stem cells; EGFR: epidermal growth factor receptor; FAK: focal adhesion kinase; MAPK: mitogen-activated protein kinase; NF-кB: nuclear factor kappa B; PI3K/Akt: phosphatidylinositol 3-kinase/protein kinase B; p53: tumor protein; ROS: reactive oxygen species; TNF-α: tumor necrosis factor alpha; TRAIL: tumor necrosis factor related apoptosis inducing ligand; TRAIL-R2: tumor necrosis factor related apoptosis inducing ligand-receptor 2
ROS are the by-products resulting from the partial reduction of molecular oxygen during the cellular metabolism processes [127]. The term “ROS” is collectively used to denote free radicals such as the hydroxyl radical (.OH), superoxide anion radical (O2.-), singlet molecular oxygen (1O2), and hypochlorous acid (HOCl) [128]. While ROS serve as intracellular signaling molecules for various biological processes [129], excessive levels can cause cellular damage. Under normal physiological conditions, the presence of anti-oxidant enzymes helps maintain intracellular ROS levels and biological system homeostasis [130]. However, when ROS concentration surpasses normal levels, cellular homeostasis is disrupted, leading to oxidative stress implicated in various pathological conditions like cardiovascular and metabolic disorders [131]. It has also been reported that increased intracellular ROS plays a pivotal role in mediating carcinogenesis by regulating the various hallmarks of cancer progression [132]. Conversely, toxic levels of ROS production exhibit anti-tumorigenic effects by inducing oxidative stress and triggering tumor cell death [133]. Consequently, therapies aimed at either reducing ROS levels or enhancing ROS production beyond toxic concentration hold promise as potential cancer treatments. In this regard, psoralidin showed anti-cancer activity by increasing the intracellular ROS concentration beyond the toxic level that resulted in tumor cell death. At a concentration of 5 µM, psoralidin induced a 50-fold increase in ROS production in PC-3 and C4-2B prostate cancer cell lines compared to the control which led to a dose-dependent reduction in cell viability and proliferation [116]. However, psoralidin treatment did not result in a substantial increase in ROS concentration in the normal prostate cell line PzHPV-7, suggesting its selective targeting of cancer cells. Moreover, the psoralidin treatment did not compromise the activity and expression of anti-oxidative enzymes superoxide dismutase and catalase indicating that it specifically induced ROS generation without affecting the expression of antioxidants. In addition, excessive induction of ROS by psoralidin treatment resulted in altered expression of EMT markers E-cadherin, Slug, and Vimentin, leading to inhibition of migration and invasion ability of prostate cancer cells. Another study demonstrated that psoralidin induced ROS generation in a concentration-dependent manner in the lung cancer cell line A549, leading to cell death [119]. Furthermore, N-acetylcysteine (NAC), a protective agent against oxidative stress, reversed psoralidin-induced ROS generation in lung cancer cells. Likewise, psoralidin treatment at 2.5–10 µM induced a time-dependent and concentration-dependent increase in intracellular ROS level in the MCF-7 breast cancer cell line which was reversed by the treatment of ROS scavenger NAC [120]. The increased ROS level induced DNA damage and cell death by increasing the expression of NOX4. Sun et al. [123], showed that treatment with psoralidin rapidly increased ROS generation, leading to DNA damage, and reduction in mitochondrial membrane potential in colon cancer and ultimately leading to cell death. These findings indicate the potential of psoralidin to induce oxidative stress selectively in cancer cells by elevating intracellular ROS levels without influencing cellular anti-oxidant mechanisms to prevent pathogenesis.
Apoptosis is a programmed cell death mechanism to regulate embryonic development [134]. Any dysregulation in apoptosis promotes carcinogenesis by enabling genetic instability, mutations, immune evasion, evasion of cell cycle checkpoints, uncontrolled cell proliferation, metastatic potential, and resistance to anti-cancer treatments [135]. A significant progress has been made in the last two decades to prevent cancer progression by targeting apoptosis. Psoralidin displayed pro-apoptotic activity in different cancer cell types by different mechanisms that prevented tumor growth. In prostate cancer, psoralidin induced apoptosis in PC-3, and DU-145 cell lines, as well as in xenograft models at concentrations ranging from 45 µM to 60 µM by upregulating the expression of death receptors and decreasing tumor growth [115, 117]. It downregulated TNF-α-mediated NF-кB signaling and EGFR/MAPK signaling pathways that suppressed cell proliferation and promoted apoptosis [113, 114]. Breast cancer cell lines MCF-7 and MDA-MB-231 treated with 10–20 µM of psoralidin showed reduced cell proliferation, DNA damage, and autophagy induction, accompanied by increased ROS generation and NOX4 expression [120]. Psoralidin also inhibited cell proliferation and promoted apoptosis in esophageal [121] and colon cancer [122] through suppression of NF-кB and PI3K/Akt signaling pathways. Psoralidin induced apoptosis and disrupted mitochondrial membrane potential in colon cancer cell lines HT-29, and HCT-116 at inhibitory concentrations ranging from 1–20 µM [123]. Furthermore, psoralidin at 64 µM induced apoptosis in HepG2 cells of liver cancer by upregulating the expression of tumor suppressor gene p53 [124]. It inhibited cell proliferation, induced G2/M cell cycle arrest, and promoted apoptosis in HepG2 cells and xenograft models at 9 µM [125]. In osteosarcoma cell lines 143B and MG63 cells, psoralidin at 30 µM inhibited cell proliferation, migration, invasion, induced apoptosis, and arrested the cell cycle progression through the suppression of PI3K/Akt and FAK signaling pathways [126]. Overall, the studies have demonstrated the effectiveness of psoralidin in targeting aberrantly active NF-кB and PI3K/Akt signaling and upregulation of death receptors across different cancer models that induced apoptosis. The dysregulated apoptosis pathway also contributes to CaCx progression. HPV16E6/E7 oncoproteins inhibit the cellular apoptosis rate for mediating cancer progression in CaCx [136]. Therefore, psoralidin can also serve as an effective therapeutic molecule in CaCx progression by promoting apoptosis.
Autophagy is a highly conserved cellular pathway that plays a pivotal role in cell survival and maintenance by removing the dysfunctional components of the cell [137]. Consequently, dysregulation of autophagy is implicated in various pathological conditions such as age-related neurodegeneration [138] aging [139], endothelial dysfunctions [140], and infectious diseases [141]. In the last decade, the role of autophagy in tumorigenesis has also been elucidated [142]. Initially, autophagy was believed to have a protective effect against cancer development [143, 144]. In contrast, a study revealed an increased basal level of autophagy in melanoma [145] and colon cancer [146]. Consequently, the role of autophagy in cancer is still debated, with it being viewed as a double-edged sword. Therefore, modulation of autophagy has emerged as a pivotal therapeutic target for cancer prevention. Psoralidin was demonstrated to induce autophagy that prevented lung cancer progression [119]. Psoralidin at 20 µM induced the formation of autophagosomes, granular structures evident by monodansylcadaverine (MDC) staining. Moreover, the expression ratio of autophagic markers light chain 3 (LC3) I and II showed a time- and concentration-dependent increase in post-psoralidin-treated cells, which was reversed by the presence of autophagy inhibitor 3-MA. Similarly, psoralidin in a 24 h treatment with 10 µM concentration, induced the formation of autophagic vesicles in breast cancer cell lines [120]. Additionally, the expression of autophagic markers LC3II, p-ULK1, and Beclin-1 increased post-psoralidin treatment. In prostate cancer, psoralidin at 4 µM induced autophagic death of RWPE-1 cells by elevating the expression of Beclin-1, Atg-3, -5, -7, and -12 along with a reduction in the expression of Plac8 [117]. Furthermore, treatment with psoralidin induced the formation of autophagosomes in liver cancer cell line HepG2 detected by transmission electron microscopy [125] and increased expression of Beclin-1 and light chain 3B (LC3B)-II. Autophagy is emerging as a therapeutic target in CaCx too. The downregualted expression of Beclin1 and LC3 is correlated with high-grade CaCx and poor survival [147, 148]. Another study reported that the infection of high-risk HPV is often correlated with reduced expression of autophagic markers Beclin1 and LC3B in cervical specimen obtained from CaCx patients [149]. Hence, there is strong evidence that psoralidin may exert its anti-cancer effects through the induction of autophagy, and therefore can also prevent CaCx progression by this mechanism.
CSCs are a small population of tumor cells which possess the properties of self-renewal [150] and multi-lineage differentiation potential [151] like normal stem cells. Their slow rate of proliferation allows them to exist in a semi-dormant state, rendering them less susceptible to various anti-proliferative cancer drugs [152]. After the treatment regimen ceases, it has been documented that CSCs repopulate tumor cells through asymmetric cell division, leading to tumor recurrence [153]. The initial understanding of the role of CSCs in cancer initiation, progression, and recurrence emerged from the study in 1994 that demonstrated a distinct subset of cells, with characteristic surface markers CD34+/CD38–, could initiate leukemia in immune-compromised mice [154]. Later on, the role of these CSCs in mediating cancer progression was observed in other solid tumors also [155]. Owing to their stemness properties, CSCs pose a formidable threat to cancer management and their therapeutic targeting can be of immense clinical significance [156]. Available literature has shown the effective targeting of these CSCs by psoralidin to prevent pathogenesis and metastasis of breast cancer [157, 158]. Breast CSCs (bCSCs) are distinguished by the presence of the functional marker aldehyde dehydrogenase (ALDH), which plays a crucial role in maintaining their stemness properties through the modulation of Notch1 expression [159]. Psoralidin showed a substantial decrease in cell viability in both ALDH-negative and ALDH-positive cells, with IC50 values ranging from 18–21 µM [158]. Moreover, within this IC50 range, psoralidin significantly inhibited the colony-forming and mammosphere-forming abilities of both ALDH-negative and ALDH-positive cells, which are hallmark features of CSCs. Its anti-CSCs activity is attributed to its effective targeting of Notch1, a key player in maintaining the stemness characteristics of CSCs. A subsequent study conducted by the same research team revealed that these ALDH-positive cells exhibited high tumorigenicity, leading to the formation of large tumors in a xenograft mice model. Administration of psoralidin orally to these mice resulted in a significant reduction in tumor size and volume showcasing the effective anti-CSCs activity of psoralidin in in vitro and in vivo model systems as well [157]. The role of CSCs in mediating CaCx progression is also well established. The CaCx initiating cells enriched with stemness properties displayed a higher degree of resistance against radiation [160]. Another study reported that Bmi1-positive CCSCs were involved in cervical tumor initiation, growth, and metastasis [161]. Due to the significant role of CCSCs in CaCx progression and tumor recurrence, they can be targeted therapeutically by psoralidin to prevent cervical carcinogenesis.
Neo-angiogenesis, the formation of new blood vessels from existing vasculature, naturally occurs during wound healing [162], embryonic development [163], and the female reproductive cycle [164]. Interestingly, neo-angiogenesis plays a crucial role in tumor progression by providing enhanced nutrition for growth and facilitating the disposal of metabolic waste through newly formed vessels [165]. It is also a hallmark of CaCx growth and progression [166]. Due to the significant role of neo-angiogenesis in cancer development, there have been ongoing efforts to identify small molecule inhibitors and targeting agents to control this process [167]. Psoralidin has shown an anti-angiogenic property. In an in vivo study, psoralidin treatment reduced the tumor volume in 4T1-tumor-bearing BALB/c mice. Moreover, it inhibited angiogenesis by downregulating the expression of pro-angiogenic molecules VEGF, Ki67, and CD31 [168].
Overall, psoralidin exhibited a multifaceted mechanism of action across various cancer types, making it a promising candidate for further exploration and development as a potential anti-cancer therapeutic in CaCx.
In a solitary study, psoralidin has also been tested for its anti-CaCx property against HeLa cells. It induced cytotoxicity in HeLa cells in a concentration-dependent manner with approximately 14% apoptotic cell population at 50 µM [118]. Interestingly, the cytotoxic potential of psoralidin was enhanced when it was used in combination with TRAIL that increased the cytotoxicity from 13% to ~70% (Figure 5A). Psoralidin combined with TRAIL induced cytotoxicity in cancer cells through apoptosis. The percentage of necrotic HeLa cells was nearly 0% in the untreated population. However, the treatment of 50 μM Psoralidin with TRAIL at 100 ng/mL increased the proportion of apoptotic cells to 67.1% ± 3.3% after 48 h of treatment (Figure 5B). In addition, psoralidin treatment induced the activation of death receptors 1 (DR 1) and DR 2 after 48 h of treatment (Figure 5C). Likewise, treating HeLa cells with either 100 ng/mL TRAIL or 50 μM Psoralidin alone resulted in a minimal effect on mitochondrial membrane potential (ΔΨm) ranging from 7–10% (Figure 5D). However, the combination of TRAIL and Psoralidin significantly increased the loss of ΔΨm, affecting a large percentage of cancer cells (58.38% ± 1.41%) and causing a major disruption of the mitochondrial membrane potential. Despite showing the significant anti-cancer property against CaCx in conjunction with TRAIL, there has been no further exploration of psoralidin as a lead or adjunct molecule against CaCx treatment.
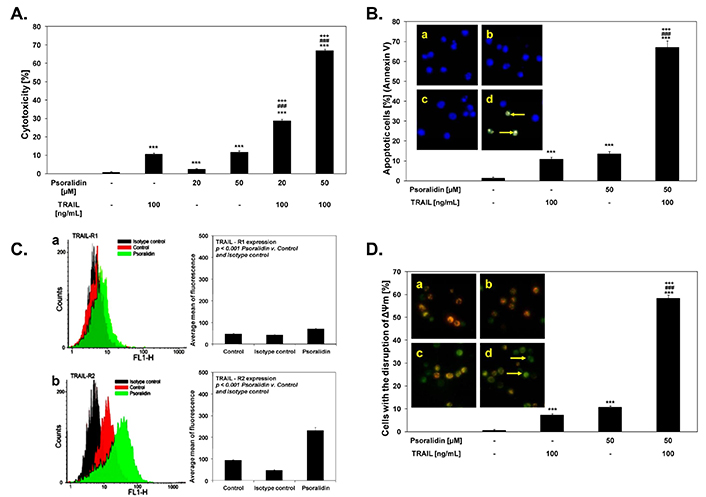
Anti-CaCx activity of psoralidin as displayed by dose-dependent cytotoxicity alone or in combination with TRAIL (A), Induction of apoptosis (B), increased death receptor expression (C) leading to (D) loss of mitochondrial membrane potential (ΔΨm). For details see [118]
Note. Adapted from “The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)” by Bronikowska J, Szliszka E, Jaworska D, Czuba ZP, Krol W. Molecules. 2012;17:6449–64 (https://doi.org/10.3390/molecules17066449). CC BY.
The metabolism and pharmacokinetic assessments of a drug is essential to establish its safety and effectiveness as a prescribed medication [169]. Psoralidin has been shown to dose-dependently inhibit the activity of cytochrome P450 3A4 (CYP3A4), the most prevalent metabolic enzyme that plays a vital role in both activating and detoxifying drugs, in differentiated HuH-7 and HepaRG cells, as well as human recombinant CYP3A4, with IC50 values of 57.7 μM, > 200 μM, and 30.8 μM, respectively. Notably, no apparent cytotoxic effects were observed within the tested concentration range of psoralidin (1–200 μM) [170]. Another study showed that psoralidin showed moderate inhibition of CYP2E1 with a K value of 2.8 µM in rat liver microsomes [171]. Conversely, an oral preparation Xian-Ling-Gu-Bao (XLGB) commonly used to treat osteoporosis in China has psoralidin as one of the main ingredients that cause mitochondrial dysfunction and liver injury [172]. The treatment significantly reduced the reserve capacity of liver and respiratory control ratios by activating the PI3K/mTOR signaling pathway. However, the adverse effect could be due to other constituents or due to prolonged exposure and high inhibitory doses used.
The pharmacokinetic behavior of psoralidin was analyzed in the liver microsomes of rats post intravenous psoralidin administration at a dose of 2 mg/kg [173]. The pharmacokinetic parameters such as total drug exposure across time i.e., area under the curve (AUC0–∞), half-life t1/2, volume of distribution (V), clearance (CL), and Cmax calculated after drug administration were 1.94 mg/L/h, 4.45 h, 6.22 L/kg, 1.12 L/h/kg, 2.19 mg/L, respectively. This UPLC-MS/MS assay-based study has also demonstrated that the enzyme CYP2C19 is the primary metabolic enzyme for psoralidin in the human liver, and the metabolism of psoralidin varies among different species, with monkeys showing the closest similarity to humans. Yang et al. [174], studied the pharmacokinetics and distribution of psoralidin in cerebral nuclei of rats post-oral administration at 1.2 g/kg. There was a quick absorption of psoralidin in the plasma of rats with a high concentration in cerebral nuclei. Another study showed that the pharmacokinetic behavior of psoralidin varied depending on the gender of mice [175]. The AUC0–t of psoralidin was higher in males than females, however, the mechanism behind this gender-specific variation is not yet elucidated. Therefore, this area remains to be explored further as understanding the pharmacokinetic and metabolism is crucial for gaining further insight into the clinical effectiveness and safety of psoralidin.
Despite psoralidin’s significant potential in addressing cancer and other pathological conditions, no specific products containing psoralidin have been commercialized, apart from the original plant extract. The studies in the clinical trials for psoralidin are also lacking. This can be primarily attributed to the hydrophobic nature, inadequate pharmacokinetic profile of psoralidin, and intestinal efflux, which hampers its clinical application [176, 177]. Hence, it is paramount to explore alternatives that enhance psoralidin’s bioavailability within the biological system. Nanoformulations have emerged as promising solutions to overcome the challenges associated with psoralidin’s limited bioavailability, offering the potential to develop a tailored nanosystem capable of addressing the constraints hindering its clinical utility [178, 179]. The bioavailability of psoralidin significantly improved with a value of 339% w.r.t to reference through its nanoencapsulation (NCs) using chitosan and Eudragit S100 [176]. The psoralidin-NCs were prepared using solvent diffusion and high-pressure homogenization techniques, resulting in nanocapsules with a particle size of less than 150 nm, ideal for the delivery of poorly water-soluble psoralidin via oral administration. Furthermore, the nanoencapsulated psoralidin exhibited remarkable gastrointestinal stability and anti-mucin binding properties. Similarly, the oral delivery of psoralidin via bilosomes coated with chitosan showed enhanced necrotic and apoptotic effects on MCF-7 and A549 cells [180]. Bilosomes are newly developed lipid nanovesicles enriched with bile salts. Due to their composition, they are not damaged by intestinal secretions of enzymes and thus offer an alternative to traditional lipid vesicle-based systems for oral delivery of drugs [181]. Hence, exploring additional methods to improve the bioavailability of psoralidin is necessary, potentially paving the way for successful oral chemotherapy with psoralidin.
Overall, psoralidin showed potential in promoting various health benefits with its unique mechanism of action such as induction of ROS and autophagy. With its wide-ranging benefits and promising therapeutic potential, psoralidin stands as a candidate therapeutic. However, limited studies of psoralidin for its anti-cancer effects demand detailed investigation of its safety and efficacy in elaborate pre-clinical and clinical studies. This knowledge gap presents an opportunity for further research into psoralidin’s therapeutic potential and offer new avenues for CaCx treatment and management.
5-HIAA: 5-hydroxy indoleacetic acid
ACTH: adrenocorticotropic hormone
ALB: albumin
ALDH: aldehyde dehydrogenase
ALP: alkaline phosphatase
Arc: activity-dependent cytoskeleton associated protein
AST: aspartate aminotransferase
AST: aspartate transaminase
BMSCs: bone marrow mesenchymal stem cells
BSP: bone sialoprotein
BUN: blood urea nitrogen
CaCx: cervical cancer
CCSCs: cervical CSCs
c-fos: fos proto-oncogene
CIN1: cervical intraepithelial neoplasia grade 1
CK: creatine kinase
CLP: cecal ligation and puncture
COX-2: cyclooxygenase-2
CRF: corticotropin-releasing factor
CSCs: cancer stem cells
EGFR: epidermal growth factor receptor
Egr1: early growth response protein 1
EMT: epithelial-mesenchymal transition
ERK: extracellular signal-regulated kinase
Fabp4: fatty acid-binding protein 4
FAK: focal adhesion kinase
HO-1: heme oxygenase-1
HPV: human papillomaviruses
LDH: lactate dehydrogenase
LPL: lipoprotein lipase
MAPK: mitogen-activated protein kinase
NAC: N-acetylcysteine
NF-кB: nuclear factor kappa B
NO: nitric oxide
OCN: osteocalcin
p-ERK1/2: phosphorylated extracellular signal-regulated kinase 1/2
PLpro: papain-like protease
PPAR: peroxisome proliferator-activated receptor
PSD: post-synaptic density
PTP1B: protein tyrosine phosphatase 1B
RANKL: receptor activator of nuclear factor kappa-B ligand
ROS: reactive oxygen species
Runx-2: runt-related transcription factor 2
SARS-CoV: severe acute respiratory syndrome coronavirus
TNF-α: tumor necrosis factor alpha
TRAIL: tumor necrosis factor-related apoptosis-inducing ligand
TRAP: tartrate-resistant acid phosphatase
VGLUT1: vesicular glutamate transporter 1
ACB: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing—review & editing. TT and ACB: Data curation, Visualization, Writing—original draft. TT, AC, DJ, UJ, NA, and CCK: Formal analysis. TT, DJ, AC, and UJ: Investigation. TT, UJ, DJ, AC, and NA: Methodology.
The authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
This work was funded by Indian Council of Medical Research-India Cancer Research Consortium (ICMR-ICRC) [No.5/13/4/ACB/ICRC/2020/NCD-III], ICMR AdHOC [2021-10573/GENOMIC/ADHOC-BMS]; Central Council for Research in Homeopathy (CCRH) [F.No.17-30/2023-24/CCRH/Tech/Coll./DU Cervical Cancer Phase-II/498]; Institution of Eminence, University of Delhi [Ref. No./IoE/2023-24/12/FRP] to ACB is thankfully acknowledged. In addition to the above funding support, the research has received several non-funding financial support such as Senior Research Fellowship to TT [764/(CSIR-UGC NET JUNE 2019)] by University Grants Commission (UGC), and Senior Research Fellowship to DJ [09/0045/(11635)/2021-EMR-1] and AC [09/0045(12901)/2022-EMR-1] by Council of Scientific and Industrial Research (CSIR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3145
Download: 52
Times Cited: 0
Sasikumar Jalajakumari Soumya ... Perumana R. Sudhakaran
Shifana C. Sadiq ... Ruby John Anto
Paradentavida Prathyusha ... Edakkadath R. Sindhu
Julia K. Opara ... Shrikant Anant
