Affiliation:
1Cátedra de Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires 1113, Argentina
2Instituto de Nanobiotecnología (NANOBIOTEC), Universidad de Buenos Aires (UBA)-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires 1113, Argentina
ORCID: https://orcid.org/0000-0002-0313-2039
Affiliation:
1Cátedra de Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires 1113, Argentina
2Instituto de Nanobiotecnología (NANOBIOTEC), Universidad de Buenos Aires (UBA)-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires 1113, Argentina
ORCID: https://orcid.org/0000-0002-7855-7074
Affiliation:
1Cátedra de Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires 1113, Argentina
2Instituto de Nanobiotecnología (NANOBIOTEC), Universidad de Buenos Aires (UBA)-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires 1113, Argentina
ORCID: https://orcid.org/0009-0002-9287-1382
Affiliation:
1Cátedra de Biotecnología, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires 1113, Argentina
2Instituto de Nanobiotecnología (NANOBIOTEC), Universidad de Buenos Aires (UBA)-Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires 1113, Argentina
Email: scamperi@ffyb.uba.ar
ORCID: https://orcid.org/0000-0003-1795-5519
Explor Drug Sci. 2024;2:648–665 DOI: https://doi.org/10.37349/eds.2024.00066
Received: June 13, 2024 Accepted: August 05, 2024 Published: September 30, 2024
Academic Editor: Ricardo P. Garay, French National Council of Scientific Research, France
The article belongs to the special issue Bioactive Peptides: Pioneering Innovations in Latin American Research
Envenomation caused by snakes, scorpions, and spiders represents a serious public health problem in Latin America. The antivenoms used for its treatment are produced by immunizing horses repeatedly with sublethal doses of animal venoms along with the adjuvant. However, venom availability is a bottleneck. Furthermore, toxin-neutralizing antibodies are only a few of the total produced with this classical method. Therefore, high doses of antivenom are required to achieve the neutralization power, which usually causes adverse reactions in the patient. With the aim of obtaining a higher proportion of toxin-neutralizing antibodies while reducing the dependency on venom availability, alternative immunization protocols have been explored using synthetic peptides with epitopes from clinically relevant toxins. The process to design an immunogenic peptide entitles: (a) choice of the medical relevant toxins in the venom; (b) identification of the epitopes in the selected toxins; (c) improvement of peptide immunogenicity; (d) immunogen synthesis; and e) in vitro and in vivo evaluation. The present article aims to review the advances in the design of immunogenic synthetic peptides for their application in antivenom production in Latin America during the 21st century. Epitopes have been identified from many clinically important toxins in Latin American snakes (snake venom metalloproteinases, snake venom serine proteases, crotamine, phospholipases A2, and three-finger toxins), scorpions (beta-mammal/insect toxin Ts1, alpha-mammal toxin Ts2, alpha-mammal toxin Ts3, toxin Ts4, and beta-mammal Tt1g neurotoxin), and spiders (dermonecrotic toxin and delta-ctenitoxin-Pn2a). Nevertheless, their application is still experimental, even though they are ideal for large-scale and low-cost antivenom production, factors that are necessary to meet national and regional demands.
Envenomation caused by venomous animal bites or stings represents a serious public health problem in tropical countries and therefore it is included in the World Health Organization (WHO) list of: “Neglected Tropical Diseases” [1]. An estimated 5.4 million people worldwide are bitten by snakes each year with 1.8 to 2.7 million cases of envenoming [2]. However, the number is probably underestimated, since most accidents occur in poor rural areas often devoid of proper data registry. In Central and South America, most accidents are caused by snakes of the genus Bothrops, Crotalus, and Lachesis (Viperidae family). Although accidents caused by snakes of the Elapidae family (Micrurus, Leptomicrurus, and Micruroides) occur with less frequency, they cause severe symptoms. In contrast, snakes of the family Colubridae are less dangerous to humans and only in a few cases induce mild envenomation. Other venom animals with medical importance are scorpions of the genus Tityus and Centruroides (Buthidae family) and spiders of the genus Phoneutria, Latrodectus, and Loxosceles [3, 4].
The main strategy for the prevention and control of envenoming is the timely administration of the appropriate antivenom produced as described in 1894, by Phisalix, Bertrand, and Calmette [5]. Antivenoms are produced in public laboratories in many countries as shown in Table 1 [6].
Antivenom production in Latin America
| Country | Laboratory |
|---|---|
| Argentina | Administración Nacional de Laboratorios e Institutos de Salud |
| Brazil | Instituto Butantan, Fundação Ezequiel Dias, Instituto Vital Brazil, and Centro de Produção e Pesquisa em Imunobiológicos |
| Bolivia | Instituto Nacional de Laboratorios de Salud |
| Colombia | Instituto Nacional de Salud |
| Costa Rica | Instituto Clodomiro Picado, Universidad de Costa Rica |
| Mexico | BIRMEX, Laboratorio de Biológicos y Reactivos de México |
| Peru | Instituto Nacional de Salud |
| Venezuela | BIOTECFAR, Universidad Central de Venezuela |
Their production involves the immunization of big mammals, such as horses, with sublethal doses of venoms along with the adjuvant. Subsequently, the antibodies produced are purified from the plasma of the immunized donor animals to obtain IgG molecules that specifically bind and neutralize the toxins that compose the venom. Alternatively, F(ab’)2 or Fab fragments are obtained by enzymatic cleavage with pepsin or papain respectively to reduce the immunogenicity of those heterologous proteins [7]. To have enough venom to immunize the donor mammals, venomous animals must be captured and kept in captivity. Venom extraction from animals is a dangerous and low yield process specially with Micrurus snakes, since they are relatively smaller than Viperidae snakes and with small-sized venom glands. The yield is even much lower with scorpions and spiders. This laborious, risky, and expensive production process limits its availability and affordability in most Latin American countries and therefore represents an important bottleneck for antivenom production [8]. Furthermore, when the donor animal is immunized, it produces antibodies for all the components of the venom. Many of these components are highly immunogenic but non-toxic to humans. Additionally, many highly toxic molecules are poorly immunogenic. Hence, toxin-neutralizing antibodies are only a few of the total amount produced, resulting in a low titer of therapeutically relevant IgG molecules [9]. The high dose of antivenom, with heterologous proteins, administered to achieve the neutralization power usually causes adverse reactions in patients [10].
With the aim of obtaining a higher proportion of toxin-neutralizing antibodies and, at the same time, reducing the dependency on venom availability, alternative immunization protocols have been explored based on immunizing mammals with pure recombinant or synthetic toxins or by using DNA-based immunogens [9, 11, 12]. Instead of using the whole toxin, synthetic antigens can be designed only with the parts that are recognized by the neutralizing antibodies (epitopes) [9, 11–13]. After epitope mapping or prediction, minimal fragments of clinically relevant toxins can be obtained by solid-phase peptide synthesis (SPPS) with high purity and yield [14, 15]. Many synthetic peptides containing one or more epitopes from those medically important venom toxins have been designed and evaluated in vitro and in vivo in experimental animals such as mice and rabbits. The present article aims to review the experimental evaluation of synthetic immunogenic peptides for their application in antivenom production in Latin America during the 21st century. A search on the Scopus and Google Scholar database from Jan. 2000 to Jul. 2024, for articles having the words “peptide”, “antivenom” and/or also “synthetic” in their title, abstract, or keywords was performed and only those articles corresponding to Latin American species were manually selected.
Peptide immunogens are applied as vaccines to combat infectious and malignant diseases as has been reviewed by many authors [16–21]. The key advantage of them is that they are produced by chemical synthesis. They can be obtained by SPPS with high purity and yield in laboratories not strictly oriented to organic chemistry [14, 15]. Most peptides are water-soluble and much more stable than the venom or the recombinant toxins. Additionally, they can be freeze-dried facilitating their distribution. Their characterization and quality control are as simple as other chemical entities and without all the problems associated with biological products. Also, peptides are less likely to induce allergies due to the lack of redundant elements [17]. Furthermore, the immune responses can be directed against naturally less immunogenic toxins or epitopes in the venom and consequently neutralizing antibodies against the most dangerous toxins are obtained [13, 22]. However, to design peptide-based immunogens, first the epitopes on the venom toxin must be identified. In addition, small peptides are poor immunogens, and they are susceptible to enzymatic degradation. Therefore, many strategies have been developed to increase their immunogenicity and enzymatic resistance. The process to design an immunogenic peptide entitles: (a) choice of the medical relevant toxins in the venom; (b) identification of the epitopes in the selected toxin; (c) improvement of the peptide immunogenicity; (d) immunogen synthesis; (e) in vitro and in vivo evaluation (Figure 1).
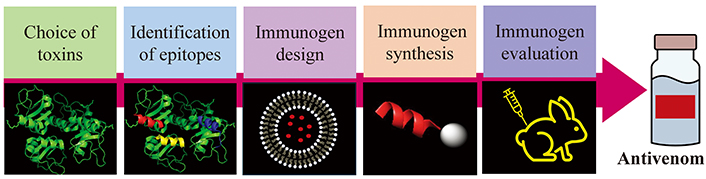
General process for immunogenic peptide development for antivenom production. Scheme drawn with PowerPoint and PyMOL software
Snake and arachnid venoms have evolved to immobilize or kill their prey and to aid in their digestion [23–25]. Only some of their components are of medical relevance for humans and therefore candidate toxins for antivenom production. In Latin American snake venoms, the most clinically important toxins are: the snake venom metalloproteinases (SVMPs), which are a major venom component in the Viperidae family, the snake venom serine proteases (SVSPs), and phospholipases A2 (PLA2s) found in Bothrops, Crotalus, Lachesis, and Micrurus spp., the crotamine of Crotalus spp. and the three-finger toxins (3FTxs) found in Micrurus spp. [3, 9]. Besides, scorpions and spiders have developed venom for insects, which are their natural prey, and only a few components are toxic to humans. Venoms of the scorpions of the genus Tityus and Centruroides comprise mainly α and β-neurotoxins cross-linked by many disulfide bonds and Phoneutria spp. venom neurotoxins are Cys-rich peptides with the so-called cystine knot structural motif [9, 13]. Most of these neurotoxins are insect-specific and only a small number of them cause medically relevant envenomation in humans [13, 22]. In contrast, alpha-latrotoxin and phospholipases-D (PLDs) are the main ones responsible for the pathophysiological symptoms caused in patients bitten by spiders from the genus Latrodectus and Loxosceles, respectively [9].
Venom composition can vary not only between species but also between venomous animals of the same species in different geographical regions. Although, in some cases an antivenom obtained from a different geographical origin than the animal responsible for the envenomation cross-recognizes its venom, there has been reported a reduction in its effectiveness [26, 27]. Therefore, when choosing the toxin, it is necessary to consider the region of the venom animal.
These target toxins can be isolated from the venom, chemically synthesized, or obtained by recombinant DNA technology and used to immunize mice or rabbits to obtain toxin-neutralizing antibodies used to identify the toxin epitopes.
A toxin epitope, the small site on its surface to which a complementary antibody specifically binds, can be a continuous amino acid sequence (linear epitopes) or discontinuous residues on the toxin that come together to form a three-dimensional structure (conformational epitope) [28]. Toxins have several epitopes that can be identified by different mapping methods thoroughly described elsewhere [13, 29]. Conserved sequence motifs are preferred to obtain antibodies that cross-recognize venoms from distinct locations. Mapping methods are based on the screening of peptide libraries composed of many different peptide sequences prepared by either chemical or biological methods. For instance, in “simultaneous multiple peptide synthesis” techniques, short linear overlapping immobilized peptides (approximately 15-mer) covering the complete sequence of the target toxin are simultaneously synthesized. In “multipin/PEPSCAN” [30] polyethylene sticks are used as solid support for each peptide entity while in the “spot-synthesis” [31], library members are synthesized onto a cellulose membrane. Both methods allow the fast simultaneous synthesis of only the required amount of each peptide on an array so that the sequence in each position can be spatially located. Subsequently, the library is screened to determine which peptides bind to the toxin-neutralizing antibodies. For example, spot synthesis has been applied for the identification of epitopes in SVMP from Bothrops atrox [32] and Bothrops jararaca [33], PLA2 and crotamine from Crotalus durissus terrificus [34], PLA2 and 3FTx from Micrurus corallinus [35], as well as many toxins from Tityus serrulatus [36–38] and Loxosceles intermedia dermonecrotic protein 1 (LiD1) from L. intermedia [39–41].
Alternatively, “phage display combinatorial peptide libraries” can be used to select amino acid sequences that mimic natural protein epitopes recognized by specific antibodies (mimotopes). In these combinatorial libraries, peptides are expressed on the surface of bacteriophages. The biopanning procedure is performed with the neutralized antibodies immobilized on a surface. Phage particles that bind to toxin-neutralizing antibodies are isolated and peptides from those phages, mimicking epitopes, are identified by DNA sequencing [42]. Phage display libraries have been applied for the identification of epitopes in SVMP from Bothrops neuwiedi [43], Lachesis muta [44], and LiD1 L. intermedia [45].
Although there are other methods described such as: “Simultaneous synthesis using tea-bags” [46] and “Mass spectrometry epitope mapping” [47], among others, they have not yet been applied to identify toxin epitopes in Latin American venoms. In addition to the different epitope mapping methods, in silico bioinformatic approaches to predict epitopes in an amino acid sequence help to accelerate, in a much more economical way, the identification of epitopes [48, 49]. These methods have been successfully applied for the identification of epitopes in SVMP from B. atrox [50] and L. muta [51], SVSP from Lachesis stenophrys [52], Tt1g from Tityus trivittatus [53] and δ-ctenitoxin-Pn2a from Phoneutria nigriventer [22].
Although epitope peptides are satisfactory antigens that bind to antibodies or B cell receptors, they are poor immunogens. To increase their immunogenicity, peptides can be coupled to an immunogenic protein called a “carrier”. The most commonly used protein carriers are bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), ovalbumin (OVA), tetanus toxoid, etc. [28]. However, those animals immunized with peptide-carrier conjugates not only produce antibodies against the target toxins but also against the carrier. Also, liposomes are used as carriers for immunogen adjuvants due to their inherent biocompatibility and versatility as delivery vehicles. Liposomes enhance antigen delivery to antigen-presenting cells, leading to improved immunization efficacy [54]. Another straightforward strategy to effectively increase peptide immunogenicity is dimerization or multimerization of peptides using synthetic polylysine cores containing two to eight branched Lys to which epitopes can be attached through their alpha and epsilon amines. This design is called: “multiple antigenic peptide” (MAP) [55, 56]. Moreover, lipopeptides are synthesized as potent immunogens using diverse linear or branched structures such as Toll-like receptor agonist lipopeptides. Likewise, self-assembled lipopeptide structures, including spherical and worm-like micelles, have been shown to act as immunogen agents [57, 58]. These modified peptides usually are more stable than venoms, recombinant toxins, or peptide-protein conjugates and are easier to prepare and store for future use. Many other strategies to improve peptide immunogenicity have been described in detail in many reviews [16–21].
Designed epitopes can be easily synthesized at a large scale by SPPS, method that allows to obtain peptides with high purity and yield. It consists of successively coupling α-amino and side chain-protected amino acids on solid support [15]. With the introduction of microwave synthesizers, the yield has been increased even further and synthesis time has been greatly shortened [59]. Peptides can be synthesized and then encapsulated in liposomes or coupled with carrier protein [13, 60]. Likewise, branched peptides and lipopeptides can be obtained (Figure 2).
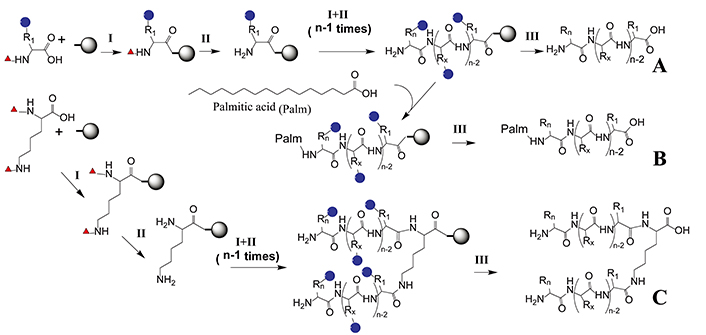
Epitope solid-phase synthesis. An N-α-protected and side chain-protected amino acid is coupled to a solid phase through a linker (I). After removing the N-α-protected group (red triangle), the second N-α-protected amino acid is coupled (II). Coupling (I) and deprotection (II) steps are repeated until the desired amino acid sequence has been elongated. Finally, side chain protecting groups (blue circles) are removed and the peptide is cleaved from the solid support by a global deprotection step (III). (A) Linear peptide synthesis that can be subsequently coupled to a carrier protein or incorporated in liposomes; (B) lipopeptide synthesis where palmitic acid is incorporated at the Nt; (C) multiple antigenic peptide synthesis where the α and ε amino groups of Lys are deprotected simultaneously to synthesize branched peptides. Scheme performed with ChemDraw 18.0 software
With the aim of reducing the number of experimental animals, first in vitro assays are performed with monocyte cell line cultures. Peptide cytotoxicity can be evaluated by performing a cell viability assay. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as well as other equivalent tests can be used to evaluate the activity of the mitochondria respiratory chain as an indicator of the number of viable cells [61]. Afterwards, monocyte activation is evaluated by incubating the cells with sublethal concentrations of immunogen and then measuring the production of cytokines [62, 63]. When immunogens activate cells, the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) migrates from the cytosol to the nucleus to upregulate cytokine gene expression. Therefore, to determine the capacity of a peptide to activate monocytes, immunofluorescence studies to evaluate NF-κB cellular distribution can also be performed [22, 64, 65].
In addition, in vivo tests performed in experimental mammals are still necessary to assess the immunogenic capacity of these peptides, and the neutralizing ability of anti-peptide antibodies obtained. Usually, before administrating venoms or toxins, the quantity that leads to the death of 50% of the animals after an established period (median lethal dose LD50), must be assayed. However, epitope-based immunogens are generally much less toxic. Therefore, their LD50 is generally much higher than the dose needed for immunization [32–41, 43–45, 50–52]. Experimental mammals, mainly mice or rabbits, are immunized with sublethal doses of the immunogen along with the adjuvant. To reduce the number of animals required for testing and discard those antivenoms that are likely to lack efficacy during in vivo assays, first in vitro binding efficacy of the antivenom produced is assessed by dot blotting, immunoblotting, enzyme-linked immunosorbent assay (ELISA) or surface plasmon resonance [66, 67]. Then in vivo assays are performed with those selected antivenoms. Initially, LD50 of a particular venom is determined. Then, mixtures containing a fixed concentration of a lethal dose of venom (typically between 2.5–5 × LD50) with various doses of the tested antivenoms are prepared and incubated. Then each mixture is injected into experimental animals while controls receive only the venom. The number of surviving animals in each group is recorded and neutralization is expressed as the median effective dose (ED50) of the antivenom, defined as the volume of antivenom at which 50% of injected animals survived. The efficacy of an antivenom is a measure of the in vivo or in vitro neutralizing potency against a specific activity of a venom and the effectiveness of an antivenom is a measure of its ability to produce a clinically effective outcome when used to treat envenoming [68–71].
Peptides that constitute the epitopes of venom toxins have been evaluated in vitro and in vivo in experimental animals to produce antivenom for Latin American snakes, scorpions, and spiders.
In Central and South America, most accidents are caused by snakes of the family Viperidae (Bothrops, Crotalus, and Lachesis) and only a few accidents are caused by snakes of the Elapidae family (Micrurus, Leptomicrurus, and Micruroides).
Many authors have identified epitopes from SVMPs. These proteases are responsible for many of the symptoms of envenomation caused particularly by species belonging to the Viperidae family such as proteolytic degradation of fibrinogen and fibrin, inhibition of platelet aggregation, and hemorrhage [72].
Cardoso et al. [43] studied epitopes from the SVMP neuwiedase (UniProt: Q9I9R4) isolated from the venom of Bothrops neuwiedi (“Yarará chica”), endemic to South America and the major source of snakebite in Argentina [73]. In this study, a phage display peptide library was used to identify mimotopes that bind to anti-neuwiedase polyclonal antibodies: NTXNGFFRSXN and HNLGMEHDGKDXL (X means any one of the 20 amino acids). Mice were immunized with phages displaying those mimotopes and the antibodies produced were able to efficiently recognize the toxin in dot blotting, immunoblotting, and ELISA tests. Moreover, Schneider et al. [32] and Kozlova et al. [50] identified epitopes of SVMP atroxlysin-1 (UniProt: P85420) isolated from the most important snake involved in human envenoming in the Amazon, B. atrox (also known as “common lancehead” or “barba amarilla”) [74]. Schneider et al. [32] using the spot-synthesis technique identified two linear epitopes, located at the N-terminus of the toxin, recognized by anti-atroxlysin-1 neutralizing rabbit antibodies: Y22NGNSDKIRRRIHQM36 and G55VEIWSNKDLINVQ68. Further, they designed and synthesized by SPPS a peptide that encompasses the two identified sequences using two Gly between them as spacers: NGNSDKIRRRIH-GG-GVEIWSNKDLINVQ. Next, they encapsulated the peptide into liposomes to immunize mice. The anti-peptide antibodies obtained were able to partially neutralize the digestion of fibrinogen by atroxlysin-1. On the other hand, hemorrhage induced by atroxlysin-1 was fully inhibited when the toxin was incubated with anti-peptide antibodies and the mixture was injected into mice [32]. Also, Kozlova et al. [50] identified, by computational prediction, the linear epitope V9DLFIVVDHGMFMKY23. The epitope obtained by SPPS was encapsulated into liposomes to immunize mice and specific antibody production was detected by ELISA. Also, they tested the enzymatic activity of atroxlysin-1 in the presence of the produced antibodies, and an activity reduction of 70–80% was observed, demonstrating their neutralizing properties. Furthermore, the mice challenged with the toxin mixed with anti-peptide serum showed a clear reduction in atroxlysin-1 hemorrhage activity when compared to the positive control group [50]. Besides, Molina et al. [33] identified a linear epitope in bothropasin (UniProt: O93523), an SVMP from the highly venomous B. jararaca (“yarará” or “jararaca”) endemic to southern Brazil, Paraguay, and northern Argentina [75]. Using the spot-synthesis technique, they identified an epitope located in the catalytic domain of bothropasin: K202ARMYELANIVNEILRYLYMH222, a sequence conserved among different SVMPs. That peptide was afterward obtained by SPPS, polymerized with glutaraldehyde, and used to immunize mice. The serum obtained cross-reacted with bothropasin and crude venoms from B. jararaca and B. atrox and neutralized the hemorrhagic activity induced by a purified fraction from B. jararaca venom in mice. In addition, Machado-de-Ávila et al. [44, 51] studied SVMP from L. muta (or “buchmaster”) the largest venomous snake in South America [76]. They found epitopes in SVMP hemorrhagic factor 2 (UniProt: P22796), also known as mutalysin-II [44, 51]. First, they found mimotopes using a phage-display library: QCTMDQGRLRCR, TCATDQGRLRCT, HCFHDQGRVRCA, HCTMDQGRLRCR, and SCMLDQGRSRCR. These peptides were synthesized by SPPS and incorporated into liposomes to immunize rabbits. Sera from rabbits were tested in an indirect ELISA for their reactivity toward mutalysin-II. The strongest reactivity toward the SVMP was obtained with the sera of rabbits immunized with peptides TCATDQGRLRCT and QCTMDQGRLRCR. In contrast, those sera of rabbits immunized with the peptides HCFHDQGRVRCA and HCTMDQGRLRCR reacted poorly with the enzyme. The neutralizing properties of the anti-peptide antibodies were evaluated in vivo by testing the hemorrhagic-inducing activity of L. muta venom in animals immunized with the four target peptides. The rabbits immunized with TCATDQGRLRCT and QCTMDQGRLRCR were completely protected, while those rabbits immunized with the HCFHDQGRVRCA and HCTMDQGRLRCR peptides were only partially protected [44]. Afterwards, using a predicted epitope database and molecular modeling they found the epitope PYCQCLNKPYL [51]. The peptide was synthesized by SPPS and incorporated into liposomes to immunize rabbits. The designed immunogen also induced antibodies that recognized mutalysin-II and protected against the hemorrhagic effects of Lachesis venom [51].
Another clinically relevant toxin to humans is the SVSPs, thrombin-like enzymes, found in the venom of Viperidae and Elapidae families, that affect hemostasis and thrombosis in the prey or victim [77]. Madrigal et al. [52], using a bioinformatic approach, predicted epitopes that included residues from the SVSP catalytic site of the Central American bushmaster L. stenophrys (UniProt: Q072L7). Those epitopes (W54VLTAAHCDR74, K109DKDIMLIKLDSPVSNS123, and L193EGGKDSCKGDSGGPLI209) were synthesized by SPPS, and an equimolar peptide mixture of them was used to immunize rabbits. The rabbit antibodies induced by the peptide mixture recognized recombinant L. stenophrys SVSP produced in E. coli as well as epitopes from L. stenophrys venom, as demonstrated by ELISA.
PLA2 enzymes are also key toxins in snake envenoming symptoms. They disrupt the cell membrane’s integrity, inducing necrosis, cardiorespiratory arrest, oedema, and anticoagulation [78]. Crotoxin and crotamine are the main responsible toxins for the pathological effects of the envenomation caused by C. d. terrificus (South America rattlesnake or “cascabel”). Crotoxin is a toxic protein complex that comprises two subunits. One with PLA2 activity, is the presynaptic neurotoxic PLA2 crotoxin basic subunit CBc (CB) (UniProt: P62022), which exerts its lethal action by blocking neuromuscular transmission. The other is an acidic non-enzymatic, and non-neurotoxic subunit called crotapotin or PLA2 homolog crotoxin acid subunit CA (CA) (UniProt: P08878) which increases the lethal potency of CB. Crotoxin accounts for 70–90% of C. d. terrificus venom proteome. In contrast, the level of the neurotoxic crotamine peptide (UniProt: Q9PWF3) varies from being absent to up to 19% [78, 79]. Melo et al. [34] identified three epitopes of CB: one in the N-terminus (L10LVGVEGHLLQFNKMIKFETR30), the second in the central part (Y43CGWGGRGRPKDATDR-CCFVH63), and the third one in the C-terminal region (T118YKYGYMFYPDSRCRGPSETC138). Likewise, they identified one epitope from crotamine (F12PKEKICLPPSSDFGKMDCRW32). These sequences were obtained by SPPS, incorporated into liposomes. Rabbits were immunized with a mixture composed of the four synthetic peptides entrapped in liposomes. Antibody response after immunization was evaluated by ELISA. The epitope from crotamine was not immunogenic. On the other hand, those epitopes from the C- and N-terminal regions of CB, with great exposure and flexibility degree, had higher antigenicity compared to the one located in the middle of the protein sequence. Anyway, the three epitope sequences elicited a strong antibody response. Moreover, mice that received C. d. terrificus venom pre-incubated with plasma from immunized rabbits showed an enhanced survival rate when compared to the control group, suggesting that the immunization induced the production of neutralized antibodies.
The 3FTx and PLA2 are abundant toxins in Micrurus spp. (coral snakes) venoms and are the major ones responsible for envenomation symptoms such as neuromuscular blockage, paralysis, and respiratory failure. Castro et al. [35] identified epitopes in four different 3FTx and one PLA2 from M. corallinus snake venom by the spot method. They found: P39DDFTCVKKWEGGGRRV55 in 3FTx Mcor 0100c (UniProt: C6JUP3); T37CPAGQKICFKKWKKG52 and P64KPKKDETIQCCTKNN79 in 3FTx Mcor0039c (UniProt: C6JUP0); L22ECKICNFKTCPTDELRH39 and T54HRGLRIDRGCAATCPTVK72 in 3FTx Mcor0604c (UniProt: Q9PRI1), and R28HASDSQTTTCLSGICYKK45 and G58CPQSSRGVKVDCCMRDK75 in Mcor0599c (UniProt: Q9PRI1). They also identified the epitopes N28LINFQRMIQCTTRRSAW45 and N119CDRTAALCFGRAPYNKNN137 in PLA2 (UniProt: Q8AXW7). Epitope sequences were chemically synthesized by SPPS. All the internal cysteine residues were replaced by serine, and tyrosine was added to the N-terminus of the sequences which did not possess aromatic residues, to allow quantification of the peptides by absorbance at 280 nm. Afterwards, antigenicity and immunogenicity were characterized. All the peptides were used together as immunogens in rabbits. A good antibody response against individual synthetic peptides and M. corallinus venom was achieved. Anti-peptide antibodies cross-reacted with M. frontalis and M. lemniscatus crude venoms. In addition, they inhibit the lethal and phospholipase activities of M. corallinus crude venom. Afterwards, they developed a combined immunization protocol, using priming doses of M. frontalis venom and booster doses of the synthetic epitopes previously obtained from M. corallinus toxins (four of 3FTx and one of PLA2) to obtain coral antivenom in a rabbit model. Immunized animals elicited a humoral response against both M. frontalis and M. corallinus venoms. Relevant cross-reactivity of the obtained sera with other Micrurus species venoms was also observed. The antibodies produced by the immunized animals were able to neutralize the PLA2 activity of both M. frontalis and M. corallinus venoms [80]. Table 2 resumes the epitopes identified in toxins responsible for pathological phenotypes in snake envenomation in Latin America.
Epitopes identified in Latin American snake toxins
| Venom animal | Toxin(UniProt) | Epitope | Epitope mapping | Immunogen | Reference |
|---|---|---|---|---|---|
| Bothrops neuwiedi | SVMP neuwiedase (Q9I9R4) | NTXNGFFRSXN, HNLGMEHDGKDXL | Phage display | Peptides displayed on phages | [43] |
| Bothrops atrox | SVMP atroxlysin-1 (P85420) | NGNSDKIRRRIH-GG-GVEIWSNKDLINVQ | Spotsynthesis | Peptide in liposomes | [32] |
| Bothrops atrox | SVMP atroxlysin-1 (P85420) | V9DLFIVVDHGMFMKY23 | Informatic | Peptide in liposomes | [50] |
| Bothrops jararaca | SVMP bothropasin, (O93523) | K202ARMYELANIVNEILRYLYMH222 | Spot synthesis | Polymerized peptide | [33] |
| Lachesis muta | SVMP hemorrhagic factor 2, (P22796) | QCTMDQGRLRCR, TCATDQGRLRCT, HCFHDQGRVRCA, HCTMDQGRLRCR, SCMLDQGRSRCR | Phage display | Peptides in liposomes | [44] |
| Lachesis muta | SVMP hemorrhagic factor 2, (P22796) | PYCQCLNKPYL | Informatic | Peptide in liposomes | [51] |
| Lachesis stenophrys | SVSP (Q072L7) | W54VLTAAHCDR74, K109DKDIMLIKLDSPVSNS123, L193EGGKDSCKGDSGGPLI209 | Informatic | Individual peptides | [52] |
| Crotalus durissus terrificus | PLA2 crotoxin basic subunit, (P62022) | L10LVGVEGHLLQFNKMIKFETR30, Y43CGWGGRGRPKDATDRCCFVH63, T118YKYGYMFYPDSRCRGPSETC138 | Spot synthesis | Peptides in liposomes | [34] |
| Crotamine (Q9PWF3) | F12PKEKICLPPSSDFGKMDCRW32 | ||||
| Micrurus corallinus | PLA2 (Q8AXW7) | N28LINFQRMIQCTTRRSAW45, N119CDRTAALCFGRAPYNKNN137 | Spot synthesis | Polymerized peptides | [35] |
| 3FTX (C6JUP3 and others) | P39DDFTCVKKWEGGGRRV55, T37CPAGQKICFKKWKKG52, P64KPKKDETIQCCTKNN79, L22ECKICNFKTCPTDELRH39, T54HRGLRIDRGCAATCPTVK72, R28HASDSQTTTCLSGICYKK45, G58CPQSSRGVKVDCCMRDK75 |
SVMP: snake venom metalloproteinase; SVSP: snake venom serine protease; PLA2: phospholipase A2; 3FTx: three-finger toxin; X: any one of the 20 amino acids
Scorpion venoms have Cys-rich peptide toxins. Principally, potassium channel-specific neurotoxins, with a constrained structure of three or four disulfide bridges. However, the main toxins responsible for the symptoms caused by stings in humans (scorpionism) are sodium channel-specific neurotoxins, despite being a minority components in scorpion venom. They are polypeptides comprising 61–76 amino acids, tightly bound by four disulfide bridges. These toxins can be divided into two groups: alpha-neurotoxins (old-world scorpion toxins) and beta-neurotoxins (new-world scorpion toxins) [13, 81].
Alpha-mammal toxin Ts3 (UniProt: P01496), also known as TsIV, is a sodium channel toxin and the major lethal component of T. serrulatus (Brazilian yellow scorpion) venom. In contrast, toxin Ts4 (UniProt: O77463) also called TsNTxP, is a non-toxic but very immunogenic peptide. Alvarenga et al. [36] identified epitopes recognized by anti-Ts3 and anti-Ts4 antibodies by the spot-synthesis method. They selected a C-terminal epitope from Ts3 (L50PDSEPTKTNGKCKS64) and three epitopes from Ts4 (the N-terminal G1REGYPADSKGCKIT15, the central T29LKKGSSGYCAWPAC43 and de C-terminal P49DSVKIWTSETNKCG63). The four epitopes were synthesized by SPPS and were mixed and coupled to KLH and then used to immunize rabbits. Anti-peptide antibodies obtained neutralized the effects of the toxic fraction of T. serrulatus venom in mice. The antigenic specificities of the anti-peptide antibodies were assessed by ELISA using crude venoms from different scorpions. While a high reactivity was observed with T. serrulatus venom, the binding to venoms from T. bahiensis, T. cambridgei, T. stigmurus, and Centruroides sculpturatus was moderate and antibodies were unable to recognize the venom of Androctonus autralis hector. Afterwards, Avila et al. [82] immunized mice with the toxic fraction of the T. serrulatus venom conjugated to BSA with glutaraldehyde. The mice developed neutralizing antibodies which protected them from the venom fraction. They afterward, using the spot method, looked for epitopes on Ts3, and on other two sodium channel toxins: the beta-mammal/insect toxin Ts1 (UniProt: P15226) also called TsVII and on the alpha-mammal toxin Ts2 (UniProt: P68410) also known as TsII. The major antigenic regions were found in the C-terminus of the three toxins and in the helical part of the Ts2 and Ts3 toxins. Subsequently, Maria et al. [37] recognized epitopes on toxin Ts1, Ts2, and Ts3 by the spot-synthesis method: K1EGYLMDHEGSKLSSAGC15, I17RPSGYSGRESGIKKAGC31, and L47PNWVKVWDRATNKAGC61 on Ts1; K1EGYAMDHEGSKFSSAGC15 and V48PDHIKVWDYATNKSAGC62 on Ts2 and K1KDGYPVEYDNSAYIAGC15, W16NAYSDKLSKDKAGC30, and G48LPDSEPTKTNGKSKAGC62 on Ts3. The eight peptides were synthesized by SPPS, then coupled to KLH, and immobilized on ELISA plates. Those plates were used to evaluate the potency of horse immune sera obtained by immunizing them with T. serrulatus venom. Nevertheless, they observed a poor correlation between the ELISA based on linear epitopes and the potency of the antivenom. Although they detected horse antibodies that interacted with the linear peptide epitopes, and those antibodies neutralized up to a certain extent the whole venom toxic effect, probably the antibodies associated with the high neutralizing potency of the antivenoms were those that interacted with conformational epitopes on the toxins.
Duarte et al. [38] search for discontinuous epitopes in the non-toxic but very immunogenic peptide Ts4, since neutralizing antibodies are often associated with conformational epitopes. Octadecapeptides with the general formula P1-Gly-Gly-P2 were synthesized by the spot method. P1 and P2 were octapeptides from the Ts4 N-terminal and C-terminal sections, respectively. The more reactive peptide sequence was the epitope G1REGYPAD8-GG-G47LPDSVKI54. That peptide was subsequently obtained by SPPS, conjugated to OVA with glutaraldehyde, and then used to immunize mice. In vivo, protection assays showed that immunized mice could resist a challenge by T. serrulatus whole venom. The peptide design matched with a discontinuous epitope exposed at the toxin molecular surface, which contains residues known to be important for the bioactivity of the toxin.
Beta-mammal Tt1g neurotoxin (UniProt: P0DMM8) has been described as responsible for the intoxication symptoms caused by a sting on humans of the Argentinean scorpion first described as Tityus trivittatus [83, 84] but recently renamed as Tityus carrilloi [85]. Tt1g peptide is similar to toxin Ts1 from the Brazilian scorpion T. serrulatus (95% identity) [66]. Rodríguez et al. [53] identified epitopes from Tt1g using the “MHC-II Binding Predictions” tool from “Immune Epitope Database Analysis Resource” [86]. The linear epitope predicted to have the highest score, L67PNWVKVWERATNRC81, corresponding to Tt1g C-terminus, was synthesized by SPPS. Cys was replaced by α-aminobutyric acid (Abu) to avoid Cys bond formation. To increase its immunogenicity, branched or N-terminal palmitoylated peptides were synthesized. Synthesized peptides were currently tested in vitro and in vivo to assess their capacity to produce antivenoms. Table 3 resumes the epitopes identified in Latin American scorpion toxins.
Epitopes identified in Latin American scorpion toxins
| Venom animal | Toxin (UniProt) | Epitope peptide | Epitope mapping | Immunogen | Reference |
|---|---|---|---|---|---|
| Tityus serrulatus | Ts3 (P01496) | L50PDSEPTKTNGKCKS64 | Spot synthesis | Peptide bound to KLH | [36] |
| Ts4 (O77463) | G1REGYPADSKGCKIT15, T29LKKGSSGYCAWPAC43, P49DSVKIWTSETNKCG63 | ||||
| Tityus serrulatus | Ts1 (P15226) | K1EGYLMDHEGSKLSSAGC15, I17RPSGYSGRESGIKKAGC31, L47PNWVKVWDRATNKAGC61 | Spot synthesis | Peptide bound to KLH | [37] |
| Ts2 (P68410) | K1EGYAMDHEGSKFSSAGC15, V48PDHIKVWDYATNKSAGC62 | ||||
| Ts3 (P01496) | K1KDGYPVEYDNSAYIAGC15, W16NAYSDKLSKDKAGC30, G48LPDSEPTKTNGKSKAGC62 | ||||
| Tityus serrulatus | Ts4 (O77463) | G1REGYPAD8-GG-G47LPDSVKI54 | Spot synthesis | Peptide bound to OVA | [38] |
| Tityus trivittatus | Tt1g (P0DMM8) | LPNWVKVWERATNR-Abu | Informatic | Branched or palmitoylated peptide | [53] |
Abu: α-aminobutyric acid; KLH: keyhole limpet hemocyanin; OVA: ovalbumin
L. intermedia, L. laeta, and L. gaucho, popularly known as “brown spiders”, are considered a serious public health issue in South America. Although the dermonecrotic toxins PLDs are not the most expressed toxins in Loxosceles venoms, they trigger most of the major clinical symptoms of Loxosceles envenomation (loxoscelism) such as: dermonecrosis, thrombocytopenia, hemolysis, and acute renal failure [87].
Felicori et al. [39], using the spot-synthesis method, found linear epitopes on the LiD1, also known as dermonecrotic toxin LiSicTox-alphaIA1bi (UniProt: P0CE81). The linear epitopes: M13VNAIGQIDEFVNLG27, I31ETDVSFDDNANPEY45, S58KKYENFNDFLKGLR72, D100NQANDAGKKLAKNL114, D160KVGHDFSGNDDISD174, and N247YPDVITDVLNEAAY261, were synthesized by SPPS. Rabbits were immunized with either: a) recombinant LiD1 (rLiD1) alone; b) a mixture of all the peptides or; c) rLiD1 together with all the peptides. Animals immunized with these different immunogens were protected from the dermonecrotic, hemorrhagic, and oedema forming activities induced by rLiD1. Nevertheless, the protection conferred by peptides was lower than that provided by rLiD1 or by the mixture of peptides and rLiD1. Subsequently, Dias-Lopes et al. [40] synthesized by SPPS the epitope N25LGANSIETDVSFDDNANPEYTYHGIP51 previously found by Felicori et al. [41] using the spot method. That epitope had residues implicated in the active site of LiD1 and was used as an immunogen in mice and rabbits. Immunized mice showed increased resistance to the lethal effect of the crude venom, as compared with non-immunized control mice, demonstrating the capacity of the antibodies anti-epitope to neutralize the lethal activity of L. intermedia venom. Also, about 70% of protection against necrotic and hemorrhagic activities caused by the recombinant protein rLiD1 was conferred to the immunized rabbits [40]. Additionally, Moura et al. [45] search for mimotopes using a phage display library. The mimotope NCNKNDHLFACW and its analog NSNKNDHLFASW, in which Cys were substituted by Ser, were encapsulated in liposomes and used as immunogens in rabbits. The resulting antibodies could neutralize some of the biological effects induced by crude L. intermedia venom. Those rabbits immunized with the two mimotopes showed 60% protection against the dermonecrotic and 80% protection against the hemorrhagic-inducing activities of the venom, indicating that both immunizing peptides had a high capacity for neutralization. Negative control animals were not protected, whereas about 90% protection against necrotic and hemorrhagic-inducing activities was observed in the rabbits that were immunized using the whole venom.
P. nigriventer (banana or wandering spider) is found in South America (mainly in Brazil and northern Argentina). It is among the most venomous spiders of medical significance for humans. Its venom comprises mainly Cys-rich peptides neurotoxins with the so-called cystine knot structural motif, that have significant effects on the sodium channels of insects. Although present in low proportion in the venom, delta-ctenitoxin-Pn2a (UniProt: P29425) and delta-ctenitoxin-Pn2c (UniProt: O76199) are responsible for the clinically relevant symptoms caused by P. nigriventer bite in humans [88].
Rodríguez et al. [22] identified epitopes from delta-ctenitoxin-Pn2a using the “MHC-II Binding Predictions” tool from “Immune Epitope Database Analysis Resource” [86]. The linear epitopes predicted to have the highest score were found in its C-terminal region. Of these, C-terminal sequence G34YFWIAWYKLANCKK48 was selected because, this fragment does not form part of the Cys-knot, thus making it more accessible for subsequent recognition by antibodies. It also contains most of the residues that interact with sodium channels and therefore the antibodies that recognize this sequence could neutralize the toxin [89]. The linear epitope was synthesized by SPPS where Cys was replaced by Abu to avoid Cys bond formation. To increase its immunogenicity branched and N-palmitoylated versions were synthesized: [(Ac-GYFWIAWYKLAN-Abu-KK)2-KG-NH2 and Palm-GYFWIAWYKLAN-Abu-KKG-NH2]. Synthesized peptides were evaluated in vitro on a murine macrophage cell line. The cellular distribution of NF-κB was examined by immunofluorescence after exposing macrophages to the peptides. Early activation was observed for all three peptides, thereby indicating that they are promising immunogens for antivenom production. Nevertheless, in vivo tests are still required to assess their immunogenic capacity and whether the generated antibodies can confer protection against the venom. Table 4 resumes the epitopes identified in toxins responsible for pathological phenotypes in spider envenomation in Latin America.
Epitopes identified in Latin American spider toxins
| Venom animal | Toxin (UniProt) | Peptide epitope | Epitope mapping | Immunogen | Reference |
|---|---|---|---|---|---|
| Loxosceles intermedia | LiD1 (P0CE81) | M13VNAIGQIDEFVNLG27, I31ETDVSFDDNANPEY45, S58KKYENFNDFLKGLR72, D100NQANDAGKKLAKNL114, D160KVGHDFSGNDDISD174, N247YPDVITDVLNEAAY261 | Spot synthesis | Peptide mixture with or without rLiD1 | [39] |
| Loxosceles intermedia | LiD1 (P0CE81) | N25LGANSIETDVSFDDNANPEYTYHGIP51 | Spot synthesis | Peptide | [40] |
| Loxosceles intermedia | LiD1 (P0CE81) | NCNKNDHLFACW, NSNKNDHLFASW | Phage display | Peptide in liposomes | [45] |
| Phoneutria nigriventer | δ-ctenitoxin-Pn2a (P29425) | GYFWIAWYKLAN-Abu-KKG | Informatic | Branched or palmitoylated peptide | [22] |
Abu: α-aminobutyric acid; LiD1: Loxosceles intermedia dermonecrotic protein 1; rLiD1: recombinant Loxosceles intermedia dermonecrotic protein 1
In most of the articles here described the authors have demonstrated the neutralization of the target toxin with the antibodies raised by immunizing experimental animals with epitope-based immunogens, proving their potential for antivenom production. Although peptide immunogens are applied as vaccines to combat infectious and malignant diseases, their application in antivenom production is still experimental, even though they are ideal for large-scale and low-cost antivenom production necessary to meet national and regional demands. Envenoming caused by snakes and arachnids are well-known medical emergency in Latin America, especially in rural areas where workers and children are the most affected. Conventional antivenom production has not always meet the regional demands specially when the venomous specimen is difficult to find, capture, and maintain in captivity. Furthermore, the geographical venom variation requires local manufacturers, each with a restricted geographical focus. This leads to a fragmented and largely unsustainable market that has resulted in the commercial withdrawal and the restricted availability of many antivenoms. On the other hand, peptides can be designed considering those geographical differences in toxin sequences and can be distributed throughout the different regions according to toxins prevalence. Therefore, it is worth continuing active research in the field to achieve their approval and application for future antivenom production. Furthermore, their sustainable benefit for manufacturers would encourage their synthesis by pharmaceutical industries complementing the governmental organization production.
3FTxs: three-finger toxins
Abu: α-aminobutyric acid
CB: crotoxin basic subunit CBc
ELISA: enzyme-linked immunosorbent assay
KLH: keyhole limpet hemocyanin
LD50: median lethal dose
LiD1: Loxosceles intermedia dermonecrotic protein 1
NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
PLA2s: phospholipases A2
rLiD1: recombinant Loxosceles intermedia dermonecrotic protein 1
SPPS: solid-phase peptide synthesis
SVMPs: snake venom metalloproteinases
SVSPs: snake venom serine proteases
JAR, GRBV, and JAE: Writing—original draft, Writing—review & editing. SAC: Funding acquisition, Conceptualization, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was partially supported by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación de la República Argentina [PICT-2021-I-A-00236]; the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) [PIP 11220210100075CO] and the Universidad de Buenos Aires, Argentina [20020220200001BA]. SAC is a researcher at the CONICET and a professor at the UBA. JAR, GRBV, and JAE are teachers at the UBA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3361
Download: 74
Times Cited: 0
Constanza Cárdenas ... Fanny Guzmán
Andrea Barragán-Cárdenas ... Javier García-Castañeda
Natalia Ardila-Chantré ... Javier Eduardo García-Castañeda
Lucero Ruiz-Mazón ... Mireya de la Garza
Vaezeh Fathi Vavsari, Saeed Balalaie
Vanesa Herlax
Karen Johanna Cárdenas-Martínez ... Javier Eduardo García-Castañeda
Daniela Perdomo-Joven ... Mauricio Urquiza-Martinez
