Affiliation:
1Molecular Bioassay Laboratory, Institute of Advanced Virology, Thiruvananthapuram 695317, Kerala, India
2Division of Cancer Research, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram 695014, Kerala, India
†These authors contributed equally to this work.
Affiliation:
2Division of Cancer Research, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram 695014, Kerala, India
†These authors contributed equally to this work.
Affiliation:
2Division of Cancer Research, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram 695014, Kerala, India
Affiliation:
1Molecular Bioassay Laboratory, Institute of Advanced Virology, Thiruvananthapuram 695317, Kerala, India
Affiliation:
2Division of Cancer Research, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram 695014, Kerala, India
Affiliation:
1Molecular Bioassay Laboratory, Institute of Advanced Virology, Thiruvananthapuram 695317, Kerala, India
2Division of Cancer Research, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram 695014, Kerala, India
Affiliation:
3The Shraga Segal Department of Microbiology, Immunology and Genetics, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel
ORCID: https://orcid.org/0000-0002-1412-0957
Affiliation:
1Molecular Bioassay Laboratory, Institute of Advanced Virology, Thiruvananthapuram 695317, Kerala, India
2Division of Cancer Research, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram 695014, Kerala, India
4Centre of Excellence in Nutraceuticals, KSCSTE, Government of Kerala, Thiruvananthapuram 695317, Kerala, India
Email: cs.coen@kerala.gov.in; rjanto@iav.res.in
ORCID: https://orcid.org/0000-0001-5856-0076
Explor Drug Sci. 2024;2:744–784 DOI: https://doi.org/10.37349/eds.2024.00073
Received: May 21, 2024 Accepted: August 05, 2024 Published: October 30, 2024
Academic Editor: Aamer Saeed, Quaid-I-Azam University, Pakistan
The article belongs to the special issue Remedial benefits of natural products in inflammation and cancer
Phytochemicals, the bioactive compounds derived from plants, play a significant role in modulating pathways leading to cancer and inflammation, rendering themselves promising candidates for therapeutic interventions. This review explores the multifaceted potential of various phytochemicals in modulating key mechanisms involved in the development and progression of cancer and inflammation. The diverse array of phytochemicals discussed here encompasses polyphenols, flavonoids, alkaloids, terpenoids, and many others, each with distinct molecular targets and modes of action. This review is an attempt to elucidate and correlate the regulatory role of phytochemicals on cellular signaling pathways implicated in oncogenesis and inflammatory responses, highlighting the significance and potential of phytochemical-based therapies for cancer prevention and treatment, as well as for managing inflammatory conditions. By exploring the promising potential of phytochemical-based remedies for cancer prevention, treatment, and inflammatory conditions and emphasizing their diverse roles in modulating critical regulatory mechanisms, this review addresses the current research landscape, challenges, and future directions in utilizing phytochemicals as effective agents against cancer and inflammation.
Phytochemicals are bioactive compounds that are produced by plants as a means of protection from pathogens, such as bacteria, viruses, fungi, or parasites. They play an important role during stressful conditions and tend to accumulate in fruits and vegetables. During growth and development, plants produce numerous phytochemicals that help resist environmental stresses [1]. The richness of phytochemicals in plants has made them integral parts of traditional healing practices across diverse cultures for centuries. The earliest documented use of plant-based medicines can be traced back to ancient civilizations, including China (circa 5,000 BC), India (circa 2,000 BC), Mesopotamia (circa 2,600 BC), and Egypt (circa 1,550 BC). However, the dominance of synthetic medications surged in the initial decades of the twentieth century, due to their perceived efficacy and profitability [2].
Phytochemicals from a diverse array of plants have been found to confer various health benefits, exhibiting a spectrum of medicinal activities encompassing anti-inflammatory, antidiabetic, anti-aging, antibiotic, antiparasitic, antidepressant, anti-cancer, and antioxidant properties. Apart from these, they also contribute to combating wounds, cardiovascular abnormalities, Alzheimer’s, Parkinson’s, epilepsy, migraine, arthritis, schizophrenia, acne vulgaris, and various age-related diseases [3, 4]. Phytochemicals such as terpenes, carotenoids, monoterpenes (including perillyl alcohol and limonene), saponins, phenols (including polyphenols and flavonoids), organo-sulphur compounds (such as indoles, isothiocyanates, and thiosulfonates), organic acids, polysaccharides, and lipids (such as isoprenoids and omega-3/omega-6 fatty acids), sourced from fruits and vegetables, offer a diverse spectrum of health benefits [5].
Inflammation represents an evolutionary strategy in higher organisms, serving as a protective response against microbial infections and tissue injuries. This immune process is essential for eliminating harmful agents and facilitating tissue repair [6]. This key mechanism of post injury tissue repair has been linked to a broader spectrum of diseases beyond infection and injury [7]. Tumor-promoting inflammation emerges as a critical driver in cancer pathogenesis, affecting neoplastic transformation, tumor progression, metastasis, and recurrence. The induction of wound healing responses in tumor tissue stimulates cell survival, angiogenesis, and extravasation, with certain immune responses promoting tumor progression. Understanding the intricate interplay between inflammation and disease processes offers valuable insights into the development and progression of various pathological conditions, underscoring the importance of targeted therapeutic strategies aimed at modulating inflammatory responses [8].
Phytochemicals such as dihydrotanshinone, curcumin, and quercetin have demonstrated remarkable efficacy against inflammatory responses, in experimental models (in vitro) as well as living organisms (in vivo). This exceptional anti-inflammatory potential positions them as promising contenders for the development of safe anti-inflammatory medications [9]. The phytochemicals curcumin and resveratrol (RSV) have also been found to down-regulate major signaling pathways contributing to cancer and inflammation. Traditional remedies like Saccharomyces cerevisiae and plant-derived substances like colchicine, down-regulate inflammation by modulating cytokine production, showcasing their potential in mitigating inflammation and pain associated with chronic ailments [10, 11]. Similarly, many flavonoids have been shown to impede tumor growth by intervening with inflammatory mechanisms, suggesting their potential applications in the prevention of diseases like breast and colorectal cancer (CRC), where chronic inflammation plays a significant role [11].
Cancer is a major global health issue, accounting for 10 million deaths in 2020. Common types of cancer include lung, breast, colon, prostate, and rectal cancers [12]. The GLOBOCAN database predicts over 35 million new cancer cases in 2050, a 77% increase from 20 million cases in 2022. Cancer is primarily caused by gene mutations, which change cell behavior and promote sustained cell proliferation [13]. Chemical compounds, smoke, viruses, bacteria, and radiation can contribute to carcinogenesis. Mutations in proto-oncogenes, which are responsible for cell division and growth, can convert them to oncogenes, which disrupt the normal cell division mechanism leading to cell transformation [14]. Mutations that inactivate tumor suppressor genes may also result in uncontrolled cell division and tumor formation. Furthermore, epigenetic modifications such as DNA methylation, histone modifications, and nucleosome position, which are also crucial for the proper regulation of cell growth and development, can also contribute to the neoplastic process [15].
A large variety of plant-derived chemicals have been found to possess properties that impede tumor invasion and metastasis by various mechanisms, such as disruption of signaling pathways that regulate epithelial-mesenchymal transition (EMT) processes or prevent migration and invasion of cancer cells [16]. Moreover, specific phytochemicals such as saponins, phenolic compounds, and terpenoids, including genistein and curcumin, can target molecular pathways regulating angiogenesis, thereby restricting nutrient and oxygen supply to tumors [17]. Additionally, alkaloids, terpenes, and polyphenols can inhibit cancer cell proliferation and growth, while many other plant extracts appear to modulate cell cycle and cell death regulating proteins such as BH3 interacting-domain death agonist (Bid), B-cell lymphoma 2 (Bcl-2)-associated protein x (BAX), and Bcl-2, inducing cancer cell death via apoptosis [18]. Figure 1 illustrates the major hallmarks of cancer and inflammation, targeted by phytochemicals. Many plant-derived compounds have been reported to hinder tumor progression by modulating biochemical pathways, such as glycolysis, the pentose phosphate pathway (PPP), the tricarboxylic acid (TCA) cycle, and serine metabolism [19]. Phytochemicals like shikonin and hypericin promote immune mediated cancer cell destruction [20]. In summary, the multifaceted anti-inflammatory actions of phytochemicals underscore their significance as both preventive and therapeutic agents in cancer and inflammation.
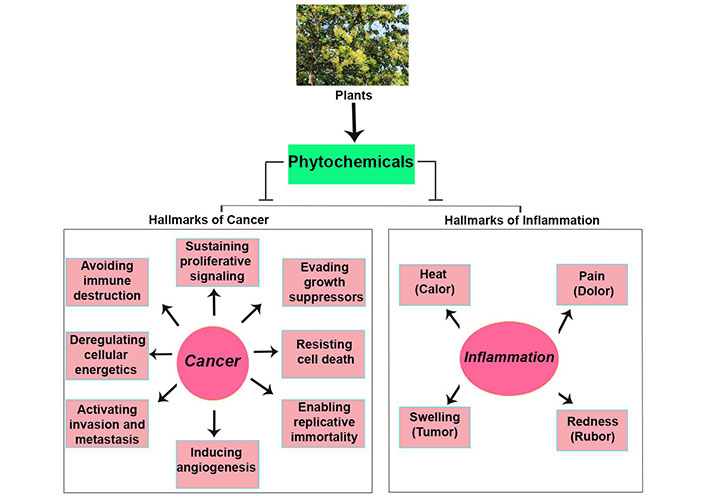
Involvement of phytochemicals in modulation of cancer cell growth and inflammation-dependent diseases
Generally, phytochemicals have been classified as primary or secondary metabolites based on their role in plant metabolism. Primary metabolites are directly involved in growth and metabolism and include common sugars, amino acids, proteins, nucleic acid purines and pyrimidines, and chlorophylls [21]. Secondary metabolites are products of the primary metabolism and are involved in a range of metabolic activities. They are grouped into different classes depending on their chemical structure, botanical origin, biosynthesis pathway, or biological properties [22]. Based on the chemical structure of the compounds, they are classified into polyphenols (flavonoids and non-flavonoids), alkaloids, terpenoids, and saponins [2, 5, 10, 23, 24]. Plant derived alkaloids, polyphenols, and terpenoids constitute the most significant group of pharmacologically active compounds [3]. The terpenoids can be subcategorized into carotenoids and non-carotenoid terpenoids. Other phytochemical groups, which share some properties with the above-mentioned groups, have been classified into a miscellaneous category, which consists of organo-sulphur compounds and non-sulphur-containing indoles [25].
The major classes of phytochemicals are listed in Table 1, while the chemical structure of the major phytochemicals that are discussed in this review is presented in Figure 2.
List of major classes and subclasses of phytochemicals and other sources
| Major class | Subclass | Compounds | Source |
|---|---|---|---|
| Polyphenols-flavonoids | Flavanols | Catechins, proanthocyanidins, epigallocatechin | Apricots, apples, peaches, green tea, and chocolate |
| Flavonols | Kaempferol, quercitin | Broccoli, onions, radish, and blueberries | |
| Flavones | Luteolin, apigenin | Celery, lettuce, and capsicum peppers | |
| Isoflavones | Genistein, daidzein | Soya and its processed products | |
| Flavanones | Hesperetin, naringenin | Citrus fruits like oranges and grapefruits | |
| Anthocyanidin | Cyanidin, delphinidin | Red wine, berry fruits, and colored fruits | |
| Polyphenols-non-flavonoids | Lignans | Pinoresinol | Flaxseeds and sesame seeds |
| Stilbenes | Resveratrol | Grapes, pomegranate, berries | |
| Phenolic acids | Ferulic acid, caffeic acid, ellagic acid, vanillic acid | Coffee, berries, onions, cereals, and legumes | |
| Curcumins | Curcumin, demethoxycurcumin, cyclic curcumin | Rhizomes of turmeric | |
| Saponins | Cycloartane saponins | Uttroside B, timosaponin A-III | Medicinal plants |
| Dammarane saponins | |||
| Oleananes | |||
| Spirostanes | |||
| Furostanes | |||
| Terpenes | Monoterpenoids | Thymoquinone | Nigella sativa |
| Diterpenoids | Paclitaxel | Bark of the Pacific yew tree | |
| Triterpenoids | Ursolic acid | Marjoram leaves, oregano leaves, rosemary, and wax layer of edible fruits | |
| Cucurbitacin | Cucurbitaceae plants | ||
| Alkaloids | Tryptanthrin | Bacteria, fungi, and plants like Couroupita guianensis, Wrightia tinctoria | |
| Cepharanthine | Stephania tubers | ||
| Oxysophocarpine | Robinia genus plants | ||
| Miscellaneous compounds | Organosulfur compounds | Sulforaphane, allicin, isothiocyanates | Garlic, onions, and cruciferous vegetables like broccoli, cauliflower, and cabbage |
| Polysaccharides | Pectin | Citrus fruits and apples | |
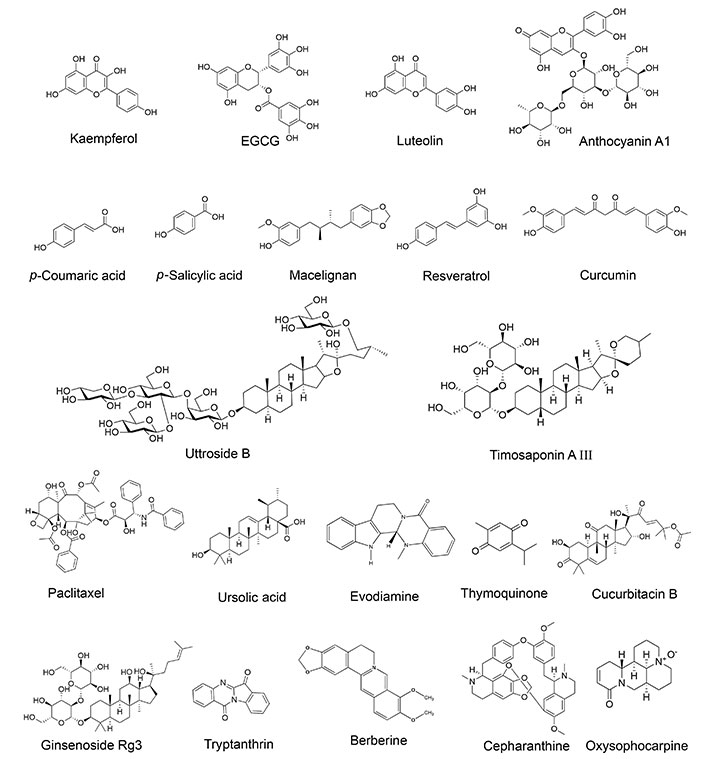
Chemical structures of phytochemicals discussed in this review. EGCG: epigallocatechin 3-gallate. The Figure was generated using ChemDraw with images sourced from Pubchem [kaempferol (PubChem Identifier: CID 5280863); EGCG (PubChem Identifier: CID 65064); luteolin (PubChem Identifier: CID 5280445); anthocyanin A1 (PubChem Identifier: CID 154824275); p-coumaric acid (PubChem Identifier: CID 637542); p-salicylic acid (PubChem Identifier: CID 135); macelignan (PubChem Identifier: CID 10404245); resveratrol (PubChem Identifier: CID 445154); curcumin (PubChem Identifier: CID 969516); uttroside B (PubChem Identifier: CID 44566638); timosaponin A III (PubChem Identifier: CID 155887663); paclitaxel (PubChem Identifier: CID 36314); ursolic acid (PubChem Identifier: CID 64945); evodiamine (PubChem Identifier: CID 442088); thymoquinone (PubChem Identifier: CID 10281); cucurbitacin B (PubChem Identifier: CID 5281316); ginsenoside Rg3 (PubChem Identifier: CID 9918693); tryptanthrin (PubChem Identifier: CID 73549); berberine (PubChem Identifier: CID 2353); cepharanthine (PubChem Identifier: CID 10206); oxysophocarpine (PubChem Identifier: CID 161544)]
Natural biophenols, synthesized by plants as secondary metabolites, predominantly for defense against pathogens, exhibit diverse roles and contribute significantly to the flavor, color, and nutritional value of plant-derived foods. These compounds are broadly categorized into two major groups: flavonoids and non-flavonoids. Research suggests that polyphenols, which are abundant antioxidants in the diet, may improve insulin resistance, reduce inflammation and oxidative stress, and slow aging process [25, 26]. They also show promise in addressing age-related symptoms such as oxidative stress, inflammation, proteostasis impairment, cellular senescence, thrombosis, and tumor development.
Flavonoids are a large group of plant secondary metabolites, with a common phenyl benzopyrone structure. They are one of the largest groups of secondary metabolites, with over 2,000 naturally occurring compounds. They are classified into two main groups based on the saturation level of the central heterocyclic ring [27]. Unsaturated flavonoids include anthocyanidins, flavones, flavonols, and isoflavones, while saturated flavonoids include flavanones, dihydroflavonols, and flavan-3-ols [28]. Flavonoids are generally found to be conjugated with sugars as glycosides or in ester or polymer forms [29]. The antioxidant capacity of flavonoids is determined by the positioning of the catechol B-ring on the pyran C-ring, as well as the quantity and arrangement of hydroxy groups on the B-ring [30]. Flavonoids originate from two distinct biosynthetic pathways viz; the phenyl propanoid pathway, which is responsible for the phenyl propanoid skeleton, and the polyketide pathway, which generates polymeric building blocks [31]. These compounds offer various therapeutic benefits, such as antioxidant activity, cardiovascular protection, anti-inflammatory effects, neuroprotection, and anti-platelet aggregation [32]. Additionally, flavonoids play a crucial role in cancer prevention by influencing processes such as apoptosis, necrosis, cell cycle arrest, and autophagy [33]. Some selected compounds from this category are discussed below.
Flavonoids are classified into seven subclasses, based on their structural differences. This includes flavanols, flavones, isoflavones, anthocyanidins, flavanones, flavonols, and chalcones [34, 35]. Flavones are characterized by a double bond between C2 and C3 and a ketone group at C4 position. They are commonly found in parsley and celery, with examples like apigenin and luteolin which are known for their anti-inflammatory and anticancer properties [36]. Flavanols have an additional hydroxyl group at C3 and are present in onions, kale, and berries, exhibiting strong antioxidant activities. Examples of this include quercetin and kaempferol [37]. Flavan-3-ols, like catechin and epicatechin, have a hydroxyl group at C3 and are found in green tea and cocoa, noted for their cardiovascular benefits [37]. Anthocyanidins are flavonoids without a ketone group at C4 position. They are responsible for red, blue, and purple pigmentation in plants such as berries and grapes, and include cyanidin and delphinidin, which are well-known for their antioxidant properties [38]. Isoflavanoids, with the B ring attached to C3, are found in soybeans and include genistein and daidzein, beneficial for menopausal symptoms and osteoporosis [39]. Chalcones, with an open-chain structure, are present in apples and strawberries, with examples like phloretin, and are known for their anti-inflammatory and antimicrobial activities [40].
Some selected compounds from this category are discussed below.
Kaempferide, a compound with multifaceted therapeutic potential, exhibits a range of anti-inflammatory properties that make it a promising candidate for treating various inflammatory conditions [41]. Specifically, it has been shown to suppress the expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 1β (IL-1β), and IL-6, which play crucial roles during the inflammatory response. By inhibiting the nuclear factor kappa B (NF-κB) pathway, a central regulator of inflammation, kaempferide effectively reduces the production of inflammatory mediators, thereby mitigating the inflammatory process [41]. Kaempferide also exhibits anti-obesity, hypoglycemic, and hypolipidemic effects, which are achieved through inhibition of the Toll-like receptor 4 (TLR4)/inhibitor of NF-κB α (IκBα)/NF-κB pathways [42]. Kaempferide demonstrates potent anti-photoaging properties, exemplified by its ability to inhibit collagen degradation, inflammatory responses, subcutaneous fat, UVB-induced reactive oxygen species (ROS)-mediated signaling pathways, and inhibit signaling effector proteins such as mitogen-activated protein kinases (MAPKs) [extracellular signal regulated kinase (ERK), p38, and C-Jun N-terminal kinase (JNK)] and protein kinase B (AKT) [41]. The kaempferide anti-inflammatory effects also contribute to preserving cardiac steady-state function [43].
In addition to its myriad anti-inflammatory attributes, kaempferide is extensively investigated for its anti-cancer properties. To the best of our knowledge, our group was the first to report the anti-cancer potential of kaempferide against cervical cancer, wherein the compound isolated from Chromolaena odorata was found to inhibit the growth of HeLa cells in vitro [44]. It emerges as a potent agent against various types of cancer, including lung adenocarcinoma, hepatocellular carcinoma (HCC), and cervical cancer [45]. The mechanism of action of kaempferide involves the regulation of onco-proteins and tumor suppressor proteins, which results in the inhibition of cancer cell growth. Kaempferide acts as a promising alternative against HCC by down-regulating transforming growth factor-β1 (TGF-β1), a crucial marker of liver cancer metastasis [46]. It also exhibits remarkable efficacy in attenuating UVB-induced skin aging processes and in enhancing cancer cell sensitivity to doxorubicin [47]. A recent study from our lab has revealed that in cervical cancer cells, kaempferide suppresses the expression of the human papilloma virus (HPV) onco-proteins, E6 and E7, two proteins that are pivotal regulators of cervical tumorigenesis [48].
Epigallocatechin 3-gallate (EGCG), a potent catechin and a major component of green tea, is derived from the formal condensation of gallic acid with (-)-epigallocatechin. It is naturally present in an array of plant sources, including Limoniastrum guyonianum and Scurrula atropurpurea. EGCG is a prominent polyphenol that has garnered significant attention due to its multifaceted attributes, including antioxidant, anti-inflammatory, anti-cancer, and anti-diabetic properties [49].
EGCG exhibits broad anti-inflammatory effects. It was found to ameliorate brain inflammation and demyelination damage in experimentally-induced autoimmune encephalomyelitis (EAE). Through a complex interplay of cellular mechanisms, EGCG not only displays neuroprotective benefits but also showcases remarkable efficacy against a spectrum of infections [50]. From battling viruses like human immunodeficiency virus (HIV), influenza, and hepatitis C to combating drug-resistant bacterial strains such as methicillin-resistant Staphylococcus aureus (MRSA) and even human-pathogenic yeasts like Candida albicans, EGCG emerges as a versatile health-promoting compound [51, 52]. Its anti-inflammatory prowess is evidenced by its ability to inhibit cyclooxygenase (COX) enzymes and reduce the expression of pro-inflammatory cytokines [53]. Moreover, by obstructing the activation of the epidermal growth factor receptor (EGFR) and its downstream signaling mechanisms, EGCG offers promising prospects for alleviating neuroinflammation in neurodegenerative diseases [53].
EGCG demonstrates potent cancer preventive and therapeutic potential by impacting on diverse signal transduction pathways. It modulates the ERK, inhibits PI3K-AKT, and sensitizes cells to TNF related apoptosis inducing ligand (TRAIL) thereby protecting cells from oxidative stress-induced apoptosis [50]. In breast cancer, EGCG exhibits multiple benefits by controlling cell proliferation, invasion, and apoptosis, and inhibiting inflammation and DNA demethylation. Its chemotherapeutic effects involve micro-RNA regulation, cellular communication network factor 5 (CCN5) activation, and inhibition of estrogen receptor-alpha (ER-α). EGCG sensitizes triple negative breast cancer cells to estrogen by activating ER-α, resulting in decreased cell viability [54, 55]. In HCC, EGCG prevents and inhibits tumor development through mechanisms that include reduction of oxidative stress and modulation of cell proliferation, invasion, and apoptosis. It enhances the efficacy of chemotherapy, radiotherapy, and targeted therapy in HCC [56, 57]. It has been reported that EGCG decreases the expression of androgen receptor (AR) and prostate-specific antigen (PSA) in the prostate cancer cell line, LNCaP. It inhibits vasculogenic mimicry by down-regulating the twist/VE-cadherin/AKT pathway in human prostate cancer cell line, PC-3 [58, 59]. In CRC, EGCG was found to induce ubiquitination and subsequent degradation of the large tumor suppressor kinase 1 (LATS1)/LATS2 kinase via the E3 ligase, carboxyl terminus of Hsp70 interacting protein (CHIP), resulting in the activation and nuclear translocation of Yes-associated protein (YAP), which is implicated in CRC progression and chemotherapy resistance [60].
Luteolin, primarily derived from Salvia tomentosa, occurs in both aglycone and glycoside forms and exhibits a diverse repertoire of physiological benefits [57, 61]. Recognized for its tetrahydroxyflavone structure, luteolin embodies a multifunctional role and functions as an antioxidant and anti-inflammatory agent, as well as a modulator of the immune system and an inhibition of cancer progression [62]. It scavenges free radicals, shields against damage induced by ROS, and impedes tumor progression and metastasis by orchestrating cell cycle arrest and apoptosis [63].
The anti-inflammatory role of luteolin has garnered significant attention in scientific research. Luteolin has been shown to inhibit the release of lipopolysaccharide (LPS)-induced TNF-α through the inactivation of ERK and p38 MAPK signaling pathways and block NF-κB transcriptional activation [64]. Through its inhibitory effects on NF-κB and AP-1 signaling pathways, luteolin down-regulates the expression of TLR3 and impedes NF-κB p65 translocation, thus exerting a comprehensive regulatory influence on inflammatory cascades [65]. Furthermore, luteolin has been shown to mitigate the expression of inflammatory cytokines and attenuate NF-κB p65 activity within the hypothalamus, attesting to its potential effects in neuroinflammatory conditions [66]. Additionally, in conjunction with other phytochemicals, luteolin has been found to be effective in mitigating inflammatory markers such as TNF-α and COX-2 in rodent models of cadmium-induced toxicity [67]. These findings underscore the potential of luteolin as a multifaceted anti-inflammatory remedy for diverse inflammatory conditions.
Luteolin emerges as a formidable contender in the fight against cancer, targeting multiple pathways involved in oncogenesis [68]. It was shown to regulate the expression of pivotal proteins such as AKT, Polo-like kinase 1 (PLK-1), and cyclins, associated with cancer progression, while enhancing the activity of apoptotic regulators, such as BAX and caspase-3, thus tipping the balance in favor of cell death [69]. Moreover, inhibition of signal transducer and activator of transcription 3 (STAT3) signaling by luteolin renders cancer cells more susceptible to conventional radiotherapy and chemotherapy, amplifying their efficacy [68]. Similarly, in prostate cancer, luteolin hinders tumor development, reduces cell growth, and suppresses cancer stemness via the inhibition of enhancer of zeste homolog 2 (EZH2) [70]. Additionally, luteolin demonstrates notable anti-tumor activity in HCC, disrupting cell survival, angiogenesis, and cellular recycling mechanisms [71–73]. In cervical cancer, luteolin emerges as a promising candidate for therapeutic intervention by inhibiting cell invasiveness, suppressing hypoxia inducible factor 1 subunit alpha (HIF-1α) signaling, and targeting key oncogenes, including the ubiquitin conjugating enzymes E2S (UBE2S). The ability of luteolin to inhibit HPV E6 and E7 oncogene expression further underlines its potential as a vital component in the treatment arsenal against cervical cancer [74, 75]. In essence, the multifaceted actions of luteolin against various types of cancer underscore its promise as a therapeutic agent with far-reaching implications in oncology.
Fisetin (FST), is a natural flavonoid compound, isolated from Venetian sumac. It is found in various plants, including their green parts, fruits, bark, and hardwood. FST is present in various fruits and vegetables, such as strawberries, apples, persimmons, grapes, onions, and cucumbers. FST possesses various health benefits such as anti-oxidant, anti-inflammatory, anti-microbial, neuroprotective, hepatoprotective, anti-obesity, anti-depressant, and anti-cancer effects [76]. Studies have revealed that FST exerts anti-cancer activity via inhibiting the cell signaling pathways, including those involved in inflammation, apoptosis, angiogenesis, growth factor regulation, and transcription factors [77].
FST reduces inflammation, a critical factor in cancer development. It inhibits pro-inflammatory mediators and improves inflammatory injury and oxidative stress caused by LPS. Clinical trials with CRC patients showed significant decreases in inflammatory markers C-reactive protein (CRP) and IL-8 with FST supplementation [78]. FST down-regulates the NF-κB pathway, which is involved in cell survival and proliferation. It increases IκBα expression, reduces NF-κB nuclear translocation, and suppresses NF-κB target genes, leading to decreased cell proliferation and enhanced apoptosis in bladder and melanoma cancer cells [79].
FST activates apoptotic markers including poly(ADP-ribose) polymerase (PARP) and caspase 3, suppresses the anti-apoptotic proteins, and enhances pro-apoptotic ones to make cancer cells more sensitive for programmed cell death in head and neck, uveal melanoma, or renal carcinoma. FST also triggers autophagy in pancreatic and oral squamous cell carcinoma cells, marked by the up-regulation of autophagy-related proteins like microtubule-associated proteins 1A/1B-light chain 3B (LC3B) and Beclin-1. This process helps in eliminating cancer cells by promoting cellular stress responses that lead to cell death. FST causes cell cycle arrest at the G0/G1 phase, preventing the proliferation of cancer cells. It increases the expression of cell cycle inhibitors like p21 and p53 while down-regulating cyclins and cyclin-dependent kinases (CDKs) that drive cell cycle progression, effectively halting tumor growth. It down-regulates vascular endothelial growth factor receptor (VEGFR) expression and reduces VEGF levels, impairing the vascular network that supplies nutrients to tumors, thereby stunting angiogenesis. FST also inhibits pathways such as PI3K/AKT/mTOR, STAT3, and wingless-related integration site (Wnt)/β-catenin, which are essential for cell growth, cell proliferation, differentiation, metastasis, and survival [80].
Non-flavonoid molecules represent a diverse group of phytochemicals that include phenolic acids, lignans, tannins, and stilbenes, each with unique structural and functional properties. Among these, phenolic acids stand out as the most prevalent category of phytochemicals, characterized by aromatic rings linked to organic acids such as cinnamic and benzoic compounds [81].
Phenolic acids are aromatic compounds characterized by their organic carboxylic acid and phenolic ring functionalities, which play a pivotal role in plant defense mechanisms against various environmental stresses, including UV radiation, pathogens, and chemical insults [82]. This diverse group of secondary metabolites is broadly categorized into two main classes, namely benzoic acid derivatives and cinnamic acid derivatives, distinguished by the position of the carboxylic acid group relative to the phenol ring [83, 84].
The abundance of hydroxybenzoic acids in fruits such as strawberries and raspberries and hydroxycinnamic acids in cereals, legumes, vegetables, and beverages underlines their importance in human nutrition. These compounds exhibit a wide range of properties, including antioxidant, antibacterial, anti-cancer, cardioprotective, immunomodulatory, anti-inflammatory, and skin-protective effects [84, 85].
Research over the years has demonstrated the potential of phenolic acids in combating oxidative stress-related diseases, especially coronary heart disease, stroke, and certain cancers, owing to their potent antioxidant activities [86]. Moreover, their anti-inflammatory effects extend to diverse tissues like the placenta and adipose tissue, suggesting their broader therapeutic applications [86]. Notably, Moringa oleifera leaves have emerged as a promising natural source of phenolics with neuroprotective and metabolic benefits. Studies have reported their ability to reduce DNA damage, modulate the activity of enzymes like acetylcholinesterase (AchE) and caspase-3, and improve the management of diabetes through the enhancement of insulin sensitivity and lipid metabolism [87]. Furthermore, phenolic-rich extracts from common beans, quince fruit, and pomegranate peel have been found to reduce inflammatory processes [88–90].
Phenolic bioactive compounds demonstrate anti-invasion and anti-metastasis properties by modulating the activities of critical proteins like protein kinase C (PKC), focal adhesion kinase (FAK), and cell division control protein 42 (Cdc42), while orchestrating the intricate phosphorylation of signaling molecules including Ras, Ras related C3 botulinum toxin substrate 1 (Rac1), ERK, JNK, p38, PI3K, p70S6K, p21 activated kinase 1 (PAK1), AKT, Ras homolog family member A (RhoA), and RhoB [91]. Consequently, this molecular ballet leads to the suppression of key transcription factors such as AP-1, NF-κB, and STAT3 [92], thus preventing the progression of cancer cells.
Lignans represent a distinctive class of secondary plant metabolites arising from the oxidative dimerization of two phenylpropanoid units (C6-C3) via the shikimic acid and phenylpropanoid pathway [93]. These compounds, composed of two phenylpropane units, are predominantly sourced from linseed, cereals, grains, fruits, and select vegetables [94]. Plant lignans and their metabolites are recognized for their remarkable properties, which include antioxidant, anti-inflammatory, anti-cancer, neuroprotective, cardioprotective, and chemopreventive.
Novel lignan compounds, such as ecdysanols A-F, dibenzocyclooctadiene, and aryl naphthalene lignans from Kadsura coccinea roots exhibits significant anti-inflammatory effects, suggesting their potential usage in conditions such as rheumatoid arthritis [95]. Similarly, lignans extracted from Ferula sinkiangensis resin, schisandrin A from Schisandra chinensis, and aryl naphthalide lignans from Schisandra medusa exhibit antioxidative and anti-inflammatory effects, making them promising drug candidates for diabetes and for neuroprotection [93]. Extracts from the roots of Krameria lappacea, traditionally used for oropharyngeal inflammation, contain lignan derivatives that possess anti-inflammatory, anti-edematous, and antioxidant properties [96]. Lignan-enriched extracts from S. chinensis and Schisandra rubriflora show enhanced anti-inflammatory activity, with individual lignans exhibiting inhibitory effects against enzymes such as 15-lipoxygenase (15-LOX), COX-1, and COX-2 [97]. Additionally, the lignans, 7-hydroxymatairesinol and 7-hydroxymatairesinol 2, found in cereals, exhibit potent anti-inflammatory effects in endothelial cells, in which they down-regulate NF-κB and ERK [97].
Several investigations have revealed that lignans are likely to function as candidates for chemotherapeutic and chemopreventive applications. Their anti-cancer efficacy is attributed to their capacity to induce apoptosis, impede cell proliferation, and suppress metastasis and angiogenesis [98]. Western European diets rich in flavonols and lignans are associated with a 25% lower risk of bladder cancer, particularly urothelial cell carcinomas [99]. Lignan intake from sources like sunflower seeds and soybeans is linked to a reduced risk of breast cancer [100]. However, it’s crucial to note that the stability of sesame oil relies on lignans and unsaturated fatty acids, necessitating careful processing to preserve its nutritional integrity [101]. Macelignan, derived from nutmeg, has been reported to inhibit M2 macrophage polarization and suppress cancer cell metastasis [102]. Lignans abundant in flaxseed, such as secoisolariciresinol diglucoside and honokiol, exhibit anti-cancer effects against breast cancer by enhancing cytotoxicity in vitro and by promoting apoptosis in resistant tumors [103]. Furthermore, olive oil-derived compounds particularly (+)-1-acetoxypinoresinol and (+)-pinoresinol, have been reported to function as significant modulators of cancer chemopreventive activity, especially in breast, colon, and prostate cancers [104].
Stilbenes (or stilbenoids) are widely distributed in food (grapes, peanuts, and other foods) and medicinal plants [105]. Stilbenes contain a carbon backbone of C6-C2-C6 and two benzene rings joined by a molecule of ethanol or ethylene. They exist in the trans-((E)-stilbenes) and cis-((Z)-stilbenes) 1,2-diphenylethylene structures. Among them, RSV, which is abundant in grapes, exhibits remarkable health benefits and is under extensive investigation [106].
RSV (3,4’,5-trans-trihydroxystilbene) is a phenolic stilbene that is abundant in grapes as well as in blueberries, raspberries, peanuts, and red wine [107]. It is a phytoalexin and is synthesized by plants in response to stress, injury, and ultraviolet irradiation [108]. RSV possesses a unique chemical structure consisting of two phenol rings linked by a double styrene bond and consists of both cis and trans structures [109].
The versatile properties of RSV make it an intriguing candidate for the prevention and therapy of multiple human diseases. Studies have shown that RSV exhibits a broad spectrum of health benefits, encompassing cardio-protection, anti-inflammatory, antioxidant, anti-diabetic, anti-aging, skin depigmentation, cancer prevention, antimicrobial, and neuroprotective properties [110–112].
RSV exhibits beneficial health related effects in a range of neurological diseases and inflammatory conditions, including depression, Alzheimer’s disease, and inflammatory bowel disease [113].
Its mechanism of action involves the down-regulation of pro-inflammatory cytokines through modulation of the NF-κB pathway, along with a brain-specific reduction in phosphorylation levels and activity of cAMP response element binding (CREB) protein and brain derived neurotrophic factor (BDNF) [114]. Preliminary studies have demonstrated that RSV is effective in treating depression, Alzheimer’s disease, and inflammatory bowel diseases. RSV-maltol hybrids have metal chelating properties and antioxidant effects, potentially addressing Alzheimer’s disease by inhibiting aggregation of Aβ1-42 [115]. Orally administered RSV, curcumin, or simvastatin can alleviate small intestinal inflammation, prevent bacterial translocation, and maintain gut barrier functions [116]. RSV was also found to exhibit protective effects against cartilage destruction and synovial inflammation [117].
RSV has been shown to possess a wide range of anti-cancer effects, exhibiting diverse mechanisms of action against distinct types of cancer. It reduces paclitaxel-induced cell death in MDA-MB-435s, MDA-MB-231, and SKBR-3 cell lines, apparently by the inhibition of G2/M cell cycle arrest and suppression of ROS production. As a result, RSV inactivates the anti-apoptotic Bcl-2 family proteins, suggesting potential damage following the RSV co-administration with paclitaxel in selected human cancers [118]. RSV was found to modulate the LPS-induced EMT in PC-3 and LNCaP prostate cancer cells, by effectively inhibiting the expression of EMT markers and by reducing cell motility via inhibition of the hedgehog signaling pathway [119]. RSV also inhibits cell proliferation and regulates the apoptosis-related genes Bcl-2 and BAX in non-small cell lung cancer (NSCLC) cell lines, as well as in a mouse model of implanted primary gastric cancer cells, resulting in which a dose-dependent inhibition of carcinoma growth was observed [120, 121]. A study from our lab has established that RSV enhances the efficacy of docetaxel chemotherapy in breast cancer cells over-expressing HER2, through the HER-2-AKT signaling axis [122]. Moreover, it cooperatively suppresses cancer cell viability and invasion with cisplatin, via selected signaling pathways [123].
3,5-Dihydroxy-4-ethyl-trans-stilbene (DETS), which is structurally similar to RSV, was studied in our lab and was found to down-regulate key molecules involved in melanoma progression, leading to cell cycle arrest at the S-G2 transition phase. To the best of our knowledge, this marks the first report on DETS’s antioxidant and anti-cancer properties, akin to RSV, from our lab [124]. DETS exhibits anti-proliferative and pro-autophagy effects on breast cancer stem cells (CSCs) which suppresses the Wnt/β-catenin pathways [125]. In ovarian cancer, DETS has been shown to exhibit more potent anti-proliferative effects compared to RSV. Both compounds were found to impede the AKT and MAPK signaling pathways, inhibit IL-6 and EGF-dependent cell migration, and downregulate the expression of EMT markers in ascites-derived cancer cells [126].
Curcumin, also known as diferuloyl methane [1,7-bis(4-hydroxy-3-methoxy phenyl)-1,6-heptadiene-3,5-dione; C21H20O6], is a lipophilic polyphenol extracted from the rhizomes of turmeric (Curcuma longa), a member of the ginger family, which is native to Asia, particularly India [127]. The term “curcuminoid” refers to a set of compounds that resemble curcumin, such as demethoxycurcumin and cyclic curcumin [128]. Curcumin is an orange-yellow crystalline powder that turns brownish-red under alkaline conditions and light-yellow under acidic conditions [129]. Though soluble in alcohol and glacial acetic acid, its limited water solubility hinders absorption and leads to low bioavailability [130]. Despite this limitation, its safety profile has led to Food and Drug Administration (FDA) approval for high doses, and it is recognized for its multifaceted health benefits, such as blood-purifying, hepatoprotective, anti-inflammatory, antioxidant, anti-atherosclerotic, antidiabetic, and anti-cancer properties [131].
Being a lab focusing on various therapeutic aspects of natural products, we have evaluated curcumin as a chemotherapeutic, chemopreventive, and chemosensitizer and have validated its efficacy using various tumor models [132–135].
Moreover, studies have explored curcumin’s impact on conditions such as osteoporosis and osteoarthritis, where it promotes bone health and suppresses inflammatory responses. It appears to alleviate the above symptoms by reducing the production of ROS [136]. Furthermore, curcumin effectively down-regulates inflammation by modulating cellular responses through the up-regulation of MAPK phosphatase 5 (MKP5) mRNA and inhibition of p38 phosphorylation [137].
Curcuminoids have gained significant attention in cancer research. It has been reported that curcumin exerts anti-carcinogenic effects by promoting apoptosis, arresting the cell cycle, and suppressing the expression of growth factor receptors [138]. Ours is one of the pioneer studies, which has evaluated the anticancer potential of curcuminoids using in vitro and in vivo models [139]. We have also evaluated many natural and synthetic curcuminoids for their antimutagenic and anticarcinogenic potential, using murine models [140]. Curcumin niosomes (curcusomes) characterized by their small size and enhanced antioxidant potential, hold promise for treating ovarian cancer [141]. Studies of the anti-cancer effects of curcuminoids also emphasize the potential synergy with conventional anti-cancer agents, such as piperine, which significantly increases the drug’s bioavailability [142, 143]. Combination therapies involving curcumin with chemotherapeutic agents have demonstrated safety in murine models, with molecular docking analyses revealing curcumin’s targeted therapeutic approach [144]. An innovative strategy involving the synthesis of curcumin-RSV hybrid compounds has been proven to improve pharmacodynamic and pharmacokinetic properties while reducing the toxicities associated with the parent drug. Beyond oncology, research has unveiled curcumin’s potential across a range of metabolic and musculoskeletal conditions such as polycystic ovary syndrome, metabolic syndrome, cardiovascular diseases, and Achilles tendinopathy [131, 145].
Saponins are glycosidic compounds found in various plants and offer diverse applications in the food, agriculture, and pharmaceutical industries [146]. These compounds hold therapeutic promise with hypolipidemic, hypoglycemic, antioxidant, and antimicrobial activities, though they also present adverse effects such as cytotoxicity to normal cells [147]. The composition and bioavailability of saponins in food are significantly influenced by processing technologies, given their complex structure and low bioavailability [148]. Moreover, saponins interact with the gastrointestinal and gut environments, altering gut microbiota composition and its own biotransformation into sapogenins [149]. Saponins extracted from green tea exhibit therapeutic potential in mammalian cells by targeting cytotoxicity, angiogenesis, inflammation, and antioxidant activity. They exhibit cytotoxic effects on cancer cells, hinder HUVEC proliferation, scavenge free radicals, and suppress pro-inflammatory cytokines [150]. Even though saponins are traditionally considered anti-nutritional factors, recent studies highlight the pharmacological potential of saponins, emphasizing the importance of understanding their structural composition, which is categorized into triterpenoid and steroidal classes based on the carbon skeletons that dictate their biological activities [151].
Major saponins with anti-cancer potential include cycloartane saponins, dammarane saponins, oleananes, spirostanes, and furostanes. Cycloartane saponins display anti-cancer effects, along with antioxidant, neuroprotective, hepatoprotective, and antiviral properties [152]. Dammarane saponins, found in medicinal plants, exhibit anti-inflammatory properties, cytotoxicity against tumor cell lines, selectively targeting malignant cells, and inducing apoptosis via calcium-dependent pathways [153]. Oleananes show diverse antitumor effects through various signal transduction pathways [154]. Spirostanes like polyphyllin D and dioscin display robust anti-cancer and immunostimulating activity, inducing apoptosis through mitochondrial dysfunction and ER stress [144]. Furostanes, a type of steroidal saponin, show limited but significant anti-cancer activity, acting as potential antineoplastic agents [155].
Uttroside B (Utt-B), a saponin abundantly found in the roots, stem, and leaves of Solanum nigrum, has captured attention due to its potent cytotoxic properties against liver cancer cells. In vitro and in vivo studies from our laboratory have demonstrated the anti-HCC potential and pharmacological safety of Utt-B [156]. In a comparative analysis assessing the therapeutic efficacy of Utt-B against sorafenib, an established FDA-approved drug for HCC, Utt-B emerged as a superior anti-cancer agent in in vitro and in vivo experimental settings. Utt-B induced better cleavage of caspase-7 and down-regulation of the cell proliferation markers PCNA and Ki67, compared to sorafenib, resulting in a remarkable reduction in tumor size in mice harboring human HCC [157].
Furthermore, our investigations have revealed that Utt-B possesses the ability to induce pro-survival autophagy in liver cancer cells. This effect is mediated through the inhibition of mTOR signaling and activation of AMPK signaling, both pivotal in regulating autophagy in cancer cells. Interestingly, the inhibition of autophagy by chloroquine, a renowned anti-malarial drug and autophagy inhibitor, not only enhanced the anti-tumor efficacy of Utt-B but also improved the pharmacological safety, in vivo [158]. These findings strongly suggest the potential benefits of repurposing chloroquine in combination with Utt-B for the treatment of HCC, opening a promising avenue for future therapeutic interventions.
Timosaponin A-III (TSAIII) is a bioactive compound derived from the medicinal plant, Anemarrhena asphodeloides Bunge [159]. TSAIII is a white to off-white solid, soluble in methanol, butanol, 80% ethanol, and aqueous pentanol, but insoluble in water. The pharmacological effects of TSAIII are notably influenced by the sugar chain at the C-3 position. Specifically, the disaccharide form of TSAIII demonstrates greater activity compared to the monosaccharide TSAI, while the trisaccharide TSAV exhibits lower activity [160]. TSAIII exhibits efficacy against cancer development through various modes such as anti-inflammatory and antioxidant effects and inhibition of cell proliferation, induction of cell cycle arrest and apoptosis, initiation of autophagy, suppression of tumor cell migration, invasion, and angiogenesis [159].
TSAIII has been shown to inhibit the expression of various inflammatory mediators, including COX-2 and matrix metalloproteinase-9 (MMP-9) [144], and exert anti-inflammatory effects by inhibiting NF-κB, a key player in the inflammatory response [161]. The compound exhibits remarkable efficacy across various cancer types through the intricate modulation of specific signaling pathways. In pancreatic cancer cells, TSAIII inhibits the PI3K/AKT pathway, leading to G1 phase arrest and apoptosis [162]. TSAIII activates ATM/Chk2- and p38 MAPK-regulated pathways to induce apoptosis in breast cancer cells and triggers mitochondria-mediated apoptosis in HepG2 cells [163]. In glioblastoma multiforme, TSAIII inhibits cell growth by down-regulating the Wnt/β-catenin pathways [164]. Moreover, TSAIII has been reported to modulate autophagy by inhibiting the mTOR/Unc-51-like kinase 1 (ULK1) pathway, promoting autophagosome formation, and compromising tumor cell growth [165]. Furthermore, in NSCLC A549 cells, TSAIII inhibits the ERK1/2, proto oncogene tyrosine protein kinase (Src)/FAK, and β-catenin signaling pathways [166]. These findings underscore the diverse and promising therapeutic potential of TSAIII in targeting specific pathways critical for cancer progression and metastasis [167].
Ginsenoside Rg3, a dammarane-type ginsenoside, abundant in Panax ginseng and Panax japonicus, possesses hydroxy substitutions at the 3β, 12β, and 20 pro-S positions. This compound undergoes transformations, such as the conversion of the 3-position hydroxy group into β-D-glucopyranosyl-β-D-glucopyranoside and the introduction of a double bond at the 24-25 position. It represents one among the more than 100 ginsenosides derived from Panax ginseng, historically revered in East Asia for its natural tonic properties [168]. These ginsenosides, including Rg3, manifest diverse pharmacological activities encompassing hepatoprotection, neuroprotection, cardiovascular protection, immune system enhancement, anti-fatigue properties, and antioxidant properties [169].
By inhibiting nucleotide-binding domain, leucine-rich-domain containing family, pyrin domain containing-3 (NLRP3) inflammasome activation and modulating gut microbiota homeostasis, Rg3 demonstrates efficacy in alleviating ulcerative colitis [170]. Moreover, Rg3 exhibits robust antioxidant and anti-inflammatory activities, validated in inflammation and oxidative stress models [171]. Rg3 extends its protective effects to diverse organs and systems. Rg3 has been reported to attenuate lung inflammation, prevent liver and kidney function damage, mitigate neuroinflammation, and offer protection against cerebral and myocardial ischemia/reperfusion (I/R) injury. Additionally, Rg3 shows promise in improving symptoms associated with hypertension and diabetes [171]. Studies also show that Rg3 directly inhibits LPS-mediated inflammation by targeting TLR4 signaling in macrophages [172]. These collective findings underscore ginsenoside Rg3 as a promising candidate for treating conditions characterized by inflammatory and oxidative stress.
Ginsenoside Rg3 showcases potent anti-cancer effects by targeting multiple signaling pathways across various types of cancer. In nasopharyngeal carcinoma (NPC), Rg3 has been found to sensitize cells to radiation therapy, curb radiation-induced EMT, and promote apoptosis [173]. In lung cancer, Rg3 impedes cell migration, invasion, and angiogenesis by suppressing the expression of COX-2 and VEGF [174]. Similarly, in renal cell carcinoma (RCC), Rg3 restricts cell migration, invasion, colony formation, and tube formation while enhancing apoptosis. These effects are achieved by promoting the demethylation of key genes such as p53, p21, and p16, along with histone acetylation [175]. Similarly, in prostate carcinoma, Rg3 was found to inhibit cell growth via modulation of the MAPK pathway [176].
Terpenoids, also known as isoprenoids, encompass a wide range of natural compounds originating from the synthesis of isoprene and terpenes, which are polymers of isoprene. They are categorized based on the carbon arrangement formed by isoprene units, adhering to the isoprene rule [177]. The key building block of terpenoids is 2-methylbuta-1,3-diene (C5H8). Terpenoids are synthesized via the mevalonate (MVA) pathway or the 2-C-methyl-D-erythriol 4-phosphate (MEP) pathway, yielding hemi-, mono-, di-, and tri-terpenoids [178].
These compounds are further classified into subclasses such as monoterpenoids, sesquiterpenoids, diterpenoids, triterpenoids, and tetraterpenoids based on their structural characteristics [179]. Terpenoids are found abundantly in various plants, including Commelina benghalensis Linn., Azadirachta indica A. Juss., Hypoestes sp., Carapa guianensis, Erica maesta, and Hohenbergia antillana, Malagasy species like Cussonia vantsilana, Helichrysum gymnocephalum, Phyllarthron madagascariense, and Gomphocarpus fructicosus [93, 180–182]. Terpenoids have emerged as promising candidates for developing anti-inflammatory compounds, effectively addressing chronic joint inflammation, and impacting inflammatory mechanisms [183]. These compounds exhibit anti-inflammatory properties by inhibiting NF-κB activation, a key pathway in inflammation [184]. Specific terpenoids, such as carnosol and carnosic acid, have demonstrated significant inflammation-reducing effects [185]. Moreover, terpenoids possess the ability to suppress the production of inflammatory molecules like nitric oxide (NO), prostaglandin E2 (PGE2), and TNF, while also impeding the nuclear translocation of the NF-κB complex [186].
Terpenoids exert anticancer effects by inducing apoptosis, inhibiting cell proliferation, and suppressing angiogenesis [187]. They have also shown anti-cancer activities when used in combination with paclitaxel and vinblastine, which are already established effective chemotherapeutic agents [188]. These activities are attributed to their interaction with diverse molecular targets involved in cancer progression, including cell cycle regulators, signaling pathways, and DNA repair mechanisms [189].
Paclitaxel, a tetracyclic diterpenoid originally derived from the bark of the Pacific yew tree, Taxus brevifolia, emerged as a potent anti-cancer agent following a screening program by the National Cancer Institute in 1960 [190]. This compound, often obtained in needle form using aqueous methanol extraction or as a fine white powder, acts as a mitotic inhibitor in chemotherapy, specifically classified as a microtubule-stabilizing agent, a metabolite, a human metabolite, and an antineoplastic agent [191]. Originally known by the generic name “Taxol”, its usage is now restricted due to its registration as a trademark. Isolated in 1971, it was discovered that endophytic fungi within Taxus species were responsible for its synthesis [192]. Administered intravenously for cancer treatment, paclitaxel is available in various formulations, such as abraxane, which features albumin-bound paclitaxel [193]. With a melting point in the range of 415°F to 421°F, paclitaxel gained approval by the US FDA 21 years after its discovery, initially for ovarian cancer and subsequently for breast cancer, solidifying its status as a highly effective anti-cancer agent [194].
Paclitaxel is renowned for its anti-inflammatory properties. In vitro studies reveal its ability to inhibit the activities of key enzymes involved in inflammation, namely COX-1, COX-2, and 5-LOX [195]. Paclitaxel has been reported to suppress the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 and promote the synthesis of anti-inflammatory cytokines like IL-10 [196]. In vivo studies show that paclitaxel treatment reduces the levels of inflammatory mediators such as inducible NO synthase (iNOS) and COX-2 [197]. These findings suggest that paclitaxel has the potential to suppress inflammation and may be beneficial in the treatment of inflammatory conditions.
Paclitaxel exerts significant anti-cancer effects through multiple mechanisms, including the induction of apoptosis, inhibition of cell proliferation, and suppression of angiogenesis. This compound interacts with diverse molecular targets implicated in cancer progression, encompassing regulators of the cell cycle, signaling pathways, and DNA repair mechanisms [198].
Studies conducted by our group have revealed interesting findings regarding the enhancement of the therapeutic efficacy of paclitaxel when combined with curcumin [135]. Pre-treatment with curcumin synergistically decreased tumor incidence and tumor volume in animals, exceeding the effects observed with either paclitaxel or curcumin alone. Interestingly, curcumin alone did not exhibit significant effects at the same concentration [135]. Another study from our lab reported that AKT functions upstream of NF-κB in the signaling cascade triggered by paclitaxel and that complete inhibition of NF-κB abolishes the synergistic effect between paclitaxel and curcumin [199].
Paclitaxel modulates critical pathways involved in breast cancer, including PI3K/AKT signaling, the Hippo pathway, microtubule stabilization, and DNA methylation, thereby influencing cell proliferation, apoptosis, and drug resistance. Paclitaxel has been reported to inhibit the PI3K/AKT/mTOR pathway in liver cancer, leading to reduced cell proliferation and migration alongside enhanced apoptosis [200]. Similar studies in colon cancer cells revealed that paclitaxel induces apoptosis by suppressing signaling pathways such as Janus kinases (JAKs)/STAT3 and PI3K/AKT/mTOR [201].
Ursolic acid (UA), a pentacyclic triterpenoid derived from various medicinal plants, exhibits notable pharmacological properties encompassing anti-inflammatory, antioxidant, antiapoptotic, and anticarcinogenic effects [202]. This compound is widely distributed across a range of botanical sources, including orchard apple, pomegranate, marjoram leaves, oregano leaves, rosemary, sage, thyme, lavender, eucalyptus leaves, black elder, hawthorn, coffee leaves, and the wax layer of various edible fruits [203].
UA has been shown to exhibit promising anti-inflammatory properties, particularly in treating skin conditions like eczema, psoriasis, and dermatitis [204]. Studies indicate that UA and its derivatives possess robust anti-inflammatory effects, capable of suppressing the production of inflammatory mediators [205]. Moreover, UA interferes with multiple inflammation-related signaling pathways, positioning it as a potential therapeutic option for inflammatory diseases [206].
Furthermore, UA has been reported to inhibit NF-κB-mediated inflammatory pathways and enhance ROS-scavenging, elucidating the key mechanisms underlying its anti-inflammatory effects [207]. These findings highlight UA as a promising candidate for combating inflammation and suggest its potential in therapeutic interventions targeting inflammatory disorders.
UA has garnered considerable attention in research owing to its capacity to suppress the production of proinflammatory cytokines and its remarkable efficacy in combating various types of cancer. Studies show that UA not only inhibits cell viability and migration but also influences autophagy, apoptosis, and cell cycle progression. It is able to induce apoptotic effects, premature senescence, and even reverse paclitaxel resistance [208].
UA has been found to initiate pro-apoptotic signaling and disrupt mitochondrial function, leading to the activation of caspases 3, 8, and 9 in liver and gastric cancer. Moreover, in gastric cancer, UA targets the Hippo pathway, exhibiting inhibitory effects on tumor growth and promoting apoptosis [209]. In CRC, UA was found to inhibit cellular proliferation and induce apoptosis through modulation of signaling pathways crucial for cell cycle regulation and apoptosis [210]. UA also exhibits apoptotic activity against NSCLC by down-regulating the JAK2/STAT3 pathway and suppressing the expression of VEGF and PD-L1, thereby inhibiting tumor angiogenesis, migration, invasion, and metastasis [57]. Similarly, studies in skin cancer indicate that UA activates peroxisome proliferator-activated receptor α (PPARα) and AMPK and induces apoptosis in mouse squamous cell carcinoma and skin papilloma [211].
Cucurbitacin B (CuB) is a tetracyclic triterpenoid commonly found in glycosidic forms [212]. It possesses a distinctive structural framework identified as 19-(10→9β)-abeo-10α-lanost-5-ene, characterized by high unsaturation and multiple ketone groups [213]. The cytotoxicity of CuB is believed to be linked to the presence of a 25-acetoxy group, although studies indicate that saturating the conjugated 23(24) double bond may mitigate this effect [214]. Cus B, I, and E, among others, demonstrate promise in inhibiting selected types of cancer including breast, hepatocellular, laryngeal, lung, melanoma, pancreatic, and prostate cancers [215].
Remarkable anti-inflammatory properties of CuB are reflected in its ability to modulate various pivotal regulators of signaling pathways, including JAK/STAT3, Nrf2/antioxidant response element (ARE), NF-κB, AMPK, MAPK, PI3K/AKT, cancerous inhibitor of protein phosphatase 2A (CIP2A)/protein phosphatase 2A (PP2A), Wnt, FAK, Notch, and Hippo-YAP [216]. Additionally, CuB holds promise for attenuating cerebral I/R injury by inhibiting inflammation mediated by the NLRP3 inflammasome and reducing oxidative stress [217]. Moreover, Cus B, E, and I demonstrate anti-platelet and anti-thrombotic effects by disrupting cytoskeletal dynamics and integrin function [218]. CuB has been reported to down-regulate TNF receptor 1 (TNF-R1), leading to inhibition of expression of the intracellular adhesion molecule-1 (ICAM-1) and suppression of the NF-κB signaling pathway, thereby attenuating inflammatory processes [219]. These multifaceted actions highlight CuB as a promising candidate for therapeutic interventions by targeting inflammation-related disorders and ischemic injuries.
CuB exhibits potential as an anti-cancer agent across various types of cancer. In pancreatic cancer, CuB induces apoptosis both in vitro and in vivo, highlighting its role in cancer immunotherapy through inhibition of the JAK/STAT3 pathway [220]. Furthermore, the diverse anticancer effects of CuB include the reduction of tumor invasiveness, suppression of metastasis-promoting genes, and inhibition of proliferation, invasion, and migration of tumor cells. Many of these effects reflect the ability of CuB to target the EGFR/STAT3/AKT signaling pathway and inhibit the EMT, ultimately inducing apoptosis and obstruction of the critical STAT3-regulated signaling pathways [221].
CuB exhibits promising antitumor effects across various cancer types through distinct mechanisms. In liver CSCs, CuB has been reported to inhibit the JAK2/STAT3 signaling pathway, targeting a crucial pathway involved in cancer stemness [222]. Moreover, CuB demonstrates significant therapeutic potential in NSCLC by inhibiting DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), highlighting its role in epigenetic regulation and cancer cell modulation [223].
Interestingly, in vivo studies conducted in our laboratory demonstrated the effectiveness of CuB in attenuating tumor growth in a NOD-SCID murine model of human melanoma through the inhibition of MEK1/2 and ERK1/2 signaling pathways. Our study also confirmed the pharmacological safety of the compound in Swiss albino mice [224].
The term “alkaloid” originates from “alkali”, suggesting an “alkali-like” nature inherent to these compounds. Alkaloids comprise a class of nitrogen-containing compounds found naturally, often derived from amino acids [225]. Organisms synthesize alkaloids as secondary metabolites primarily for defense, leading to their classification into five types based on the structural positioning of nitrogen: true alkaloids, proto-alkaloids, polyamine alkaloids, peptide and cyclopeptide alkaloids, and pseudoalkaloids [226]. However, the classification of alkaloids lacks a standardized system due to overlapping definitions. Numerous alkaloids extracted from various plants and organisms have been utilized for their pharmacological properties in treating various diseases [227]. Alkaloids demonstrate diverse activities, including anti-cancer, antiviral, analgesic, and anti-inflammatory effects across a spectrum of health conditions [228]. Particularly, cancer, a prevalent ailment in the modern era, has been a focus of research while exploring the utility of alkaloids in treatment [229]. Published studies that delve into the exploration of alkaloids for cancer therapy have led to the discovery of several valuable alkaloids as effective drugs that target selected types of cancer [230, 231].
Alkaloids are classified into different groups based on their structural characteristics. Proto-alkaloids are a subset of plant metabolites characterized by nitrogen atoms derived from amino acid residues, typically in non-cyclic skeletons, and are often derived from tryptophan, tyrosine, or phenylalanine [232]. A coumarin-alkaloid conjugate is a hybrid molecule that combines the structural features of coumarins and alkaloids, leveraging the unique properties of both classes of compounds [233]. Coumarin, a benzopyrone phenolic compound, is essential for life processes in plants, such as photosynthesis, hormone regulation, and respiration. Indole alkaloids, characterized by their pentacyclic and pyrrole rings with a basic nitrogen atom, are the largest category of alkaloids and are widely distributed among plant families [234]. Being clinically significant for their anticancer properties, indole alkaloids like vinblastine and vincristine are used in chemotherapy. Quinoline, a benzopyridine organic molecule, forms the basis of various phytochemicals such as isoquinoline, benzylisoquinoline, and aporphine alkaloids, which have been demonstrated to have antineoplastic effects [235]. Steroidal alkaloids, the derivatives of plant steroids with one or more nitrogen atoms in their heterocyclic rings, possess the biological properties of both alkaloids and steroids, providing protection against environmental threats to plants [236].
Tryptanthrin (indolo[2,1-b]quinazolin-6,12-dione) is an alkaloid possessing an indolo[2,1-b]quinazoline core with various pharmacological properties, including anti-tumor, anti-inflammatory, and anti-bacterial activities. The name tryptanthrin originated from the fact that it was initially isolated from Candida lipolytica, a type of bacteria that grows primarily on L-tryptophan [237]. In addition to Candida lipolytica, other sources of tryptanthrin include fungi like Schizophyllum commune and Leucopaxillus cerealis, as well as plants like Couroupita guianensis, Wrightia tinctoria, Strobilanthes cusia, Polygonum tinctorium, and Isatis tinctoria [238–243].
Tryptanthrin exhibits remarkable anti-inflammatory properties across a spectrum of conditions. In LPS-treated BV-2 microglia cells, tryptanthrin has been found to reduce pro-inflammatory cytokine levels through modulating NF-κB and Nrf2/heme oxygenase-1 (HO-1) pathways, effectively mitigating inflammation in a mouse model of edema [244, 245]. Studies indicate that tryptanthrin alleviates rheumatoid arthritis by reducing proinflammatory cytokine expression and collagen-induced arthritis by down-regulating MMP-3 and IL-6 [246]. The wound healing efficacy of tryptanthrin has been demonstrated in a mouse excisional wound model, where tryptanthrin accelerated the wound healing response by lowering VEGF and MMP-9 levels [247].
Tryptanthrin ameliorates psoriasis by modulating the levels and activity of inflammatory mediators that target T helper 17 (Th17). These include IL-6, IL-8, S100A9 peptide, C-C motif chemokine ligand 20 (CCL20) chemokine, TNF-α, and IL-1β [248]. Furthermore, tryptanthrin displays promising results against neuroinflammation by reducing the levels of pro-inflammatory cytokines, iNOS, and COX-2 levels in LPS-induced neuroinflammation through inhibition of the TLR4-myeloid differentiation primary response gene 88 (MyD88)- and NF-κB-dependent pathways [249]. It is suggested that tryptanthrin guards the liver tissue by down-regulating apoptotic processes, lowering inflammatory cell infiltration, and stimulating the AMPK and p38 pathways [250].
Tryptanthrin has been found to boost anti-inflammatory factors and reduce pro-inflammatory markers in HMC-1 cells triggered by atopic dermatitis [251]. It also diminishes leukotriene production, modulates the subcellular localization of 5-LOX, and decreases the levels of its products, thereby alleviating inflammation in pleurisy [252]. Moreover, tryptanthrin significantly mitigates inflammation in dextran sodium sulfate-induced colitis, ensuring 100% survival in treated animals [242].
Tryptanthrin demonstrates potent anti-tumor actions by altering the levels of key proteins involved in cell growth and survival while inducing cell cycle arrest, apoptosis, and autophagy in cancer cells. Additionally, it has been shown to modulate the inflammatory tumor microenvironment, further contributing to its anti-tumor effects [253].
In vitro studies using MCF-7 cells have revealed that tryptanthrin inhibits cell proliferation, migration, and invasion while up-regulating E-cadherin and down-regulating MMP-2 and SNAIL [254]. Another derivative of tryptanthrin, termed benzo[b]tryptanthrin, exhibits potent inhibitory activity against multidrug resistance protein 1 (MDR1) in adriamycin-resistant breast cancer cells [255]. By activating p53 and controlling the TRAIL/death receptors (DRs) pathway, tryptanthrin induces apoptosis in lung cancer cells [256]. Tryptanthrin has been shown to have potent anti-tumor effects against HCC cells, causing S-phase arrest by down-regulating cyclin A1, B1, CDK2, and p-Cdc2, and inducing caspase-dependent apoptosis. It also regulates signaling targets and protects liver cells from oxidative injury [253]. Studies using the human chronic myeloid leukemia cell line K562 suggest that tryptanthrin inhibits the proliferation of leukemia cells and induces apoptosis in these cells through the up-regulation of cytosolic cytochrome-c, BAX, and activated caspase-3 while down-regulating Bcl-2, mitochondrial cytochrome-c, and pro-caspase-3 [257].
Studies from our laboratory have revealed that tryptanthrin from Wrightia tinctoria exhibits potent anti-tumor activity against malignant melanoma (A375) cells by targeting key molecules including BRAF, Wnt/β-catenin, AKT, and NF-κB pathways that converge on MITF-M. Furthermore, through preclinical investigations, we have demonstrated that tryptanthrin significantly reduces malignant melanoma growth in mice insert [258]. Additionally, our research has extended to explore the anti-tumor effects of tryptanthrin on non-melanoma skin cancer (NMSC) in mouse models. Here, we observed that tryptanthrin inhibits NMSC by inactivating ERK1/2 and p38 regulated pathways, resulting in the suppression of c-Myc and cyclin-D1 expression while promoting β-catenin activity [259].
Cepharanthine, derived from Stephania tubers, exhibits a variety of pharmacological effects, including antioxidant, anti-inflammatory, immunomodulatory, antitumoral, and antiviral activities [260]. Its interaction with the voltage-dependent anion channel (VDAC) results in voltage-independent channel narrowing, leading to dose-dependent cytotoxicity by altering transport between mitochondria and cytoplasm [261]. Cepharanthine boosts apoptosis in glioma and neuronal cells via ROS and alters mitochondria-to-cytoplasm transport, showcasing diverse medicinal properties, including pathway inhibition, immunomodulation, and antiviral activity against SARS-CoV-2, with over 70 years of clinical use [262]. Additionally, cepharanthine shows promise in treating HCC and alleviating ulcerative colitis by modulating the levels of pro-inflammatory cytokines as well as the gut microbiota [263, 264]. Additionally, cepharanthine has been reported to hinder the release of pro-inflammatory cytokines and the expression of aconitate decarboxylase 1 (ACOD1) in macrophages, reducing macrophage infiltration and ACOD1 levels in colon tissue [265]. Furthermore, cepharanthine diminishes the viability and promotes apoptosis of myofibroblasts while suppressing the expression of myofibroblast markers. These findings suggest that cepharanthine holds promise as an anti-inflammatory agent for a range of diseases [34].
Cepharanthine also exhibits potent anti-cancer effects against a range of cancers, including HCC, CRC, leukemia, ovarian cancer, and gastric cancer [266]. Its mechanism of action involves the induction of apoptosis, reduction of cell viability, and modulation of critical signaling pathways implicated in cancer progression. Some of its targets within cells include the Bcl-2 family proteins, PARP cleavage, ERK, STAT3, and AKT. The efficacy of cepharanthine extends to both p53 wild-type and mutant cancer cells, suggesting potential utility in treating CRC harboring mutant p53, often resistant to conventional chemotherapy [260]. In liver cancer, cepharanthine was found to inhibit cellular proliferation and invasion, promote apoptosis, and suppress the hedgehog/glioma associated oncogene homolog 1 (Gli1) and Wnt/β-catenin signaling pathways [267]. Additionally, it is cytotoxic to NSCLC cells through the induction of apoptosis, generation of ROS, and modulation of STAT3, PI3K/AKT, and MAPK/ERK signaling pathways [266].
Oxysophocarpine (OSP) serves as a prominent quinolizidine natural alkaloid present in leguminous plants such as Sophora viciifolia Hance [268], Sophora alopecuroides [269], Sophora flavescens Ait [270], and other species within the Robinia genus [271]. It is a constituent of traditional Chinese medicine, notably found in compound Kushen injection (CKI), utilized for various ailments, including several types of cancer [272]. OSP demonstrates diverse pharmacological properties, including analgesic effects, anti-inflammatory activity, antitumor properties, neuroprotective effects, and antiviral activity [268, 273–275].
OSP demonstrates remarkable anti-inflammatory effects across various disease models. It effectively attenuates inflammation by inhibiting neutrophil recruitment to lesions and reducing levels of key pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, IL-6, macrophage inflammatory protein 2 (MIP-2), granulocyte colony stimulating factor (G-CSF), and KC, in tuberculosis-associated inflammation [272]. Moreover, it mitigates inflammation in ulcerative colitis by targeting oxidative stress-related factors such as PGE2, myeloperoxidase (MPO), and superoxide dismutase (SOD), along with inflammation-associated factors like IL-6, TNF-α, and IL-1β. These effects are mediated through TNF-R-associated factor 6 (TRAF6) down-regulation and NF-κB phosphorylation [268]. In neuronal inflammation, OSP was found to modulate the expression of inflammatory markers including TNF-α, IL-1β, pERK1/2, p-JNK1/2, and p-p38 MAPK [273]. It also reduces carrageenan-induced inflammation in mice by targeting the ERK1/2-COX-2-PGE2 signaling pathway, leading to neutrophil removal and reduced pain perception, further highlighting its broad anti-inflammatory actions. Sophocarpine has been found to alleviate inflammation induced by various agents (e.g., hot plate and xylene) in mice by down-regulating proinflammatory cytokines such as IL-1β, IL-6, and PGE2, and by lowering serum NO levels [276]. Moreover, OSP significantly reduces inflammation in BV-2 microglia under conditions of oxygen-glucose deprivation/reperfusion (OGD/R) by decreasing the expression levels of COX-2, NO synthase, and various inflammatory mediators, while also preventing microglial cell apoptosis [277].
Studies in HCC cell lines, including HepG2 and Hepa1-6, suggest that OSP inhibits cell proliferation and migration while promoting apoptosis. Additionally, it enhances the responsiveness of HCC cells to CD8+ T-cell immunotherapy by modulating IL-6-mediated JAK2/STAT3 signaling [278]. It also acts as an anti-tumor agent against oral squamous cell carcinoma by suppressing cell proliferation and metastasis, mediated by the down-regulation of Nrf2 and the HO gene [279].
Berberine is an isoquinoline quaternary alkaloid (or a 5,6-dihydrodibenzo[a,g]quinolizinium derivative) extracted from various medicinal plants such as Hydrastis canadensis, Berberis aristata, Coptis chinensis, Coptis rhizome, Coptis japonica, Phellodendron amurense, and Phellodendron chinense Schneid [280]. Traditionally, it has been utilized as an antidiarrheal, antibacterial, antifungal, and antiprotozoal agent [281]. However, recent studies have highlighted its potential benefits in treating neurodegenerative diseases, primarily due to its strong antioxidant properties. Berberine, an isoquinoline alkaloid, is found in multiple plants, including species from the Coptis and Berberis genera [282].
Berberine induces apoptosis in cancer cells by activating various pathways, such as caspase-mediated pathways, mitochondrial pathways, and PI3K/AKT signaling inhibition. It can inhibit the proliferation of cancer cells by affecting cell cycle progression, particularly by arresting cells in the G1 or G2/M phases. Berberine has been reported to inhibit angiogenesis, the process by which new blood vessels are formed to supply nutrients to tumors, thereby suppressing tumor growth [283].
Berberine also exhibits potent anti-inflammatory effects, which are crucial in cancer therapy and other inflammatory conditions. Berberine can suppress the production and release of pro-inflammatory cytokines and mediators, such as TNF-α, IL-6, and COX-2. It inhibits the activation of NF-κB, a key transcription factor involved in the regulation of inflammatory responses, and can modulate immune responses, activating anti-inflammatory pathways and regulating inflammatory cascades [283].
Evodiamine is a quinolone alkaloid with a tetracyclic structure containing both indole and quinazoline ring systems. Its molecular structure includes hydroxyl, methoxy, and carbonyl groups, contributing to its pharmacological activities [284]. It is the primary component extracted from the fruit of Evodia rutaecarpa. It has been demonstrated to have multiple biological effects, such as stimulating testosterone and catecholamine secretion while exhibiting antinociceptive, anti-inflammatory, anti-obesity, vasodilatory, thermoregulatory, and uterotonic properties [285]. Nanocarriers can potentially increase the therapeutic efficacy of evodiamine in cancer therapy while minimizing adverse effects. Numerous studies have focused on developing smart nanocarriers to overcome evodiamine’s limitations [286].
Evodiamine has been demonstrated to have significant anti-tumor properties by inhibiting the proliferation of various cancer cell lines, including cervical, colon, lung, melanoma, leukemic T-lymphocyte, prostate, and breast cancer cells. One key mechanism involves arresting cell cycle progression at the G2/M phase through the activation of Cdc2/cyclin B. Additionally, evodiamine induces apoptosis in tumor cells through multiple pathways. The PI3K/AKT and ERK signaling pathways play crucial roles in the apoptotic response of tumor cells to evodiamine [287].
Evodiamine induces apoptosis by modulating NF-κB activation, which inhibits NF-κB-regulated gene products such as cyclin D1, X linked inhibitor of apoptosis protein (XIAP), Bcl-2, and Bcl-Xl. Other studies have shown that evodiamine increases the expression of the apoptosis inducer, BAX, and decreases the expression of the apoptosis suppressor, Bcl-2, thereby inducing apoptosis via the caspase pathway. ROS and NO generation also regulate p53, p21, protein tyrosine kinases (PTK), and other signaling proteins involved in evodiamine-induced apoptosis [288].
Organosulfur compounds are renowned for their beneficial effects, which include anti-inflammatory, antioxidant, and anti-cancer properties [289]. These compounds are abundant in foods such as garlic, onion, asparagus, and cruciferous vegetables like broccoli, cauliflower, and cabbage [290].
Animal studies and epidemiological research suggest that organosulfur compounds can reduce the risk of CRC by inducing mitotic arrest and apoptosis [291]. Specific sulfur-containing compounds like allicin, ajoene, and isothiocyanates exhibit antibacterial and antifungal activities [292]. One well-studied organosulfur compound is sulforaphane, classified as an isothiocyanate, which shows promising chemopreventive properties. Sulforaphane induces phase I and II detoxification enzymes and plays a role in preventing carcinogenesis. It also exerts anti-tumor effects during the post-initiation phase [293].
Pectin, a natural polysaccharide derived from plants like citrus fruits and apples, consists of a linear chain of α-(1-4) linked D-galacturonic acid residues [294]. Low-molecular weight citrus pectin has emerged as a promising anti-cancer agent by exerting multiple beneficial effects [295]. It has been reported that pectin suppresses cancer cell adhesion, aggregation, and metastasis while also inhibiting the activity of the premetastatic protein galectin-3 (GAL-3). Additionally, citrus pectin promotes caspase-mediated apoptosis and inhibits tumor cell growth, highlighting its potential in cancer therapy [295, 296].
Plant-based non-digestible carbohydrates like pectin lower the risk for CRC by inhibiting GAL-3 protein expression, which augments apoptosis and inhibits cancer cell migration [295]. Similarly, ferulic acid, a bioactive component found in rice bran, exhibits anti-inflammatory, anti-diabetic, anti-cancer, and antioxidant activities [297]. Rice bran itself demonstrates notable antitumor activities by modulating various biochemical pathways, including the inhibition of oxidative stress and the induction of apoptosis [298].
Thiazole-based peptides from marine sources are notable for their distinctive structures and varied pharmacological effects, including inducing apoptosis in cancer cells, inhibiting their proliferation, and modulating immune responses by reducing inflammation by inhibiting pro-inflammatory cytokines and enzymes [299]. Chenopodium album, a traditional Indian medicinal plant, is widely used for treating diabetes, digestive issues, and skin diseases. The plant extract exhibited significant α-glucosidase inhibitory activity and strong antioxidant properties, indicating its potential to lower blood glucose levels and reduce diabetic oxidative stress [300]. Allicin, the sulphur containing compound produced by garlic when it’s crushed, exhibits a range of biological activities, such as inhibiting cancer cell growth, inducing apoptosis, protecting cells from oxidative damage, reducing inflammation, and modulating the immune system [301].
Phytochemicals, despite their significant anticancer potential and therapeutic benefits, face several limitations that hinder their widespread clinical application. Some of the limitations include poor bioavailability, solubility, hydrophobicity, and restricted therapeutic potential, leading to reduced efficacy [302]. Apart from these, obstacles such as toxicity, high production cost, inadequate availability of raw materials, and ineffective regulatory system impede further barriers to the commercial utilization of plant-based bioactive compounds [303].
Even modern drug delivery systems, including nano drug delivery systems, encounter several limitations despite their promising advantages. A few of the challenges include limited solubility, stability of the natural products when administrated orally, short durations of effectiveness, non-specific targeting that hinders the potential of the system, and poor bioavailability. Apart from these, issues like poor patient compliance, dermal discomfort, and pain associated with parenteral routes further restrict the efficacy of protein based drug delivery systems [304].
Even though nanotechnology offers solutions to improve the pharmacological and therapeutic qualities of conventional medicines, strict regulatory standards are necessary to address drawbacks and ensure the safe and effective delivery of drugs at the cellular levels. Despite these limitations, ongoing advancements in phytochemical and nano drug delivery systems continue to demonstrate promising strategies to overcome challenges and transform the field of medicine [305].
The present review underscores the substantial role of diverse phytochemicals in exerting anti-cancer and anti-inflammatory effects. Our comprehensive analysis has illuminated the multifaceted mechanisms through which these natural compounds exhibit therapeutic effects in cancer and inflammatory diseases. By targeting pivotal signaling pathways and key effector proteins such as MAPK, PI3K/AKT/mTOR, JAK/STAT, Wnt, and NF-κB (Figure 3), phytochemicals actively modulate gene expression and orchestrate cellular processes, thus offering promising avenues for the development of novel therapeutic agents. The increasing recognition of their varied biological activities has sparked significant interest in their potential application in cancer therapy and anti-inflammatory treatment. As our understanding of the intricate molecular mechanisms underlying tumor growth and inflammation continues to evolve, there is a burgeoning optimism regarding the integration of phytochemicals into comprehensive prevention and treatment strategies. Future research endeavors are anticipated to prioritize the development of targeted therapies utilizing specific phytochemicals or their derivatives. Furthermore, the prospect of nutritional interventions tailored to individual genetic and metabolic profiles may soon be within reach. Phytochemicals, whether employed individually or in combination with conventional chemotherapeutic drugs, exhibit promise in enhancing therapeutic efficacy and mitigating challenges such as chemoresistance and inflammation. Moreover, the exploration of innovative drug delivery systems aimed at augmenting phytochemical bioavailability and tissue specificity holds significant promise. Ultimately, the future trajectory of phytochemicals in cancer therapy and inflammatory disorders represents an integrative and complementary approach to conventional therapeutic modalities, poised to make substantial contributions to disease prevention and management.
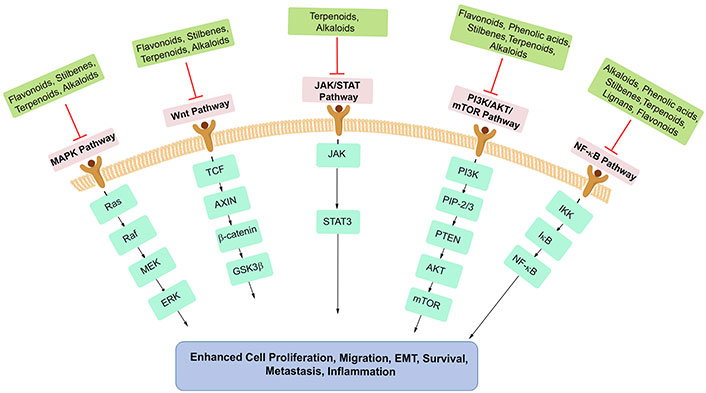
The pivotal pathways that are targeted by the major phytochemicals (ChemDraw has been used to draw some parts of this figure). MAPK: mitogen-activated protein kinase; JAK: Janus kinase; STAT: signal transducer and activator of transcription; AKT: protein kinase B; NF-κB: nuclear factor kappa B; ERK: extracellular signal regulated kinase; IκB: inhibitor of NF-κB; EMT: epithelial-mesenchymal transition; Wnt: wingless-related integration site; Raf: rapidly accelerated fibrosarcoma; TCF: T cell factor; AXIN: axis inhibition protein; GSK3β: glycogen synthase kinase 3 beta; PIP-2: phosphatidylinositol 4,5-bisphosphate; PTEN: phosphate and tensin homolog; IKK: inhibitor kappa B kinase; PIP-3: phosphatidylinositol 3,4,5-triphosphate
The exploration of phytochemicals has revealed their promising potential in cancer therapy and inflammation management. Cutting-edge techniques such as genomics, proteomics, and metabolomics can provide detailed insights into the mechanisms of action of these compounds, enabling the development of more targeted and effective therapies. The integration of nanotechnology with phytochemical research can enhance the bioavailability, stability, and targeting efficiency of phytochemicals, overcoming traditional limitations such as poor solubility and rapid metabolism. Investigating the synergistic effects of phytochemicals with conventional anti-cancer and anti-inflammatory drugs could lead to the development of combination therapies that are more effective and reduce side effects. The synthesis and evaluation of novel derivatives and analogs of flavonoids, saponins, terpenoids, and alkaloids can lead to compounds with enhanced activity. The application of phytochemicals in personalized medicine holds significant promise in treatment strategies. Well-designed clinical trials are needed to validate the therapeutic potential of phytochemicals and to establish standardized treatment protocols. Collaboration between researchers, clinicians, and pharmaceutical companies will be key to advancing this field. In conclusion, the future of phytochemicals in cancer and inflammation therapy is promising, with numerous avenues for research and development. By tackling the challenges, we can unlock the full potential of these natural compounds, leading to improved patient outcomes and significant advancements in the field of medicine.
15-LOX: 15-lipoxygenase
AKT: protein kinase B
BAX: B-cell lymphoma 2-associated protein x
Bcl-2: B-cell lymphoma 2
Cdc42: cell division control protein 42
COX: cyclooxygenase
CRC: colorectal cancer
CuB: cucurbitacin B
DETS: 3,5-dihydroxy-4-ethyl-trans-stilbene
EGCG: epigallocatechin 3-gallate
EMT: epithelial-mesenchymal transition
ERK: extracellular signal regulated kinase
ER-α: estrogen receptor-alpha
FAK: focal adhesion kinase
FDA: Food and Drug Administration
FST: fisetin
HCC: hepatocellular carcinoma
IL-1β: interleukin 1β
JAKs: Janus kinases
JNK: C-Jun N-terminal kinase
LPS: lipopolysaccharide
MAPKs: mitogen-activated protein kinases
MMP-9: matrix metalloproteinase-9
NF-κB: nuclear factor kappa B
NO: nitric oxide
NSCLC: non-small cell lung cancer
OSP: oxysophocarpine
PGE2: prostaglandin E2
ROS: reactive oxygen species
RSV: resveratrol
STAT3: signal transducer and activator of transcription 3
TLR4: Toll-like receptor 4
TNF-α: tumor necrosis factor-alpha
TSAIII: timosaponin A-III
UA: ursolic acid
Utt-B: uttroside B
VEGF: vascular endothelial growth factor
Wnt: wingless-related integration site
We acknowledge Council of Scientific & Industrial Research (CSIR) - India for the funding. We thank Dr Kavitha A. K. for the help rendered in generating high resolution images of chemical structures of the compounds in the manuscript.
SCS, MPJ, and TPR: Investigation, Visualization, Writing—original draft. SUA and CKK: Visualization. AA: Writing—original draft. NI: Writing—review & editing. RJA: Writing—review & editing, Supervision, Funding acquisition. The final version of the manuscript was read and approved by all authors.
Noah Isakov who is the Editorial Board Member and Guest Editor of Exploration of Drug Science, and Ruby John Anto who is the Guest Editor of Exploration of Drug Science, had no involvement in the decision-making or the review process of this manuscript. The other authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by Council of Scientific & Industrial Research (CSIR) – India [Grant No. CSIR – (60(0124)/20/EMR-II)]. The data collection for this manuscript was facilitated through the support of the CSIR India. The funders had no role in study design, data analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Sasikumar Jalajakumari Soumya ... Perumana R. Sudhakaran
Tanya Tripathi ... Alok Chandra Bharti
Paradentavida Prathyusha ... Edakkadath R. Sindhu
Julia K. Opara ... Shrikant Anant
