Affiliation:
Instituto de Investigaciones Bioquímicas de La Plata (INIBIOLP), CCT-La Plata, CONICET, Facultad de Ciencias Médicas, Universidad Nacional de La Plata, La Plata 1900, Argentina
Email: vherlax@med.unlp.edu.ar
ORCID: https://orcid.org/0000-0003-4218-8702
Explor Drug Sci. 2024;2:836–850 DOI: https://doi.org/10.37349/eds.2024.00076
Received: July 01, 2024 Accepted: October 30, 2024 Published: November 20, 2024
Academic Editor: Andreimar Martins Soares, Oswaldo Cruz Foundation, Brazil
The article belongs to the special issue Bioactive Peptides: Pioneering Innovations in Latin American Research
In recent years, research efforts have increased to develop new therapeutics aimed at combating antibiotic-resistant bacterial infections. These efforts focus on inhibiting the virulence factors bacteria secrete to proliferate. This review aims to highlight the advances in these antivirulence therapies, with a particular emphasis on those utilizing peptides to inhibit toxin activity. Specifically, we will review the mechanism of action of a group of toxins known as Repeat in ToXins (RTX) and the progress made regarding the use of peptides to inhibit their action. Notably, we will discuss the use of peptides mimicking either cholesterol recognition/interaction amino acid consensus (CRAC) or CARC motifs, which are similar to CRAC motifs but have the opposite orientation, to reduce their interaction with cholesterol in target cells. We will present results corresponding to the inhibition of three characteristic toxins of this group: HlyA from Escherichia coli, LtxA from Aggregatibacter actinomycetemcomitans, and CyaA from Bordetella pertussis. While these advances are very recent, they are promising for the development of new therapies. The advantage of this type of therapy is that it reduces the selective pressure for the growth of resistant bacteria.
The global advance of infectious diseases is alarming. By 2050, it is projected that more people will die annually from infectious diseases than those dying from diseases with high mortality rate, such as cancer and diabetes [1]. This alarming growth is multifactorial. On one hand, the excessive and uncontrolled use of antibiotics has led to the selective proliferation of antibiotic-resistant strains and the potential exchange of these resistance-encoding genes among bacteria. On the other hand, the development and production of new antibiotics have decreased in recent years. Additionally, the rising average age of the population results in immunosuppressive conditions in patients due to neurodegenerative and cardiological disease, chemotherapy, dialysis, or organ transplantation and/or entering healthcare environments, where resistant organisms are prevalent [2]. The increase in immunosuppression has also risen in underdeveloped countries, where poor housing and healthcare conditions further facilitate bacterial infections [1].
The development of antibiotics involves finding a substance that can inhibit bacterial growth without affecting the host’s eukaryotic cells. To achieve this, molecules have been synthesized that inhibit bacterial cell wall synthesis, protein synthesis, or bacterial proliferation [3]. Because bacteria replicate quickly, this creates a “selection pressure”, leading to the survival of the fittest. In other words, the few bacteria resistant to antibiotics will survive, and these resistant strains will rapidly proliferate due to their quick reproduction rate.
In recent years, research efforts have increased to develop new therapeutics aimed at combatting antibiotic-resistant bacterial infections. These efforts focus on inhibiting the virulence factors that bacteria secrete to proliferate, evade the immune system, and infect eukaryotic cells [4, 5]. This review aims to highlight the advances in these antivirulence therapies, with a particular emphasis on those utilizing peptides to inhibit toxin activity.
Virulence factors include a wide range of molecules that facilitate infection, proliferation, and dissemination of pathogenic microorganisms (such as bacteria, viruses, fungi, and parasites) that enable them to cause disease in a host organism. Examples of virulence factors may include adhesion molecules for attachment to host cells, toxins that damage host tissues, enzymes that facilitate invasion into host cells or tissues, and mechanisms for evasion of immune responses. The presence and expression of virulence factors play a crucial role in determining the severity and outcome of an infectious disease. Some characteristic virulence factors of different microorganisms are listed in Table 1. These are just a few examples of virulence factors, highlighting some of those implicated in various well-known bacterial infections. It is important to note that each microorganism can secrete a distinct pool of virulence factors.
Some characteristic virulence factors of pathogenic bacteria
| Virulence factor | Activity | Bacteria | Reference |
|---|---|---|---|
| Diphtheria toxin | Toxins | Clostridium diphtheriae | [6] |
| Choleric toxin | Vibrio cholerae | [7] | |
| CyaA | Bordetella pertussis | [8] | |
| HlyA, CNF-1 | Escherichia coli | [9] | |
| Type I fimbria | Adhesins | Escherichia coli | [10] |
| Pili | Neisseria gonorrhoeae | [11] | |
| Coagulase, hialurodinase | Degradative enzymes | Staphylococcus aureus | [12] |
| Enterobactin | Siderophore | Escherichia coli | [10] |
| Polysaccharide | Capsule | Streptococcus pneumoniae | [13] |
| Polyribosyl-ribitol phosphate | Haemophilus influenzae | [14] | |
| Protein A | Immune evasion proteins | Staphylococcus aureus | [15] |
| Protein M | Streptococcus pyogenes | [16] | |
| Flagellum | Motility | Helicobacter pylori | [17] |
| ActA | Listeria monocytogenes | [18] |
HlyA: alpha-hemolysin; CNF-1: cytotoxic necrotizing factor 1
In this review, we will focus on describing the mechanism of action of certain toxins, as key virulence factors secreted by bacteria, and the synthesis of various peptides to inhibit their cytotoxic effects.
Toxins encompass a wide range of proteins whose goal is to eliminate or damage the cells of the infected organism to promote bacterial development. They are secreted by both Gram-negative and Gram-positive bacteria. Often, toxins play an important role in the pathophysiology of infections, but in some cases, they are the main cause of pathology, as seen with enterotoxigenic Escherichia coli (E. coli) or Bordetella pertussis (B. pertussis) [8, 19]. Toxins are not only released by bacteria; plants and animals also produce them [20].
The mechanisms of action of toxins are diverse, but they always require an initial contact with the plasma membrane of the target cell, either to permeabilize it or to interact with a membrane protein to alter some signal transduction pathway. Sometimes their action is limited to the membrane (pore formation, destabilization of the membrane barrier with detergent-like effects), while in other cases they need to be translocated to the cytoplasm to disrupt an intracellular pathway [21].
For the development of antivirulence therapies, specifically against toxins, it is essential to have a detailed understanding of their mechanisms of action. Several interesting reviews and books have been published concerning the mechanism of action of different groups of toxins [20–25]. In this review, we will focus on certain toxins from the Repeat in ToXins (RTX) group, as our research team has extensive experience in studying the mechanism of action of alpha-hemolysin (HlyA) from E. coli, a prototype of this family [9, 26–28].
RTX toxins are exoproteins secreted by Gram-negative bacteria, grouped into this family due to shared structural characteristics: i: RTX toxins are encoded in an operon along with other genes necessary for their post-transcriptional activation and secretion. Figure 1 provides a general schematic of this operon. The rtxA genes encode the inactive protoxin (ProRTX), which is activated intracellularly by acylation of internal Lys [29]. This process is catalyzed by an acyltransferase encoded by the rtxC genes that transfers the fatty acids from an acyl-carrier protein (ACP) to ProRTX. The rtxB and rtxD genes encode bacterial membrane proteins that form a “channel-tunnel” facilitating toxin export via a Type I secretion system, which is ATP-dependent [30–33]; ii: RTX toxins possess a secretion signal peptide towards the C-terminal end [9, 34, 35]; iii: RTX toxins contain the characteristic glycine- and aspartate-rich repeats (GGXGXDXUX) that bind numerous calcium ions, typically in the carboxy-terminal portions of the molecule [9, 36]. The large multifunctional autoprocessing RTX (MARTX) toxins also bear similar repeats in the N-terminal segments. RTX toxins thus require physiologically high (> 1 mM) Ca2+ concentrations for proper folding and biological activity on host cells. This so-called RTX motifis the one that defines this class of toxins.
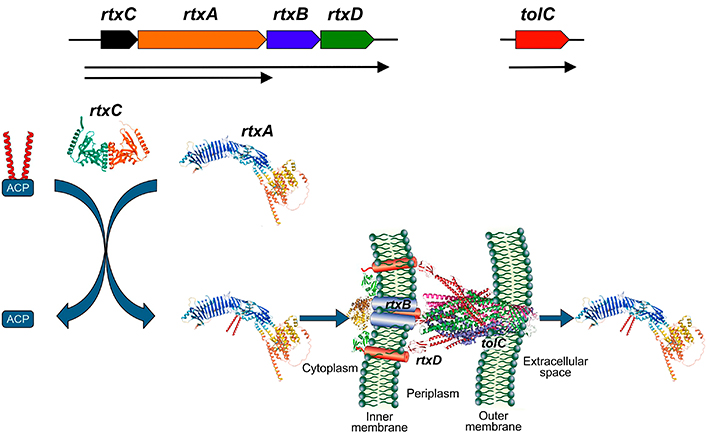
Scheme of the synthesis, activation and secretion of RTX toxins. rtxC gene encodes for an acyltransferase that transfer fatty acids from the acyl-carrier protein (ACP) to the ProRTX protein encoded by rtxA gene. Rtx B and Rtx D are part of the secretory machine with tolC. AlphaFold structures were included in the scheme using as example for the RtxA polypeptide, HlyA from E. coli (UniProtKB P09983), and for RtxC, the acyltransferase ApxC from Actinobacillus pleuropneumoniae (PDB 4WHN). Image HlyA from E. coli was adapted from UniProt of UniProtKB P09983. CC BY, and the image acyltransferase ApxC from Actinobacillus pleuropneumoniae was adapted from RCSB PDB (RCSB.org) of PDB 4WHN [37]. CC0. RTX: Repeat in ToXins; HlyA: alpha-hemolysin
It is important to highlight that the crystallization or exact structural determination of RTX toxins has not yet been achieved, neither in solution nor when bound to membranes. Current knowledge is based on the interpretation of biophysical experiments, molecular dynamics (MD), and potential structures predicted by AlphaFold.
The main members of the RTX toxin family are: HlyA from uropathogenic E. coli (UPEC), CyaA from B. pertussis, leukotoxin (LtxA) from Aggregatibacter actinomycetemcomitans, EhxA from enterohemorrhagic E. coli, ApxIA from Actinobacillus pleuropneumoniae, VcRtxA from Vibrio cholerae, among others [35].
RTX toxins interact with a wide range of cells, leading to extensive research on the existence of specific protein receptors for these toxins in target cells. Traditionally, RTX toxins have been subdivided in two categories (namely, hemolysins and LtxAs) due to their range of activity against different cell types. The so-called hemolysins were initially found to be active toward many different cell types from a wide variety of species, while cytotoxins and LtxAs display toxicity toward a narrower range of cell types and species [38–40]. The hemolytic effect, although significant for the biochemical and genetic characterization of these toxins, is not highly relevant physiologically. More significant are the sublytic effects produced by these toxins on both erythrocytes and leukocytes [21, 41–43]. The physiologically relevant target cells of most RTX toxins are leukocytes that express β2 integrins, which serve as specific receptors for many RTX toxins. Although recently, Ristow et al. [44] have demonstrated that both HlyA and LtxA interact with the extracellular domain of β2 integrins, downstream signaling from this interaction is not implicated in the sublytic effects on cells expressing these proteins. At high concentrations, RTX toxins can cause direct lysis of the target leukocytes. Alternatively, when present at sublytic concentrations (which is most likely the case during in vivo infection), they can alter membrane permeability, resulting in calcium entry, potassium efflux, or both. This can trigger various signaling cascades that may alter cell physiology, eventually leading to apoptosis. Ultimately, these damages result in inflammation and contribute to tissue or organ lesions, consequently enhancing the pathogenicity of the bacterial hosts.
On the other hand, glycophorin had been characterized as a protein receptor for HlyA in erythrocytes [45], and it was demonstrated that the region between amino acids 914–936 was involved in this interaction [46]. Recently, our group has shown that human erythrocytes lacking glycophorin A and B (GPAnull/GPBnull variant) are equally sensitive to the toxin as normal erythrocytes, indicating that the presence of glycophorin is not essential for the lytic effect [26].
Bearing in mind the concept introduced by Skals et al. [47], that none of the RTX cytolysins meet the strict pharmacological requirement of specific binding to a membrane protein to exert their effect, it is noteworthy that HlyA, CyaA, and LtxA, for instance, can disrupt protein-free liposomes [48–50]. Therefore, the authors propose the existence of “proximal molecules” in the membrane that facilitate the approach of the toxins to the cells, allowing them to insert into the membrane in the correct conformation to exert their effect. These “proximal molecules” could include glycophorins, integrins, etc.; but it can also include lipids, such as cholesterol [47].
In recent years, it has been demonstrated that the activity of many RTX toxins depends on the cholesterol content in membranes. For example, the hemolytic activity of HlyA decreases when erythrocytes are depleted of cholesterol using methyl-β cyclodextrin. Furthermore, a reduction in the oligomerization of this toxin in the membrane was observed [51]. Additionally, the release of fluorophores from liposomes varies according to their cholesterol content [52]. This was observed for both HlyA and CyaA [50, 52, 53]. Similarly, it was demonstrated that reducing the cholesterol content in J774A.1 macrophages decreases the translocation capacity of the adenylate cyclase (AC) domain of CyaA [54].
In the case of LtxA from A. actinomycetemcomitans, its ability to kill Jurkat clone Jn.9 and THP-1 cells (monocyte and T lymphocyte cell lines, respectively) decreases when these cells are depleted of cholesterol. Once cholesterol is restored to their membranes, these immune cells regain sensitivity to the toxin [55]. The hemolytic activity of RtxA from Kingella kingae (K. kingae) also showed a dependence on cholesterol content [56]. All these findings suggest that cholesterol binding may be another structural characteristic of this toxin family. Moreover, cholesterol interaction domains have been identified in the sequences of all these toxins. The cholesterol recognition/interaction amino acid consensus (CRAC) motifs have the consensus sequence: L/V-(X1-5)-Y-(X1-5)-R/K. This sequence consists of a branched apolar residue (L or V) followed by a variable segment containing 1 to 5 residues of any amino acid, then an aromatic residue Y, followed by another 1 to 5 residues of any amino acid, and finally a basic residue (K or R) [57, 58]. The CARC sites are similar to CRAC motifs but have the opposite orientation, with the sequence K/R-(X1-5)-Y/F-(X1-5)-L/V [59]. This domain contains basic residues that ensure the correct positioning of the CARC motif at the polar/apolar interface of a transmembrane domain, exactly where cholesterol is presumed to be located and can accept tyrosine (T), phenylalanine (F) or tryptophan (W) as the central amino acid residue [58]. Table 2 shows a list of the CARC and CRAC found in several RTX toxins, together with the number of residues of each protein. This information was obtained from [60].
Total number of CRAC/CARC motifs identified in the primary structure of RTX toxins [60]
| RTX toxin | Bacterium | CRAC motif | CARC motif |
|---|---|---|---|
| EhxA | Enterohemorrhagic (EHEC)Escherichia coli | 16 | 15 |
| LktA | Mannheimia haemolytica | 15 | 13 |
| PILktA | Mannheimia varigena | 21 | 16 |
| PaxA | Pasteurella aerogenes | 19 | 21 |
| MmxA | Morganella morganii | 13 | 15 |
| HlyA | UPEC | 13* | 13 |
| CyaA | Bordetella pertussis | 22 | 18 |
| LtxA | Aggregatibacter actinomycetemcomitans | 24 | 20 |
| ApxIA | Actinobacillus pleuropneumoniae | 18 | 16 |
| ApxIIA | A. pleuropneumoniae | 16 | 16 |
| ApxIIIA | A. pleuropneumoniae | 22 | 19 |
| MbxA | Moraxella bovis | 25 | 18 |
| RtxA | Kingella kingae | 12 | 14 |
* The differences observed from results published by Vazquez et al. [52] are due to the flexibility impair to the sequence of the CRAC domain. CRAC: cholesterol recognition/interaction amino acid consensus; RTX: Repeat in ToXins; HlyA: alpha-hemolysin; UPEC: uropathogenic Escherichia coli; LtxA: leukotoxin. Adapted from [60]. © 2024 by the authors. CC BY
Peptides have emerged as promising therapeutic agents for the treatment of bacterial infections due to their unique mechanisms of action, specificity, and reduced potential for resistance development [61].
Antimicrobial peptides (AMPs) are a diverse group of natural or synthetic molecules capable of targeting a broad spectrum of pathogens, including antibiotic-resistant bacteria [62, 63]. AMPs typically exert their antibacterial effects by disrupting the bacterial cell membrane, leading to cell lysis and death. This is primarily driven by electrostatic interactions between the cationic peptides and the negatively charged components of the bacterial envelope. Once the peptide reaches the bacterial membrane, it can penetrate through various models, including the barrel-stave, carpet, annular pore, aggregation channel, and sinking raft models [63]. The five main proposed mechanisms of action include the interruption of quorum sensing, disruption of membrane potential, inhibition of alarm systems, degradation of biofilm matrix and polysaccharides, and inhibition of transporter expression [64]. Additionally, the role of AMPs in inhibiting biofilm formation is significant. Bacteria within biofilms are able to evade the immune system and survive, often causing chronic infections. Conventional antibiotics are frequently unable to penetrate these biofilms; hence, the development of peptides that inhibit biofilm formation and enhance antibiotic penetration is promising. AMPs with these properties have been tested against biofilms of Staphylococcus aureus (S. aureus) [64] and Pseudomonas aeruginosa (P. aeruginosa) [65].
In recent years, AMPs have emerged as potential alternatives to traditional antibiotics due to their unique and complex antibacterial mechanisms, offering a promising solution to combat multi-drug resistant bacteria [61]. Additionally, the probability of drug resistance to peptides generated by gene mutation is low; therefore, drug resistance is more difficult to develop in AMPs than antibiotics [62, 65].
As mentioned earlier, in recent years, the design of peptides and molecules inhibiting virulence factors of various bacteria has increased to reduce antibiotic resistance [2]. The synthesis of peptides that inhibit these factors is not as widely disseminated as AMP. Therefore, I will describe some of these peptides, particularly those targeting RTX toxins.
The main goal of these therapies is to inhibit the interaction of the toxin with its target cell, either by preventing its interaction with a membrane protein or a lipid. As described above, the presence of a protein receptor for RTX toxins is quite controversial. However, CRAC and CARC motifs that interact with cholesterol have been detected in several of them [60]. For three of them, including LtxA from A. actinomycetemcomitans [66], HlyA from E. coli [67], and CyaA from B. pertussis [68], the possibility of synthesizing peptides that inhibit the interaction of these domains with cholesterol in target cells has been studied. In fact, the application arose from studying various peptides synthesized from CRAC/CARC sequences of the toxins to understand the molecular mechanism of these toxins’ binding to the membrane. Once this interaction was characterized, they were tested as potential inhibitor.
A. actinomycetemcomitans is associated with aggressive forms of periodontitis as well as systemic infections, including endocarditis. Strains of A. actinomycetemcomitans that are most closely associated with diseases have been shown to secrete the most LtxA, which has led to the description of this toxin as a “key” virulence factor of the organism.
UPEC are responsible of the 80% of urinary tract infections. The hlyA gene was detected in ~50% of the isolated UPECs, with the toxin’s expression in those cases being associated with complications in patients with urinary infection, such as the appearance of septic symptoms associated with bacteremia [69–71].
B. pertussis is the etiological agent causative of whooping cough, a highly contagious, acute respiratory illness in humans. Among its virulence factors is CyaA, a distinct member of the RTX toxin family, due to its bifunctional activity. It possesses an AC domain in addition to the core structural features common to other RTX hemolysins [72]. CyaA exhibits the same hemolytic activity as other RTX toxins [72, 73], and can deliver its catalytic AC domain into the cytosol of target cells, where it is activated by calmodulin (CaM).
Figure 2 shows the AlphaFold structure of the three toxins. In HlyA structure the main charateristic domains of the RTX toxins are marked, but as well as the LtxA, the binding calcium domain is the region predicted with high confidentiality (blue region). Instead the insertion region presents less confidentiality (orange-yellow region).
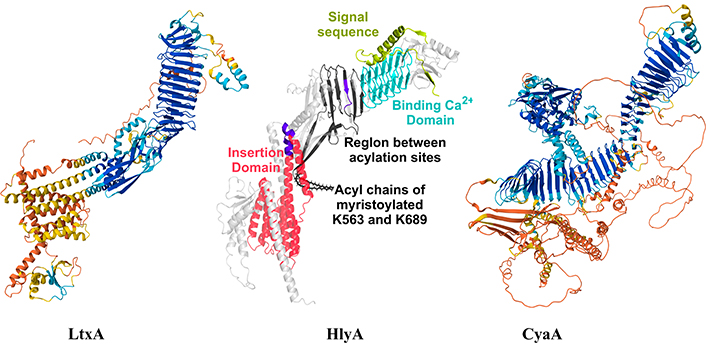
AlphaFold structure of LtxA (UniProtKB P16462), HlyA (UniProtKB P09983) and CyaA (UniProtKB J7QLC0). The main structural domains of HlyA were highlighted with different colours as in [67]. HlyA image was adapted with permission from [67]. © 2023 American Chemical Society, and LtxA and CyaA images were adapted from UniProt (UniProtKB P16462 and UniProtKB J7QLC0). CC BY. LtxA: leukotoxin; HlyA: alpha-hemolysin
In the case of LtxA, 44 CARC and CRAC motifs were identified in its sequence (Table 2). Brown and colleagues [49] demonstrated that the domain located between amino acids 334–340 is specifically involved in the interaction with cholesterol and inhibits LtxA’s ability to lyse Jurkat cells (Jn.9). Based on this finding, they synthesized a peptide containing this domain (display in bold):
327-FDRARMLEEYSKRFKKFGY-346
Using isothermal titration calorimetry (ITC), they showed that the peptide binds with high affinity to cholesterol-containing membranes and does not disrupt membrane packing, suggesting that it primarily resides near the membrane surface [74]. The peptide undergoes a conformational change upon binding to cholesterol. In solution, the peptide consists of 25% α-helices and 25% β-sheets, with the remaining structure being random coils or disordered regions. When bound to a cholesterol-containing membrane, there is a large decrease in helicity and a significant increase in β-sheet structure [74].
Preincubating giant unilamellar vesicles (GUVs) containing cholesterol with the peptide showed a decrease in LtxA binding. Additionally, pretreating THP-1 cells with the peptide resulted in reduced toxin internalization, demonstrating its efficacy in mitigating the toxin’s effects.
In HlyA, 26 CRAC/CARC motifs were identified according to Table 2, while Vazquez et al. [52] found 20. That is because the two groups used different consensus motif sequences. Researchers who compiled the data in Table 2 used the following sequence: (L/V)-X1,5-(Y/F)-X1,5-(R/K) [60], whereas Vazquez et al. [52] required that the central aromatic amino acid has to be only tyrosine (Y). Based on the location of these domains in the HlyA sequence, Cané et al. [67] synthesized two peptides:
341-RFKKLGYDGDSLL-353 (PEP 1), located in the pore-forming domain, and
639-VVYYDK-644 (PEP 2), located between the two acylated lysines (K563 and K689).
The authors explored the interaction of both peptides with membranes of different lipid compositions (pure POPC and POPC/CHOL at 4:1 and 2:1 molar ratios) using surface plasmon resonance (SPR) and MD simulations. They showed that both peptides preferentially interact with cholesterol-containing membranes, presenting a higher affinity PEP 2 than PEP 1 in all the membranes tested. However, only the peptide located between the fatty acids (PEP 2) was able to inhibit hemolysis caused by HlyA (Figure 3A) and did not exhibit lytic activity per se. Instead, PEP 1 presented hemolytic activity at high peptide concentration, and it did not inhibit toxin activity. These differences might be due to the location of the peptides in the membrane as predicted in MD snapshots in Figure 3B. In this case, two inhibition strategies were tested: 1) pre-treating erythrocytes with the peptide, and 2) adding the peptide and toxin together. The latter strategy would be more akin to an in vivo situation if these peptides were to be used as a treatment. Unfortunately, inhibition of toxin binding to erythrocyte ghosts was only observed when the erythrocytes were pre-treated with the peptide Figure 3C [67].
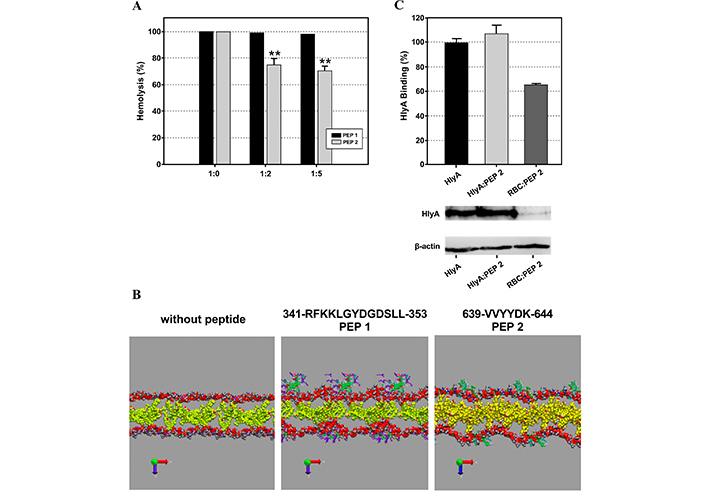
These results were extracted from the Cané et al. [67]. (A) Inhibition of alpha-hemolysin (HlyA) lytic activity by PEP 1 and PEP 2. Hemolysis percentage of 2% v/v erythrocytes as a function of different toxin/peptide molar ratios. Black bars correspond to erythrocytes preincubated with PEP 1, and gray bars correspond to erythrocytes preincubated with PEP 2. (B) Representative snapshots of the final state of POPC/Cho (2:1 molar ratio) membranes in the absence and presence of PEP 1 and 2. Tf = 200 ns. The polar headgroups of POPC (phosphate and choline groups) and Cho are represented as VDW. Peptides are represented in licorice, colored by the polarity of their residues. Water and hydrocarbon tails of POPC are not shown to improve visualization. (C) The binding percentage of HlyA was calculated as the optical density ratio between HlyA and β-actin bands of the Western blot membranes showed below. Figure A and B were reprinted with permission from [67], Figure C was adapted with permission from [67]. © 2023 American Chemical Society
CyaA contains 40 CRAC/CARC motifs in its sequence (Table 2). Masin and colleagues [75] reported that Y/F or Y/A substitutions in the respective central tyrosine residues of the four CRAC motifs (627–638, 654–661, 721–728, and 732–741) predicted in the pore-forming domain of this toxin had no effect on the translocation or hemolytic activity of the toxin. However, recently, Amuategi et al. [76] found four CRAC/CARC motifs: two located in the transmembrane helices (413–420 and 481–487) and the other two in the first helices of the membrane insertion domain (518–527 and 527–534). Surprisingly, none of these four functional CRAC/CARC motifs are conserved in the rest of the RTX toxins [60]. Studies with point mutants where F residues 521 and 532 were substituted by A in the CRAC 518–527 and CARC 527–534 motifs caused a potent inhibition of the ACT activity, while two mutants in the central F residue of the CARC 413–420 and CRAC 481–487 motifs caused a prominent inhibition of the ACT capacity to generate cAMP in the target cell cytosol. At the same time, these mutations augmented the toxin’s lytic capacity. This suggests that in cholesterol-rich membranes, such as the eukaryotic plasma membrane, binding to cholesterol through these CARC 413–420 and CRAC 481–487 drives h1 and h2 to embed into the membrane, and further, that this insertion is essential for AC translocation [76].
Additionally, Amuategi et al. [68] showed that a peptide containing the CRAC 481–487, 454-ASAHWGQRALQGAQAVAAAQRLVHAIALMTQFGR-487, undergoes a conformational change upon binding to cholesterol-containing membranes and inhibits the hemolytic effect of CyaA and the generation of AC in J774A.1 cells.
As mentioned before, some of these toxins also interact with surface proteins such as β2 integrins and glycophorins [45, 77]. It has been demonstrated that these toxins’ activity diminishes when cells are depleted in cholesterol. However, their activity remains largely unaffected when the toxin-protein receptor is compromised [26, 51]. Furthermore, it was demonstrated that many RTX toxins interact with β2 integrins, but intriguingly, the sublytic effects of the toxin do not involve β2 subunit downstream signaling [44].
Considering this evidence leads to a hypothesis proposing that these toxins first interact with proteins, which are in high concentration on membranes, acting as “proximating proteins”. Subsequently, they interact with membrane cholesterol through CRAC and CARC motifs, which stabilizes active conformations for further oligomerization or clustering in lipid rafts or facilitates toxin translocation. The mimicking CRAC peptides inhibit toxin interaction with cholesterol, affecting the adoption of the active conformation, altering in this way the action of the toxin.
Recent advances in peptide engineering have facilitated the creation of synthetic peptides with enhanced stability, efficacy, and selectivity. These engineered peptides are specifically designed to target bacterial strains, minimize toxicity to host cells, and resist degradation by host proteases [66]. Additionally, the application of peptide conjugates and delivery systems, such as nanoparticles and liposomes, has significantly enhanced the therapeutic potential of AMPs [67]. While the inhibition of RTX toxin activity by peptides is a novel approach, it shows great promise. Future studies could investigate whether some of these peptides can inhibit multiple toxins as their sequences are derived from broad and adaptable regions like the CRAC and CARC domains.
Nonetheless, much remains to be explored. Initially, in vivo assays with these CRAC/CARC peptides must be conducted. Additionally, the method of administration must be carefully evaluated, as peptides generally exhibit a short half-life and are readily degraded in the bloodstream and various tissues. Their permeability through the intestinal barrier is also typically low, presenting another challenge.
Clinical applications of AMPs are expanding, with several peptides currently undergoing clinical trials for the treatment of infections caused by multi-drug resistant bacteria, such as methicillin-resistant S. aureus and P. aeruginosa. Additionally, AMPs are being explored for their potential use in combination therapies with conventional antibiotics, enhancing the efficacy of existing treatments and reducing the likelihood of resistance development.
The synthesis of antivirulence peptides will also grow. It is necessary to continue researching the various virulence factors produced by microorganisms, aiming to diminish their action to eradicate these organisms. However, there is also a need for awareness regarding the rational use of antibiotics. Antibiotic therapy helps in the replication of microorganisms, while antivirulence therapies aid in reducing the effects produced by these microorganisms and facilitate their elimination by the immune system. Several disadvantages need to be addressed for the use of these peptides, such as reducing the cost of synthesis and improving their half-life in circulation, as they are often susceptible to degradation by proteases. Furthermore, advancements in delivery systems are necessary.
In conclusion, peptides represent a versatile and promising class of therapeutic agents for the treatment of bacterial infections. Undoubtedly, the development of new therapies against the progression of infections caused by resistant microorganisms is necessary and very promising. However, the advancement of antibiotic-resistant strains can also be reduced if health policies are implemented to decrease the misuse of these drugs and to promote global collaboration on such policies. Given the current globalization and the movement of people around the world, the transfer of pathogens from one place to another facilitates the spread of resistance through genetic exchange.
AC: adenylate cyclase
AMPs: antimicrobial peptides
B. pertussis: Bordetella pertussis
CRAC: cholesterol recognition/interaction amino acid consensus
E. coli: Escherichia coli
HlyA: alpha-hemolysin
LtxA: leukotoxin
MD: molecular dynamics
P. aeruginosa: Pseudomonas aeruginosa
RTX: Repeat in ToXins
S. aureus: Staphylococcus aureus
UPEC: uropathogenic Escherichia coli
The author thanks Mario Ramos for the graphical designs.
VH: Conceptualization, Investigation, Writing—review & editing, Writing—original draft.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by Fundación Florencio Fiorini Grant [2023-2024] and by Universidad Nacional de La Plata (UNLP) Grant number [M247]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2372
Download: 43
Times Cited: 0
Constanza Cárdenas ... Fanny Guzmán
Andrea Barragán-Cárdenas ... Javier García-Castañeda
Natalia Ardila-Chantré ... Javier Eduardo García-Castañeda
Jésica A. Rodríguez ... Silvia A. Camperi
Lucero Ruiz-Mazón ... Mireya de la Garza
Vaezeh Fathi Vavsari, Saeed Balalaie
Karen Johanna Cárdenas-Martínez ... Javier Eduardo García-Castañeda
Daniela Perdomo-Joven ... Mauricio Urquiza-Martinez
