Abstract
Lutein, a natural dihydroxy carotenoid and a member of the non-vitamin A carotenoids family, is abundant in yellow-colored fruits and green leafy vegetables such as spinach and lettuce. As the second most common type of carotenoid found in human serum, lutein offers a plethora of medicinal benefits, including anti-cancer, anti-inflammatory, and anti-oxidative properties. It is well-absorbed and systemically localized to the liver, lung, and retina, where it can cross the blood-retina barrier and accumulate in the macular pigment. Due to its anti-oxidative and singlet oxygen quenching properties, lutein is reported to reduce the risk of age-related macular degeneration (AMD). Higher concentrations of fasting plasma carotenoids and enhanced skin yellowing after lutein consumption indicate its presence in various regions of the human body, including the skin, breast, brain, and cervix. Lutein has remarkable benefits for neurodegenerative diseases, cardiovascular health, liver protection, and bone disease prevention. In the central nervous system (CNS), lutein supports brain homeostasis through its antioxidant and anti-inflammatory properties, increasing interleukin-10 (IL-10) and reducing tumor necrosis factor-α (TNF-α). It reduces the risk of coronary artery disease and exerts anti-inflammatory effects on peripheral blood mononuclear cells (PBMCs). Lutein protects against alcohol-induced liver damage by modulating the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway. Additionally, lutein promotes bone health by enhancing mineralized bone nodule development and inhibiting osteoclast production, reducing bone resorption, and suppressing soluble receptor activator of nuclear factor kappa B ligand (RANKL). These multifaceted benefits make lutein a valuable agent in disease prevention and health promotion. This review discusses the comprehensive profile of lutein as a phytochemical activity, including anti-inflammatory, antioxidant, anti-cancer, hepatoprotective, neurological, and cardioprotective effects. Additionally, it discusses lutein’s beneficial impact on macular degeneration and eye diseases, showcasing its potential as a natural, plant-based therapeutic agent.
Keywords
Lutein, antioxidant, antiinflammation, natural products, carotenoidIntroduction
Inflammation represents a ubiquitous and crucial component of the innate immune response. Functioning as the initial line of defense, it orchestrates a cascade of cellular and molecular events to combat invading pathogens, tissue injury, and irritants. It is of two types namely, acute and chronic inflammation. Acute inflammation can be caused by toxic chemicals, tissue injury from trauma, or microbial infiltration. Acute inflammation ensures healing and restoration of tissue integrity, ultimately leading to homeostasis. This process takes off quickly and can often lead to worse conditions leaving the symptoms to remain for a few days [1]. When inflammation persists for an extended period, it is named as chronic, characterized by sustained tissue damage, damage-induced cellular proliferation, and tissue repair. This eventually can lead to mutagenicity, and can even progress to tumorigenesis [2]. Several studies have demonstrated that chronic inflammation leads to several diseases like atherosclerosis, heart disease, Alzheimer’s disease, asthma, rheumatoid arthritis (RA), acquired immune deficiency syndrome (AIDS), cancer, multiple sclerosis (MS), diabetes, infections (bacteria, fungi, parasites), gout, inflammatory bowel disease (IBD), aging, congestive heart failure (CHF), and other neurodegenerative diseases [3]. Inflammation response involves the process of activation of various pathways involved in regulating the transcriptional and translational mediators in normal tissues and blood cells [4]. The inflammation response can be diverse depending on the nature of the stimulus, however, usually it involves a few common steps such as recognition by receptors, activation of the pathways, production of inflammatory markers, and mobilization of inflammatory cells. Examples of inflammation causing stimuli are various pathogenic products [e.g., lipopolysaccharide (LPS)] and cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6. These generally induce inflammation by the mediation of some of the key players such as Toll-like receptors and receptors of IL-1, IL-6, and TNF. The various receptor activation further is followed by the activation of various signaling pathways such as mitogen-activated protein kinase involving nuclear factor kappa B (NF-κB) Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, etc.
Dysregulation of one or more of these processes can result in inflammation-associated diseases [5, 6]. In such disease, there will be abnormal activation of several enzymes, such as cyclooxygenase (COX)-2, glutathione (GSH) peroxidase (GPx), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), inducible nitric oxide synthase (iNOS), high-mobility group box 1 (HMGB1), and superoxide dismutase (SOD) also observed [7, 8].
Antioxidant enzymes play a key role in inducing oxidative stress. Increased oxidative stress can lead to the formation of reactive oxygen species (ROS), malondialdehyde (MDA), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and isoprostanes. These molecules can further induce various transcription factors, like NF-κB, STAT, p53, and activator protein-1 (AP-1) [9]. These eventually promote the expression of genes responsible for chemokines, inflammatory cytokines, and growth factors [10]. Oxidative stress is implicated in the development of multiple diseases, including atherosclerosis, diabetes, cancer, cardiovascular disease, and hypertension. These markers may hence serve as potential indicators of inflammatory responses.
Since inflammation is a central process, anti-inflammatory medications play a critical role in modern medicine in the management and treatment of various diseases. These medications target the body’s inflammatory response, offering relief from pain, swelling, and fever associated with various conditions, thus controlling the symptoms and slowing the progression of diseases. Glucocorticoids and non-steroidal anti-inflammatory drugs (NSAIDs) are the two main groups of anti-inflammatory drugs. However, the deleterious effects of anti-inflammatory medications in gastrointestinal, cardiovascular, hepatic, renal, cerebral, and pulmonary problems are alarmingly indicated by data from several placebo-controlled trials and meta-analyses of research [11]. NSAIDs cause topical mucosal damage by inhibiting COX-1, resulting in a decreased level of prostaglandins, which protect the gastric mucosa, and thereby resulting in damage in both the upper and lower gastrointestinal tracts (GITs) [12]. The renal adverse effects of NSAIDs include complications like acute renal dysfunction, fluid and electrolyte disorders, renal papillary necrosis, and nephrotic syndrome/interstitial nephritis due to a significant decrease in the prostaglandin levels. Atrial fibrillation, thromboembolic events, and myocardial infarction (MI) are the complications of NSAID toxicity on the heart. Hepatic side effects are less common. Among NSAIDs, diclofenac causes potential side effects on the heart and liver [13, 14]. Because of their antiplatelet action, non-selective NSAIDs are more susceptible to hematologic side effects; it is mainly seen in patients with gastric ulcer history. Minor adverse effects include urticaria and aspirin-exacerbated respiratory illness [15].
Therefore, novel therapeutic compounds derived from plants are needed to solve these issues. Plants include a variety of phytoconstituents that have been shown to reduce inflammation become a safer alternative. Many of these activities are associated with their capacity to prevent the synthesis or function of cytokines, chemokines, adhesion molecules, and the pathways arachidonic acid and NO pathways [16–18]. Development of natural products with strong anti-inflammatory activity and no side effects can replace traditional anti-inflammatory drugs.
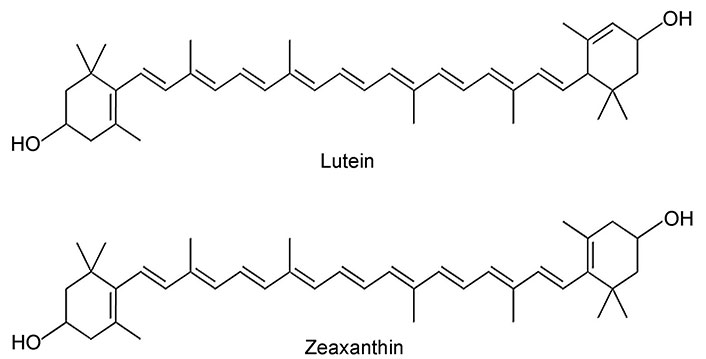
Carotenoids are of two types, a) carotenes (e.g., α-carotene, β-carotene, lycopene, torulene, isorenieratene) and their oxygen derivatives; b) xanthophylls, containing oxygen in the molecule in the form of hydroxyl, epoxy, or carbonyl groups, e.g., lutein, zeaxanthin, canthaxanthin, astaxanthin, and echinaxanthin. Lutein and zeaxanthin are plant-produced pigments that are members of the xanthophyll family, and both are tetraterpenoids composed of eight isoprenoid units. They are remarkably similar in structure; the only notable difference is in the arrangement of atoms [19].
Lutein is also called β, ε-carotene-3, 3'-diol (molecular formula-C40H56O2) (Figure 1) [20], a natural dihydroxy carotenoid and has a molecular weight of 568.886 g/mol. Because of its molecular makeup, it has a high melting point of 196°C, which allows it to withstand high temperatures while cooking. Being a fat-soluble nutrient, eating foods high in lutein should be combined with a supply of lipids for enhanced absorption. As lutein has two hydroxylations in the terminal β rings, it is more polar than β-carotene [20]. Zeaxanthin, a structural isomer of lutein, it differs from lutein in the location of the double bond in the cyclic ring (Figure 1) [20]. Due to the presence of hydroxyl groups in the carbon ring in these molecules, compared to carotenoids, xanthophylls are more polar compounds, absorbing radiation of shorter wavelengths. Xanthophylls are widely distributed in nature, with a group of compounds showing both chemical and physicochemical similarity. Both are present in a variety of green and yellow fruits and vegetables, including guava, collard greens, spinach, and cabbage. Consuming lutein as a normal food source from various fruits and vegetables is advised for the management of many inflammatory diseases. The lutein content of some of the common vegetables is listed here: spinach was the top one in the list with the highest content of lutein: 8,950 µg per serving. Pumpkin contains 2,820 µg and next was green peas amounting to 2,230 µg per serving. Broccoli, young beetroot leaves, celery, and zucchini were other rich sources of lutein consisting of 1,970 µg, 1,960 µg, 1,680 µg, and 1,140 µg respectively [21].
The hydroxyl group of xanthophyll contributed to their polarity during its absorption, metabolism, distribution, and uptake into body tissues [22]. Lutein is present in both esterified and non-esterified forms of fatty acids, primarily palmitic acid. Compared to their free counterparts, esterified carotenoids are less likely to degrade and cannot be absorbed from the GIT [23]. Lutein esters must first be de-esterified by intestinal enzymes to enter the blood stream, while crystalline lutein is easily absorbed from meals and nutritional supplements. Xanthophylls such as lutein and zeaxanthin are integrated into both high-density lipoprotein (HDL) and low-density lipoprotein (LDL) but the carotenes are only present in the LDL fraction [24, 25]. Metabolites of lutein and zeaxanthin were detected in significant concentrations, with 3'-dehydro-lutein being a common metabolite of both. Two diastereomers, (3R,6'R)-3'-dehydro-lutein and (3R,6'S)-3'-dehydro-lutein, were found and demonstrated to be present in roughly equimolar levels using chiral-phase high performance liquid chromatography (HPLC). It is suggested that they develop from either lutein or zeaxanthin. These results were similar to those of studies conducted using human plasma [26]. Higher concentrations of fasting plasma carotenoids and enhanced skin yellowing after lutein consumption demonstrate that lutein is also present in various regions of the human body, such as the skin, breast, brain, and cervix. Granado et al. [27] demonstrated that blood lutein concentration between 0.6 µM/L and 1.05 µM/L is considered to be safe. However, the recommended daily intake of lutein is 2 mg/kg body weight, or 120 mg/day for an individual weighing 60 kg. Moreover, there has been no evidence of any negative consequences in people from long-term dietary lutein supplementation. Higher doses of lutein (30 mg/kg and 40 mg/kg body weight) were proven to be safe [28, 29].
Lutein is the second most common type of carotenoid found in human serum [11]. Lutein possesses many medicinal benefits such as anti-cancer, anti-inflammatory, and anti-oxidative properties. When consumed in a fat enriched diet, the dietary absorption of lutein is good; it systemically gets localized to the liver, lung, and retina. Lutein can cross the blood-retina barrier and get accumulate in the macular pigment. Since lutein has anti-oxidative and singlet oxygen quenching properties, it is reported to reduce the risk of age-related macular degeneration (AMD) [30]. The unique structure of Lutein appears to be contributing to its superior and beneficial effects such as anti-inflammatory and antioxidant properties, especially due to the presence of conjugated double bonds and hydroxyl groups [31]. The conjugated double bond acts as a powerful antioxidant by donating electrons and neutralizing free radicals, resulting in a more stable product. This structural feature may also influence its superior absorption property by altering the polarity and flexibility of carotenoids. Lutein, with its plethora of health benefits, is thus recognized as an effective functional molecule [11].
Extensive research has demonstrated the long-lasting anti-inflammatory properties of lutein by blocking multiple inflammation pathways and mediators such as NF-κB, phosphatidylinositol 3-kinase (PI3K)/Akt, nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and sirtuin-1 (SIRT-1) signaling [32]. Lutein is reported to show protective and therapeutic effects in inflammatory and neurological disorders, various eye conditions like diabetic retinopathy, osteoporosis, cardiovascular diseases, skin diseases, liver damage, obesity, and colon diseases. Furthermore, it also displays tissue-specific functions, including controlling skin function, adipocyte differentiation in obesity, and lipid profiles in cardiovascular disorders [33]. Because of its unique structure that includes conjugated double bonds and hydroxyl group, lutein has anti-inflammatory and antioxidant properties [31]. The anti-inflammatory effect of lutein may be associated with the inhibition of NF-κB-dependent expression of genes, such as COX-2, TNF-α, IL-1β, iNOS [33].
Beneficial effects of lutein on neurodegenerative disease
In the central nervous system (CNS), maintenance of brain homeostasis and regulation of neuronal surveillance have been associated with microglia, the primary immune effector cells [34, 35]. These cells regulate the homeostasis of CNS, through the secretion of IL-10, which has anti-inflammatory and neuroprotective properties [36, 37]. Neurodegenerative diseases have been linked to microglia hyperactivation, and in vitro cultures, IL-10 has been demonstrated to prevent microglia and other glia from producing proinflammatory cytokines [38]. The JAK-STAT pathway is activated upon IL-10 binding to the receptor which suppresses the expression of cytokine genes and the ability of antigen presentation [39].
The antioxidant and anti-inflammatory properties of lutein in the presence of H2O2 were discovered in BV-2 microglia by Pap et al. [40]. Their results suggest that lutein inhibits the synthesis of ROS and reduces inflammation by raising IL-10 and decreasing proinflammatory TNF-α release. In the control group, BV-2 cells released a low level of proinflammatory TNF-α and anti-inflammatory IL-10 cytokines. The cells produced more TNF-α and less IL-10 in response to stress stimuli caused by H2O2 and also showed that lutein, either by alone or in combination with H2O2 therapy, could increase IL-10 levels in BV-2 cells without up regulating proinflammatory molecules. Compared to cells that received H2O2 alone, the lutein and H2O2 treated cells showed a time-dependent falling trend in TNF-α cytokine levels. The main anti-inflammatory, inhibitory, or self-regulating function of the cytokine through autocrine and paracrine pathways is thought to be the rise of IL-10 in response to lutein therapy [41].
In LPS-activated microglia, lutein reduced neuroinflammation by blocking inflammatory signaling pathways, including NF-κB, and regulating the expression of IL-1β, COX-2, iNOS, and TNF-α. Lutein may stimulate extracellular signal-regulated kinase (ERK, a mitogen activated protein kinase) and cause the dissociation of Kelch-like ECH-associated protein 1 (Keap1) from the Nrf2/Keap1 complex. In the nucleus, phosphorylated Nrf2 binds with small Maf proteins (sMaf) protein to form a heterodimer. This complex attaches to the regulatory region of DNA called the antioxidant response element (ARE), leading to the expression of antioxidant enzymes such as NAD(P)H quinone oxidoreductase 1 (NQO1) and HO-1. As a result, lutein provides protection against inflammation-related neurodegenerative diseases [42, 43]. In membrane lipid bilayers, lutein and zeaxanthin, unlike nonpolar carotenoids, have polar groups at both ends of their molecules, giving them a membrane-spanning configuration. It is thought that these groups take a perpendicular or semi perpendicular orientation in these situations. This property, in conjunction with their high solubility in membranes, can significantly affect membrane properties such as fluidity, ion exchange, oxygen transport, and membrane stability [44, 45]. It may also have an impact on inter-neuronal communication by influencing gap junctions [46, 47]. Lutein may be positioned in membranes to prevent the oxidation of these delicate brain lipids due to this and evidence shows that it localizes to membrane domains rich in poly unsaturated fatty acids (PUFAs) including docosahexaenoic acid (DHA) [48, 49]. Not only does inhibition of DHA oxidation contribute to preserving membrane fluidity and structure, but it also keeps DHA in an accessible state for cleavage and subsequent synthesis of anti-inflammatory compounds [50, 51]. Oxidative stress-induced inflammation and apoptosis are linked to severe traumatic brain injury (STBI) [48]. In rats, lutein prevented serious brain injury by down regulating the expression of IL-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1), as well as lowering the levels of ROS in the serum [52].
Prior research by Cheng et al. [53] revealed that lutein guards against ischemia-reperfusion injury by reducing oxidative stress, inflammation, and the expression of COX-2 and NF-κB. NF-κB plays a crucial role in controlling the release of inflammatory mediators during cerebral ischemia-reperfusion. Numerous studies have demonstrated that cerebral ischemia-reperfusion triggers NF-κB activation [54]. In STBI rat models, lutein administration dramatically inhibited the production of NF-κB protein [52]. The mechanism by which lutein inhibits oxidative stress-induced inflammatory responses in neurodegenerative diseases is illustrated in Figure 2 and Table 1 [40, 42, 43, 46, 53].
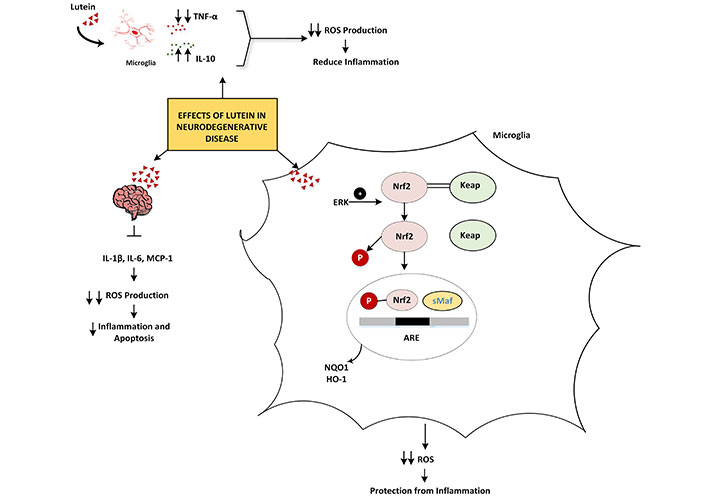
The mechanism by which lutein inhibits oxidative stress-induced inflammatory responses in neurodegenerative diseases. ↑: increasing; ↓: decreasing. TNF-α: tumor necrosis factor-α; IL-10: interleukin-10; ROS: reactive oxygen species; Nrf2: nuclear factor erythroid 2-related factor 2; ARE: antioxidant response element; HO-1: heme oxygenase-1; NQO1: NAD(P)H quinone oxidoreductase 1; MCP-1: monocyte chemoattractant protein-1; ERK: extracellular signal-regulated kinase; Keap: Kelch-like ECH-associated protein; sMaf: small Maf proteins
Anti-inflammatory and antioxidant effect of lutein
| Sl.No. | Disease | Mechanism of action | Reference |
|---|---|---|---|
| 1 | Neurodegenerative disease | In BV-microglia:Inhibit the synthesis of ROS↑IL-10↓TNF-α | [26] |
| In LPS activated microglia:Block NF-κB signaling pathwayRegulate the expression of IL-1β, COX-2, iNOS, and TNF-αStimulate ERK, finally causes the expression of antioxidant enzymes (NQO1 and HO-1) | [27, 28] | ||
| In serious brain injury rats:↓IL-1β, IL-6, MCP-1, and ROS levels in serum | [32] | ||
| In STBI rats:Inhibit NF-κB protein production | [33] | ||
| 2 | Cardiovascular disease | In PBMCs:Blocks NF-κB signalingPrevents leukocyte adherence and production of inflammatory genes, such as IL-1β, TNF, and IL-6 | [35] |
| Prevent atherosclerosis in mice and guinea pigs by ↓oxidized LDL concentrations, MDA, and cytokine production in aortic tissue | [36, 37] | ||
| HUVECs:↓Nitrotyrosine (an ONOO–) index and ROS↑NO, suppresses the production of adhesion molecules dependent on NF-κB and the interaction between monocytes | [41] | ||
| Prevents MI by modulating the MIAT/miR-200a/Nrf2 pathway | [46] | ||
| 3 | Liver disease | Protects alcohol-induced liver damage by modulating the NF-κB, Nrf2/HO-1 signaling pathway, ↓SOD and GPx | [48, 49] |
| In ethanol treated rats:↓NF-κB, COX-2, iNOS, TNF-α, MCP-1, IL-1β, IL-6, AST, ALT, LDH, and sulfhydryl content↑Nrf2 levels and activities of antioxidant enzymes (catalase, GPx, GSH) | [50] | ||
| In guinea pigs fed high cholesterol diet:PPARα/RXRα-mediated regulation or direct suppression of NF-κB activation (↓levels of mRNA and TNF-α, IL-1β, COX-2, and iNOS) | [51] | ||
| Liver ischemia-reperfusion injury in rats:↓MDA, TNF-α, NF-κB, and IL-1βEnhance ROS formation and proinflammatory cytokines | [53] | ||
| CP toxicities in the liver:Block NF-κB/p38-MAPK activation and prevent cytokine secretion | [54] | ||
| In slow-growing egg-type chicks in ovo:Regulates PPARγ/RXR pathway and I-κB/NF-κB pathway | [55] | ||
| 4 | Eye disease | In retinal neural cells:↓ROS, rhodopsin level, and upregulate GFAP expression | [57] |
| Inhibit the direct pathway of STAT3 phosphorylation and activation by ROS | [58] | ||
| In retina treated lutein-PLGA NCs (+PL):Inhibit NF-κB p65 activity and down-regulate the expression of COX-2 and iNOS | [60] | ||
| During optic nerve injury in rats:↓MDA, IL-1β, and TNF-α, ↑total TSH level in serum | [61] | ||
| Anti-cataract effect:↓Expression of NOS2 and COX-2 through NF-κB | [62] | ||
| 5 | Bone disease | Inhibits the expression of RANKL through NF-κB in osteoblasts, which suppresses osteoclast differentiation | [42] |
| Protection against osteoarthritis:Control oxidative stress and apoptosis through Nrf2 and NF-κB expression | [63] | ||
| In LPS-stimulated DC-reduced inflammation and modifying immune response:↓CD40, co-stimulatory molecule CD86, MHC class II molecule, and IL-6 and ↑production of IL-12 p40 | [65] | ||
| 6 | Diabetes | In diabetic rats:↓TNF-α and IL-1βInhibit inflammatory response by shielding the proteasome from oxidative stress induced inactivation | [59, 69] |
| In the retina of Ins2Akita/+ mice:Inhibit the release of TNF-α and IL-1β and ROS formation | [27, 71] | ||
| In diabetic rats:↓CCT levels of TBARS, caspase-3 TNF-α, IL-1β, and IL-6Downregulates BDNF, NGF, and IGF. So, oxidative and inflammatory damage as well as neuronal degeneration are also reduced in diabetes | [72] | ||
| 7 | Obesity and inflammatory bowel disease | In epididymal adipose tissue, downregulate the expression of FAS, PPARγ, and CEBP-α | [74] |
| Lutein nanoparticles, control colitis in mice:Inhibit NF-κB signaling, TNF-α, iNOS, NLRP3, and IL-1β↑Expression of ZO-1, claudin-1, and occluding | [76] | ||
| 8 | Skin inflammation | Inhibit the redox-sensitive AP-1 pathway | [77, 78] |
↑: increasing; ↓: decreasing. ROS: reactive oxygen species; IL-10: interleukin-10; TNF-α: tumor necrosis factor-α; LPS: lipopolysaccharide; NF-κB: nuclear factor kappa B; COX-2: cyclooxygenase-2; iNOS: inducible nitric oxide synthase; HO-1: heme oxygenase-1; STBI: severe traumatic brain injury; PBMCs: peripheral blood mononuclear cells; MI: myocardial infarction; MIAT: MI associated transcript; miR: micro-RNA; Nrf2: nuclear factor erythroid 2-related factor 2; SOD: superoxide dismutase; PPARα: peroxisome proliferator-activated receptor alpha; RXRα: retinoid X receptor alpha; MDA: malondialdehyde; CP: cyclophosphamide; STAT3: signal transducer and activator of transcription 3; RANKL: receptor activator of NF-κB ligand; DC: dendritic cell; TBARS: thiobarbituric acid reactive substances; BDNF: brain-derived neurotrophic factor; NGF: nerve growth factor; IGF: insulin-like growth factor; NLRP3: NOD-like receptor protein 3; ZO-1: zonula occluden-1; GPx: glutathione peroxidase; GSH: glutathione; ERK: extracellular signal-regulated kinase; NQO1: NAD(P)H quinone oxidoreductase 1; MCP-1: monocyte chemoattractant protein-1; LDL: low-density lipoprotein; AP-1: activator protein-1; HUVECs: human umbilical vein endothelial cells; AST: aspartate transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; I-κB: inhibitor of kappa B; GFAP: glial fibrillary acidic protein; PLGA: poly(lactic-co-glycolic acid); NCs: neural crest cells; PL: phospholipid; TSH: thyroid stimulating hormone; MHC: major histocompatibility complex; Ins2: insulin 2; FAS: Fas cell surface death receptor; CEBP-α: CCAAT/enhancer-binding protein alpha; CCT: cerebral cortex
Lutein—as a cardioprotective agent
Lutein is popular for its benefits for cardiovascular health, potentially reducing the risk of coronary artery disease perhaps due to its antioxidant and anti-inflammatory properties. Chung et al. [55] reported that, ex vivo studies on lutein have anti-inflammatory effects on human peripheral blood mononuclear cells (PBMCs). Adding lutein to PBMCs between 10 μM and 20 μM leads to its intracellular accumulation and suppresses NF-κB signaling. Finally prevents leukocyte adherence and the production of inflammatory genes, such as IL-1β, TNF, and IL-6 [55]. In animal models, the development of atherosclerotic lesions has been reported to be inhibited by lutein supplementation [56–58]. It has been suggested that both antioxidant and anti-inflammatory properties have atheroprotective benefits. It is commonly known that lutein can stop oxidative cell damage and cell lipid oxidation [59]. According to Dwyer et al. [56], in atherosclerosis-prone mice, lutein supplementation reduced the inflammatory response of monocytes to oxidized LDL. A lutein-rich diet reduced oxidized LDL concentrations, MDA, and cytokine production in aortic tissue, and prevented atherosclerosis in guinea pig models [57].
Complement factors C3 and C3a are found in greater concentrations in the blood of those who have a history of atherosclerosis. By using a different complement pathway, C3 generates a membrane attack complex that can kill pathogens or host cells by causing a hole or pore in the membrane. It has been demonstrated that lutein lowers plasma complement factor levels, which includes membrane attack complex. Thus, lutein may contribute to atheroprotection and cardiometabolic health by preventing or reducing tissue oxidation and preventing the blood’s harmful complement factors from getting activated [60]. By considerably lowering nitrotyrosine (an ONOO–) index and ROS, carotenes increase the bioavailability of NO. Simultaneously, it suppresses the production of adhesion molecules dependent on NF-κB and the interaction between monocytes and human umbilical vein endothelial cells (HUVECs) (Figure 3) [61].
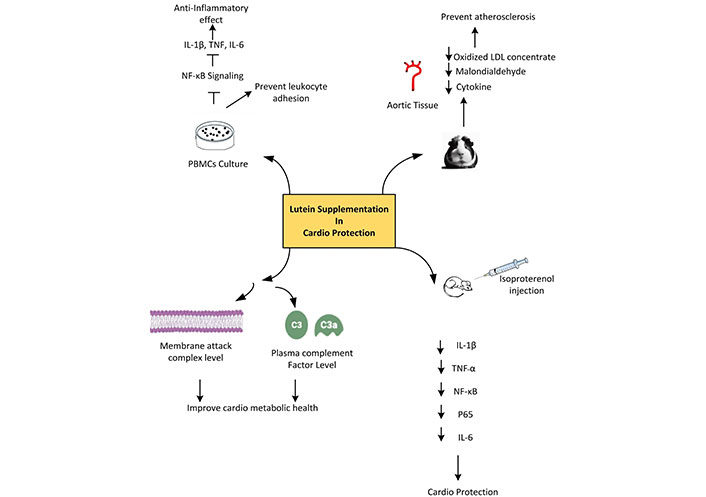
The overall mechanism of action of lutein as a cardioprotective agent. ↓: decreasing. IL-1β: interleukin-1β; TNF: tumor necrosis factor; NF-κB: nuclear factor kappa B; PBMCs: peripheral blood mononuclear cells; LDL: low-density lipoprotein
Lutein has been reported to exhibit strong cardioprotective activity against isoproterenol (ISO) induced myocardial damage in rats. Lutein pre-treatment could significantly reduce the infarct size, cardiac markers [cardiac troponin T (cTnT), creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH)], inflammatory markers (IL-1β, TNF-α, NF-κB p65 subunit, and IL-6), lipid peroxidation product, and apoptotic markers. It could also significantly up regulate the protein expression of Nrf2 and HO-1. All these are attained by preventing the activation of the NF-κB signaling pathway [62]. According to Wu et al. [42] and Li et al. [63], the protein expressions of Nrf2 and HO-1 were found to be up regulated in rats pre-treated with lutein. The activation of Nrf2 by lutein may be caused by the presence of an electrophilic group that combines with the cysteine residue thiols in Keap1 to cause the Nrf2-Keap1 complex to break down, thereby boosting the production of different cytoprotective and antioxidant proteins and supporting their cardioprotective properties.
UV-irradiation of lutein results in different fragments. Namely anhydrolutein (B, C40H54O), 2,6,6-trimethylcyclohexa-1,4-dienylium (M1, C9H13), (2E,4E,6E,8E)-9-(4-hydroxy-2,6,6-trimethylcyclohex-1-1en-1-yl)-3,7-dimethylnona-2,4,6,8-tetraen-1-ylium (M2, C20H29O), 4-[(1E,3E,5E,7E)-3,7,-dimethyldeca-1,3,5,7-tetraen-1-yl]-3,5,5-methylcyclohex-3-en-1-ol (M3, C21H30O), and zeaxanthin (M4, C40H56O) and its isomers as 13'-Z zeaxanthin, 13'-Z lutein, all-trans zeaxanthin, and 9-Z lutein. In that M1, M2, and M3, oxidative derivatives of lutein reduced acute inflammation in rats more effectively than lutein preventing the synthesis of nitrites, MDA, prostaglandin E2 (PGE2), TNF-α, and IL-6 cytokines. Derivatives may have an anti-inflammatory effect by reducing inflammatory cytokines and increasing antioxidant enzymes [catalase, SOD, GPx, GSH S-transferase (GST), GSH reductase]. This would decrease iNOS, COX-2, and MDA and consequently reduce inflammatory responses (Figure 3) [64].
Lutein in ISO-induced MI, immunohistochemical analysis showed that the lutein pre-treated group had up regulated expressions of cTnT, regulates excitation-contraction coupling [65] and desmin maintain a healthy cardiomyocyte function [66]. Additionally, it increased the contents of cardiac thioredoxin (Trx) and GSH, decreases oxidative stress micro-RNA (miR)-200a and Nrf2. It was able to down regulate lipid peroxidation, long non-coding MI associated transcript (lncRNA MIAT), and Trx-interacting protein (TXNIP) in comparison to the untreated group. Additionally, a significant inverse correlation was observed between MIAT and miR-200a. Abdelmonem et al. [67] reported that lutein could ameliorate MI in rats by modulating the MIAT/miR-200a/Nrf2 pathway. The LU-receiving group exhibited an up regulation of miR-200a, critical function of miR-200a over expression reduces many cardiac injury models, including hypoxia-induced cardiac mortality and chemotherapy-induced cardio toxicity, through the activation of Nrf2. It was demonstrated that the nuclear translocation of Nrf2 and subsequent downstream gene transcription were greatly enhanced by miR-200a’s suppression of Keap1, which in turn reduced the production of ROS and the death of cells (Table 1) [55, 56, 61, 67–69].
Hepatoprotective effects of lutein
Lutein protects from alcohol-induced liver damage by exerting anti-oxidative and anti-inflammatory capacities by modulating the Nrf2/HO-1 signaling pathway. High-dose lutein increased the expression of the antioxidant enzyme HO-1 helping to counteract the alcohol-induced reduction in SOD and GPx levels. It also raised the “total antioxidant capacity (T-AOC)”. Lutein treatment reduced the alcohol-induced elevation of cytochrome P450 2E1 (CYP2E1) and lowered levels of triglycerides (TG) and MDA, suggesting that it may help protect the liver from damage through its antioxidant effects [70]. Additionally, lutein is reported to reverse the alcohol’s impact on inflammatory protein levels, reduce inflammation, and partly improve liver health by modulating the NF-κB signaling pathway. Antioxidants like lutein may support alcohol metabolism and prevent alcohol-related liver damage by regulating the feedback between Nrf2 and aldehyde dehydrogenase 2 (ALDH2). This further depicts that high-dose lutein increased the mRNA expression of ALDH2 related to alcohol metabolism in the liver [71, 72]. Administering lutein (40 mg/kg body weight) 30 min before ethanol exposure lowered levels of inflammatory proteins (NF-κB, COX-2, iNOS), inflammatory cytokines (TNF-α, MCP-1, IL-1β, IL-6), liver enzymes (aspartate aminotransferase, alanine aminotransferase, LDH), and oxidative stress markers (ROS, lipid peroxidation, protein carbonyls, and sulfhydryls) in rats. Additionally, lutein boosted Nrf2 levels and increased the activity of antioxidant enzymes (catalase, GPx, GSH, and GST) [73]. In guinea pigs fed a high-cholesterol diet, lutein (0.1 g per 100 g) therapy prevents degenerative diseases in the liver by lowering hepatic free cholesterol, lipid peroxidation, and attenuating the inflammatory responses known to be involved in the development of non-alcoholic fatty liver disease (NAFLD) [74]. Additionally, data suggest that the reduced NF-κB, p65 DNA binding activity was the cause of the attenuated inflammatory response with lutein therapy. Lutein directly reduces the activation of NF-κB and inhibits the levels of mRNA and protein of TNF-α, IL-1β, and COX-2. Carotenoids given before hatching and in their diet after hatching help lower inflammation markers in the liver as suggested by some studies conducted in slow-growing egg-type chicks. Changes in liver weight during inflammation and IL-6 mRNA levels in the spleen were reduced. This effect may be mediated by the peroxisome proliferator-activated receptor gamma (PPARγ)/retinoid X receptor (RXR) pathway, which directly affects gene expression, or by reducing oxidative stress and modifying the inhibitor of kappa B (I-κB)/NF-κB pathway [75]. Gündoğdu et al. [76] reported that in cytokine-related liver ischemia-reperfusion injury in rats, lutein administration decreases oxidant markers like MDA and levels of proinflammatory cytokine mediators including TNF-α, NF-κB, and IL-1β. The values observed were similar to those in the group of good health. Again, in the lutein-administered group, we observed mild sinusoidal dilation in the parenchyma and portal regions, together with a histological look similar to normal liver cells, where inflammation accompanied with hepatic congestion was retreated. Lutein can protect against tissue damage induced by excess NF-κB and TNF-α. Its hepatoprotective action is attributed to its ability to inhibit the creation of ROS and the synthesis of proinflammatory cytokines (Figure 4).
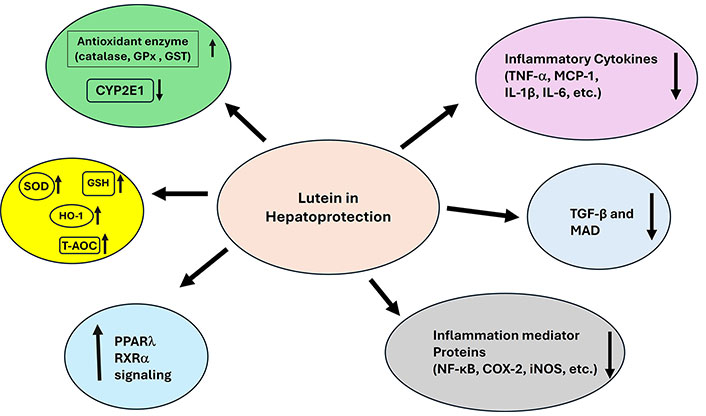
The overall mechanism of action of lutein as a hepatoprotective agent. ↑: increasing; ↓: decreasing. GPx: glutathione peroxidase; GST: glutathione S-transferase; SOD: superoxide dismutase; GSH: glutathione; HO-1: heme oxygenase-1; T-AOC: total antioxidant capacity; PPARλ: peroxisome proliferator-activated receptor λ; RXRα: retinoid X receptor α; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; NF-κB: nuclear factor kappa B; COX-2: cyclooxygenase-2; iNOS: inducible nitric oxide synthase; CYP2E1: cytochrome P450 2E1; MCP-1: monocyte chemoattractant protein-1
Strong protective effects of lutein are seen against hepatic and pulmonary toxicities brought on by cyclophosphamide (CP). Its capacity to reduce lipid peroxidation, prevent oxidative and nitrosative stress, and restore antioxidant enzyme activity is what gives it this protective action. Lutein’s strong antioxidant action was successful in blocking NF-κB/p38-MAPK activation, which in turn prevented the release of cytokines. Consequently, lutein may be clinically significant if paired with CP following additional experimental and clinical validation research [77].
In slow-growing egg-type chicks, carotenoid exposure in ovo and in the food after hatch reduces markers of inflammation in the liver (e.g., change in liver weight during acute phase response) and spleen (e.g., IL-6 mRNA abundance). These outcomes are most likely mediated by the PPARγ/RXR pathway directly influencing gene expression, or by impacts on oxidative stress and the ensuing modifications in the I-κB/NF-κB pathway. The mechanism is as follows, RXR binding and heterodimerization with PPARγ bound to fatty acids, eicosanoids, and several synthetic ligands, carotenoids influence immunological responses [78]. To influence transcription processes, PPARγ-RXR dimers bind response elements and co-activators in DNA. Specifically, these dimers inhibit the transcription factor NF-κB, which in turn controls the expression of genes related to inflammation and immunity, such as iNOS (Table 1) [71–73, 75, 77–79].
Lutein in preventing eye disease
In addition to its well-known effect on rhodopsin preservation, lutein suppresses STAT3 activation in the retinal cells [80]. The inflammatory cytokines like IL-6 activate STAT3, and STAT3 in turn regulates IL-6. Therefore, the IL-6-STAT3 pathway enters a vicious loop that intensifies the pathogenic situation after STAT3 activity over a particular threshold. However, lutein inhibits STAT3 activation, which stops this vicious cycle and effectively guards against tissue damage in the eye. In terms of the mechanism preventing STAT3 activation, lutein may inhibit the direct pathway of STAT3 phosphorylation and activation by ROS. The reduction of ROS by lutein may suppress the rapid activation of JAK and STAT3, leading to the efficient preservation of rhodopsin protein. Thus, lutein has an anti-inflammatory impact because oxidative stress and inflammation are linked and can be controlled by lutein [81].
Oxidative stress and rapid cytokine transmission in the retinal neural cells and associated inflammation responses impair vision. Lutein treatment effectively decreased ROS, thus facilitating the reduction of rhodopsin levels, and the up regulation of glial fibrillary acidic protein (GFAP) expression; this in turn prevents the impairment of vision during inflammation. Lutein administration also may offer neuroprotection against retinal inflammatory disorders [80].
Another mechanism linking oxidative stress to altered gene expression associated with inflammation is the through inactivation of proteasomes. The proteasome was shielded from inactivation and the alterations in the expression of these inflammation-related genes were mitigated when lutein or zeaxanthin was added to retinal pigment epithelium (RPE). This could be one of the ways that lutein and zeaxanthin consumed as food affect systemic and ocular inflammation and lower the risk of AMD [82].
Studies using lutein encapsulated in poly(lactic-co-glycolic acid) (PLGA)-phospholipid nano-carrier demonstrated improved anti-inflammatory action in the serum and retina of LPS-induced lethal dose (LD) mice. They also inhibited the NF-κB p65 activity and down-regulated the expression of COX-2 and iNOS. Nanoparticles loaded with lutein effectively increased the solubility, stability, and bioavailability of lutein, hence increasing its bio efficacy. In addition to target delivery to the retina, these findings will support the application of lutein-PLGA neural crest cells (NCs) (+PL) (PLGA nanocarrier with phospholipid), against oxidative stress and ocular inflammatory consequences [83].
According to Karakurt et al. [84], lutein treatment for two weeks (0.5 mg/kg) was found to be highly efficient in reducing toxic optic neuropathy caused by isoniazid (50 mg/kg, p.o., 14 days) and ethambutol (50 mg/kg, p.o., 14 days). Blood samples and tissues from the lutein-administered group of rats showed significantly lower levels of MDA, IL-1β, and TNF-α. The lutein administered group also had considerably greater total GSH levels in their serum and tissue, suggesting that lutein plays a key role in guarding against optic nerve injury.
Lutein therapy along with higher concentrations of eicosapentaenoic acid and DHA is thought to contribute to its anti-cataract effect. The inflammatory mediators of eicosanoids of fatty acids and the antioxidant activity of lutein combine to produce preventive effects against cataract formation. According to the findings, lutein and omega-3 fatty acids can effectively control inflammation and oxidative stress when used together to avoid cataracts. The delivery of lutein inhibited the PGE2-mediated inflammatory response, so highlighting the critical role of fatty acids, particularly those in the omega-3 family, and the need to prevent inflammation. NF-κB regulates several genes implicated in the inflammatory response, such as iNOS2 and COX-2. And administration of lutein prevents the expression of these genes and reduces the risk of cataracts [85]. In a randomized double-blind placebo controlled parallel group comparison trial, the continuous intake of lutein (12 mg) for 16 weeks as a formulation, considering its bio-accessibility, increased macular pigment optical density (MPOD), improved contrast sensitivity, and prevented decreases in visual function caused by a glare from light (Table 1) [80, 81, 83–86].
Lutein in preventing and curing bone disease
Lutein promoted the development of mineralized bone nodules in osteoblast cultures. Conversely, lutein distinctly inhibited 1α, 25-dihydroxyvitamin induced vitamin D3 bone resorption. Lutein inhibited the production of osteoclasts caused by 1α, 25-dihydroxyvitamin D3 in co-cultures of osteoblasts and bone marrow cells. Lutein also suppresses soluble receptor activator of NF-κB ligand (RANKL), which is responsible for increasing the production of osteoclasts in bone marrow macrophage cultures. In a study, five-week-old male mice were given lutein orally for four weeks, leading to a significant increase in femoral bone mass, particularly in cortical bone, as measured by bone mineral density using dual X-ray absorptiometry and micro-computed tomography (μCT) scans. Lutein primarily works by inhibiting RANKL-dependent osteoclast production, acting on both macrophages and osteoclast precursor cells. Additionally, lutein inhibits the expression of RANKL in osteoblasts, thereby suppressing osteoclast differentiation. This effect is attributed to lutein’s ability to reduce the mRNA expression of RANKL in osteoblasts, which is typically induced by bone-resorbing factors. By decreasing bone resorption and promoting bone formation, lutein effectively increases bone mass in developing mice. So, lutein may be a naturally occurring substance that supports bone turnover and is good for human bone health [87].
Lutein promotes the growth of new bone while inhibiting osteoclastic bone resorption. It seems plausible that NF-κB is one of the molecular targets of lutein in bone resorption since NF-κB activation is essential for RANK-dependent differentiation into osteoclasts in macrophages and for IL-1-induced RANKL production by osteoblasts. Sclerostin [a sclerosteosis (SOST) gene product] is an essential component of the negative regulator in bone development, and osteocytes and osteoblasts may play a major role. Therefore, by inhibiting canonical wingless-related integration site (Wnt)/β-catenin signaling, the augmentation of bone production by lutein may need the reduction of sclerostin expression. Research on this potential connection would be needed to establish any direct influence of lutein on sclerostin level [88].
Lutein has the ability to activate Nrf2, treating chondrocyte cells with it, demonstrated a strong protective effect against oxidative stress events produced by monosodium iodoacetate (MIA). The transcription factor Nrf2, also known as nuclear factor erythroid 2-related factor 2, is a crucial regulator of redox imbalance by up regulating ARE. Nrf2 provides cytoprotective action in response to oxidative stress, however, excessive redox circumstances cause Nrf2 to be down regulated, which promotes modifications to regular cellular functioning. In addition to a notable elevation of Nrf2, the administration of lutein demonstrated elevated expression levels of downstream genes HO-1 and NQO-1. Lutein lowered oxidative stress via increasing Nrf2 and the cytoprotective antioxidant system. A significant down regulation of inflammatory proteins (NF-κB and COX-2) and the generation of proinflammatory cytokines were seen upon lutein administration. In LPS-stimulated macrophages, lutein reduced intracellular H2O2 formation by scavenging superoxide and H2O2 as well as the NF-κB-regulated inflammatory genes, iNOS, TNF-α, IL-1β, and COX-2. In a strong correlation with its inhibitory effect on LPS-induced I-κB kinase (IKK) activation, I-κB degradation, nuclear translocation of NF-κB, and binding of NF-κB to the κB motif of the iNOS promoter, lutein suppressed LPS-induced NF-κB activation. This substance suppressed the upregulation of IKK activation caused by LPS and H2O2 in PI3K activity, phosphatase and tensin homolog (PTEN) inactivation, NF-κB-inducing kinase (NIK), and Akt phosphorylation [89, 90].
The administration of lutein considerably controlled the caspase-3 activation and loss of membrane potential brought on by MIA, hence averting chondrocyte cell death. By controlling oxidative stress and apoptosis through Nrf2 and NF-κB expression, lutein protects against osteoarthritis [91].
For the first time, the inhibitory effects of lutein on dendritic cell (DC) development and activation were identified. The expressions of three maturation-associated surface markers [CD40, co-stimulatory molecule CD86, and major histocompatibility complex (MHC) class II molecule] in LPS-stimulated DCs were first considerably and dose-dependently reduced by lutein. The most significantly impacted phenotypic change brought on by lutein exposure was CD86 expression. Furthermore, when DCs mature, they release a lot of inflammatory cytokines, which are thought to be functional indicators of DC maturation. LPS-stimulated DCs’ production of the proinflammatory cytokines IL-12 p40 and IL-6 effectively lowered following lutein administration [92]. Reducing inflammation and modifying immune responses may also be achieved therapeutically through suppressing IL-6 expression [93] and IL-6 receptors (Table 1) [86, 88, 92, 94].
Anti-diabetes effects of lutein
High levels of HDL-cholesterol (HDL-C) in the bloodstream support lutein absorption and biological activities. HDL-C is the major carrier of lutein in the circulatory system. It has been noted that inflammatory indicators including leukocyte count and C-reactive protein (CRP) are adversely correlated with serum lutein levels [95].
In the diabetic rats, proinflammatory cytokines such as TNF-α and IL-1β were found to be greatly lowered in the testicles when lutein was administered [96]. The anti-inflammatory qualities have previously been demonstrated [97]. It is hypothesized that lutein inhibited the inflammatory response by shielding the proteasome from oxidative stress-induced inactivation [83].
The Ins2Akita/+ mice activation of microglia is suppressed by lutein. This conclusion is in line with an in vitro study’s findings that lutein inhibited microglial activation, TNF-α and IL-1β release, and ROS formation [44]. Because of its ability to scavenge ROS, lutein may shield the diabetic retina from inflammatory damage. Thus, long-term lutein treatment had positive effects on the retina of Ins2Akita/+ mice, suppressing retinal inflammation, shielding the retinal vasculature, and maintaining retinal function. These findings suggest that lutein may be used as a long-term therapeutic intervention to help patients with early-stage diabetic retinopathy to avoid inflammation and retinal degeneration [98].
A dietary supplement high in lutein dramatically reduced the cerebral cortex (CCT) levels of thiobarbituric acid reactive substances (TBARS), caspase-3, TNF-α, IL-1β, and IL-6 that were elevated due to diabetes. When lutein was given as a dietary supplement to rats fed a regular diet (three different doses—40 mg/kg, 80 mg/kg, and 160 mg/kg), the inhibition of non-protein sulfhydryl groups (NP-SH), DNA, and RNA levels was dramatically reduced. This effect was shown in a dose-dependent way. After five weeks of lutein dietary supplementation, the diabetes-induced down regulation of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and insulin-like growth factor (IGF) were mitigated in the diabetic animal [99]. These factors are essential for the survival of neurons and the growth of axons. They eliminate oxidative stress and protect neurons from neuronal and myelin damage via secreting enzymes like GSH and SOD [100–102]. The results from previous studies indeed elucidated that lutein is neuro protective, in addition to its popular benefits such as antioxidant and anti-inflammatory properties. It also centralized corneal thickness associated with diabetes (Table 1) [42, 82, 96, 98, 99].
Lutein in the management of obesity and IBD
As natural agonists of the nuclear retinoic acid receptor, lutein and its metabolites may activate retinoic acid response elements found in the retinoic acid-responsive genes, regulating transcription of several genes related to adipocyte metabolism, including PPAR and CCAAT/enhancer-binding protein (CEBP). Furthermore, lutein might have an impact on how PPAR target genes are expressed. PPARα attenuates inflammation in adipose tissue, reduces adipocyte hypertrophy, and promotes β-oxidation of fatty acids. Exercise significantly increased carnitine palmitoyl transferase 1 and AMP-activated protein kinase levels while significantly lowering the serum level of lutein in an animal model treated with the supplement. Because they are involved in energy metabolism and lipid utilization, suggesting that lutein causes elevated lipid metabolism, most likely as a result of increased cellular levels of these enzymes. The lutein-treated group exhibited a significant reduction in visceral fat, as well as in serum levels of total cholesterol and LDL cholesterol. However, other parameters such as TG, HDL cholesterol, glucose homeostasis, non-esterified fatty acids (NEFA), and appetite sensations remained unchanged. When combined with low calorie diet, lutein supplements may help middle aged obese people to maintain their lipid profile and body composition [103]. Lutein, when combined with the medication, effectively inhibited the elevation of hepatic TG and cholesterol levels caused by the high-fat diet. Similarly, lutein and the combination of its medications effectively reduced the high blood glucose levels caused by high fat diet. The expression of Fas cell surface death receptor (FAS), PPARγ, and CEBP-α was down regulated in the epididymal adipose tissue by lutein. Therefore, lutein supplementation may be able to reduce diet-induced obesity and related problems in the human population [104].
In the animal model [105], lutein and zeaxanthin were found to modulate anti-inflammatory cytokine (IL-10) in the liver and jejunum as well as proinflammatory cytokines (IL-1β, IL-6, and IFN-γ) in the liver, duodenum, jejunum, and ileum. Lutein hydrogel more successfully reduced the levels of inflammatory factors, colon tissue damage, and fecal heme content in mice with colitis produced by dextran sodium sulfate (DSS). To preserve the integrity of the intestinal barrier, lutein hydrogel also inhibited the NF-κB signaling, TNF-α, iNOS, NOD-like receptor protein 3 (NLRP3), and IL-1β, it also increased the expression of intestinal tight junction proteins zonula occluden-1 (ZO-1), claudin-1, and occludin. Sodium alginate-based hydrogel as a slow-release carrier to load lutein nanoparticles was found to be really efficient in controlling colitis in mice models [106]. Lutein hydrogel also regulated the ecological imbalance of the intestinal microbiota by regulating the relative abundances of Desulfovibrionaceae (decreased), Erysipelotrichaceae and Rikenellaceae (both are increased). The sodium alginate hydrogel-based delivery system might enhance the interaction between lutein and gut microbiota to modulate colonic inflammation [107].
Huang et al. [108] designed a gallic acid/sodium alginate hybrid hydrogel (GAS) with added AG, which demonstrated enhanced resistance to gastric acid and improved adhesion in the GIT. Alginate hybrid hydrogel can help lessen ulcerative colitis by controlling the polarization of macrophages, reducing the expression of inflammatory markers, and enhancing the function of the intestinal mucosal barrier. But there was no significant alteration in the lutein’s ability to suppress the expression of inflammatory factors when the lutein nanoparticles were filled in sodium alginate hydrogel. In a recent investigation involving 56 individuals in remission from ulcerative colitis, when monitored for gastrointestinal symptoms, one of the main concerns for many of them was recurring constipation. Increased intake of lutein and zeaxanthin may help reduce the likelihood of constipation and thus contribute to enhancing quality of life (Table 1) [104, 106, 109].
Lutein suppressed skin inflammation
Studies have shown that lutein effectively suppressed various skin inflammatory responses in UV-irradiated keratinocytes, IFN-γ/TNF-α-stimulated human keratinocyte cell line (HaCaT) cells, and LPS-treated macrophages, reducing the production of IL-6, COX-2, and matrix metalloproteinase-9 (MMP-9). Lutein also mitigates cutaneous inflammation by preventing neutrophil accumulation, which is induced by the activation of the transient receptor potential ankyrin 1 [110]. Further analysis of nuclear transcription factor levels and intracellular signaling pathways revealed that lutein can inhibit the activation of the redox-sensitive AP-1 pathway. This AP-1-targeted anti-inflammatory effect is attributed to lutein’s antioxidant activity, which involves scavenging free radicals and ROS. Consequently, lutein is proposed as a potential anti-inflammatory and cosmetic agent for treating inflammatory skin conditions due to its anti-oxidative properties (Table 1) [32, 107, 110].
Anti-cancer effects of lutein
Lung cancer
Studies by Zhang et al. [111] elucidated the anti-cancer properties of lutein in lung cancer cell line A549. Lutein induced apoptosis in A549 via inhibiting the PI3K/Akt signaling pathway [111]. In non-small cell lung cancer cells, lutein dose-dependently reduced cell division, arrested cells at G0/G1 phase, and triggered apoptosis. The mechanical stimulation of DNA damage and subsequent activation of the ataxia telangiectasia mutated and Rad3-related kinase (ATR)/checkpoint kinase 1 (Chk1)/p53 signaling pathway in A549 cells treatment with lutein increases the level of pro-apoptotic protein B-cell lymphoma 2 (BCL-2) and reduces the BCL-2-associated X protein (BAX) protein which is antiapoptotic. Moreover, lutein suppressed the expression of the SRY-related HMG-box protein 2 (SOX2) and Nanog homeobox (NANOG) genes in A549. It also increased CD44 and CD133 gene expression but decreased solute carrier family 11 member A1 (SLCA11) gene expression (Table 2) [32, 111, 112].
Anti-cancer effects of lutein
| Sl.NO | Type of cancer | Mechanism of action | Reference |
|---|---|---|---|
| 1 | Lung cancer, A549 cell lines | Induce apoptosis—PI3K/Akt signaling pathway and ATR/Chk1/p53 signaling pathway↑Expression of BCL-2 and ↓expression of BAX proteinSuppress expression of SOX2 and NANOG genes in A549↑Expression of CD44 and CD133, ↓expression of SLCA11 gene | [79–81] |
| 2 | Gastric cancer, AGS cell lines | Induce apoptosis—↑DNA fragmentation, caspase-3 activity, the BAX/BCL-2 ratio, and NADPH oxidase-mediated ROS generation | [82] |
| 3 | Hepatic cancer, HepG2 cell lines | G2/M phase cell cycle arrest and the phosphorylation of Cdk1 and p53Alter the phosphorylation of ERK, JNK, and Akt in hyperglycemic cell lines | [83, 84] |
| 4 | Prostate cancer, PC-3 cell lines | ↑Drug-induced cell cycle arrest and apoptosis, and also increased the expression of biomarker genes linked to death and proliferation in PC-3 cells | [85] |
↑: increasing; ↓: decreasing. PI3K: phosphatidylinositol 3-kinase; ROS: reactive oxygen species; ATR: ataxia telangiectasia mutated and Rad3-related kinase; Chk1: checkpoint kinase 1; BCL-2: B-cell lymphoma 2; BAX: BCL-2-associated X protein; SOX2: SRY-related HMG-box protein 2; NANOG: Nanog homeobox; SLCA11: solute carrier family 11 member A1; NADPH: nicotinamide adenine dinucleotide phosphate; ERK: extracellular signal-regulated kinase; AGS: gastric adenocarcinoma; Hep: hepatic; Cdk1: cyclin-dependent kinase 1; JNK: c-Jun N-terminal kinase; Akt: protein kinase B; PC-3: prostate cancer cell line
Gastric cancer
In human gastric cancer cell line gastric adenocarcinoma (AGS), lutein inhibited viability and colony formation in a dose-dependent manner. DNA fragmentation, caspase-3 activity, and the BAX/BCL-2 ratio were elevated, suggesting apoptosis induced by Lutein. Intracellular ROS and the activity of NOX were also elevated. Lutein induces apoptosis in stomach cancer cells by increasing NOX-mediated ROS generation (Table 2) [113].
Hepatic cancer
Sindhu et al. [114] reported that both in vitro and in vivo, lutein has the ability to potently block phase I enzymes, which may prevent carcinogen activation. In the in vitro study, the concentration of lutein needed to inhibit phase I enzymes 50% was less than 12 mg/mL for CYP1A1, CYP1A2, CYP2B1/2, aniline hydroxylase, and aminopyrine-N-demethylase. In in vivo studies, lutein markedly and dose-dependently enhanced its inhibition of 7-ethoxyresorufin-O-deethylase (EROD), 7-pentoxy-resorufin-O-depentylase (PROD), and 7-methoxyresorufin-O-methylase (MROD) activity. Enzymes involved in phase II drug metabolism are responsible for converting a foreign substance into its excretable metabolite. In rats, lutein significantly and dose-dependently boosted the activity of phase II enzymes, including GST and uridine diphosphate (UDP) glucuronyl transferase. These findings suggest that lutein’s chemo preventive effect in N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinoma (HCC) in rats may be attributed mainly to lutein’s inhibitory activity in CYP enzymes, both in vitro and in vivo, as well as increases in phase I enzyme activity in vivo in rats [114, 115]. According to Moreno et al. [116], lutein functions as a suppressor of hepatocarcinogenesis by inhibiting, but not blocking, the processes involved in the early stages of the disease. Suppression of high glucose-induced cell proliferation in HepG2 cell line model by lutein was linked to increased expression of high glucose-mediated repressed HO-1 and prevention of hyperglycemia-mediated elevated ROS, but it was not associated with the induction of apoptosis. Furthermore, it was discovered that lutein-mediated inhibition of hyperglycemia-mediated cell proliferation was connected to G2/M phase cell cycle arrest and the phosphorylation of Cdk1 and P53. It also altered the phosphorylation of ERK, c-Jun N-terminal kinase (JNK), and Akt in hyperglycemic HepG2 cells and reduced the hyperglycemia-induced activation of P38, which is related to high glucose-induced ROS-mediated suppression of growth (Table 2) [114–117].
Prostate cancer
The effect of lutein on genes linked to survival and proliferation in using cell line model-prostate cancer [prostate cancer cell line (PC-3)] cells revealed lutein-induced inhibition of PC-3 cell proliferation in vitro, and this inhibition was enhanced by PPARγ agonists and other chemotherapeutic drugs. Lutein enhanced drug-induced cell cycle arrest and apoptosis, and also increased the expression of biomarker genes linked to death and proliferation in PC-3 cells. These results demonstrate that in PC cells, lutein regulates the expression of genes linked to growth and survival (Table 2) [118].
Conclusions
Lutein, a prevalent carotenoid found in fruits, vegetables, and flowers, has emerged as a promising candidate for promoting human health. This review summarizes the multifaceted properties of lutein, exploring its potential applications in various medical conditions. It efficiently scavenges free radicals, protecting cells from oxidative damage implicated in numerous chronic diseases. This antioxidant action may contribute to its potential benefits for eye health, cardiovascular function, and age-related cognitive decline. Its abundance in the macula underscores its significance in eye health. Research suggests that lutein may help prevent AMD and cataracts by filtering blue light and neutralizing free radicals that damage retinal cells. Studies indicate its potential role in reducing inflammation, a key player in various diseases. Lutein may modulate inflammatory pathways, offering benefits for conditions like arthritis and IBD. Additionally, research suggests its potential to protect against cognitive decline, cardiovascular diseases, and various cancers. The mechanism of action of the various effects is also summarized. Many of these effects are through its antioxidant and anti-inflammatory properties. Lutein’s interaction with specific cellular signaling pathways could contribute to its protective effects across various organ systems.
Considerations and future directions
The diverse biological activities of lutein position it as a valuable nutrient for promoting overall health while managing chronic diseases through its antioxidant and anti-inflammatory properties. Further studies are needed to fully elucidate its mechanisms of action and optimal dosages for specific health conditions. Additionally, exploring the synergistic effects of lutein with other chemo therapeutic drugs and dietary components can provide valuable insights into developing an efficient therapeutic regime and optimizing dietary strategies. The role of lutein in eye health is well established, and research into its border benefits for the brain, cardiovascular system, skin, and other organs is expanding. Further studies and clinical trials are needed to fully understand its therapeutic potential and the improved bioavailability of lutein via nanocrystals incorporated into rapidly dissolving films for oral consumption is a new area of exploratory research. Thus, lutein paves the way for its integration into preventive and treatment strategies.
Abbreviations
| AMD: | age-related macular degeneration |
| AP-1: | activator protein-1 |
| BCL-2: | B-cell lymphoma 2 |
| COX: | cyclooxygenase |
| CYP2E1: | cytochrome P450 2E1 |
| DC: | dendritic cell |
| DHA: | docosahexaenoic acid |
| GITs: | gastrointestinal tracts |
| GPx: | glutathione peroxidase |
| GSH: | glutathione |
| GST: | glutathione S-transferase |
| HDL: | high-density lipoprotein |
| HO-1: | heme oxygenase-1 |
| IBD: | inflammatory bowel disease |
| IL-10: | interleukin-10 |
| iNOS: | inducible nitric oxide synthase |
| I-κB: | inhibitor of kappa B |
| JAK: | Janus kinase |
| Keap1: | Kelch-like ECH-associated protein 1 |
| LDL: | low-density lipoprotein |
| LPS: | lipopolysaccharide |
| MDA: | malondialdehyde |
| MI: | myocardial infarction |
| MIAT: | myocardial infarction associated transcript |
| miR: | micro-RNA |
| NADPH: | nicotinamide adenine dinucleotide phosphate |
| NF-κB: | nuclear factor kappa B |
| NOX: | nicotinamide adenine dinucleotide phosphate oxidase |
| Nrf2: | nuclear factor erythroid 2-related factor 2 |
| NSAIDs: | non-steroidal anti-inflammatory drugs |
| PC-3: | prostate cancer cell line |
| PI3K: | phosphatidylinositol 3-kinase |
| PPARα: | peroxisome proliferator-activated receptor alpha |
| RANKL: | receptor activator of nuclear factor kappa B ligand |
| ROS: | reactive oxygen species |
| RXRα: | retinoid X receptor alpha |
| SOD: | superoxide dismutase |
| STAT: | signal transducer and activator of transcription |
| TG: | triglycerides |
| TNF-α: | tumor necrosis factor-α |
Declarations
Acknowledgments
The authors thank all colleagues who contributed to the preparation of this manuscript, providing support through its development, assisting in the formatting, and making final corrections. During the preparation of this work, authors used the Microsoft CoPilot for generating the guinea pig image. After using the tool, authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Author contributions
PP, ATT, PPB, and SVB: Writing—review & editing. GV: Visualization, Writing—original draft, Writing—review & editing. ERS: Conceptualization, Writing—review & editing.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.