Affiliation:
1University Clinical Centre of the Republic of Srpska, 78000 Banja Luka, Bosnia and Herzegovina
ORCID: https://orcid.org/0009-0003-0657-5112
Affiliation:
1University Clinical Centre of the Republic of Srpska, 78000 Banja Luka, Bosnia and Herzegovina
ORCID: https://orcid.org/0009-0009-7168-5258
Affiliation:
1University Clinical Centre of the Republic of Srpska, 78000 Banja Luka, Bosnia and Herzegovina
ORCID: https://orcid.org/0009-0007-0087-9173
Affiliation:
2Market Support Sector, Bosnalijek d.d., 71000 Sarajevo, Bosnia and Herzegovina
ORCID: https://orcid.org/0000-0002-9217-7473
Affiliation:
3Medical support department, Bosnalijek d.d., 71000 Sarajevo, Bosnia and Herzegovina
ORCID: https://orcid.org/0000-0003-3378-6954
Affiliation:
3Medical support department, Bosnalijek d.d., 71000 Sarajevo, Bosnia and Herzegovina
ORCID: https://orcid.org/0000-0003-0002-7150
Affiliation:
4Scientific Research Unit, Bosnalijek d.d., 71000 Sarajevo, Bosnia and Herzegovina
5Department of Pharmaceutical Biochemistry and Laboratory Diagnostics, University of Sarajevo-Faculty of Pharmacy, 71000 Sarajevo, Bosnia and Herzegovina
Email: UnaG@Bosnalijek.ba
ORCID: https://orcid.org/0000-0003-1206-6990
Explor Drug Sci. 2025;3:100886 DOI: https://doi.org/10.37349/eds.2025.100886
Received: October 03, 2024 Accepted: December 04, 2024 Published: January 24, 2025
Academic Editor: Fernando Albericio, University of KwaZulu-Natal, South Africa, Universidad de Barcelona, Spain
Aim: The aim of this study was to examine the effectiveness and safety of lysozyme-based spray in the treatment of oral mucositis in patients undergoing head and neck radiotherapy.
Methods: A prospective, open-label study was conducted on patients with ulcerative inflammation of the oral cavity and pharynx mucous membranes clinically assessed for oral mucositis according to the World Health Organization (WHO) Oral Toxicity Scale. Patients were randomly divided into a lysozyme group (using a spray containing lysozyme + cetylpyridinium + lidocaine) and a control group (using a compounded preparation containing gentamicin + dexamethasone + lidocaine). The efficacy and safety of therapy were evaluated on the baseline and three follow-up visits (7, 14, and 21 days after the baseline visit).
Results: The total number of participants was 56, of which 26 were in lysozyme and 30 in the control group. The efficacy parameters were similar between the groups and there was no deterioration of symptoms during the follow-up period of 21 days. A significantly lower pain intensity when eating solid food was observed after 21 days in lysozyme compared to the control group. No adverse reactions were observed.
Conclusions: This study showed the efficacy and safety of lysozyme-based spray for treating radiotherapy-induced oral mucositis. The availability of new treatment options based on lysozyme, a natural enzybiotic present in the saliva of healthy subjects, could bring added value to the treatment of oral mucositis and the prevention of its complications. However, a larger randomized, blinded study is needed to confirm our results [the study was registered at the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina (https://klinicka.almbih.gov.ba/pages/klinicka-registar-javni) under the protocol number LCS-OM-01].
Oral mucositis represents ulcerative inflammatory changes of oral mucosa due to the toxic effects of chemo- or radiotherapy. It is among the most important challenges in oncology patients’ treatment, especially in head and neck carcinoma [1]. It is initiated by injury of epithelial cells leading to inflammatory changes in the oral mucosa. Depending on the grade, ulcerations can develop and affect the deepest submucosal tissues with microbial infections leading to exacerbation. Among the key aspects of treatment are tissue regeneration, immunomodulation, antimicrobial activity, and pain management [2]. Adequate therapy timing (chronotherapy) could decrease the risk of severe oral mucositis development [3]. Hospital pharmacists and nurses have a crucial role in managing oral mucositis. They implement oral care protocols and treatments and educate patients about self-care at home [4, 5]. Despite the influence of oral mucositis on quality of life and anti-cancer treatment success, prevention and treatment options are scarce [1]. Guidelines combine good oral hygiene, nutrition, and various therapeutics [6]. Palifermin is the only approved drug for the treatment of oral mucositis in hematologic patients or as supportive care for preparative regiments in patients predicted to develop oral mucositis of grade 3 according to the World Health Organization (WHO) Oral Toxicity Scale [7]. It is a recombinant human keratinocyte growth factor 1 which is stimulating the proliferation of epithelial cells [1]. There are many compounded preparations, usually specific for certain clinical sites, that are also used. Benzydamine mouthwashes and oral glutamine [8, 9] together with povidone-iodine, sodium bicarbonate, and natural compounds [10] are often recommended. Photobiomodulation is also a successful and cost-effective approach to the prevention and therapy of oral mucositis [11–13]. However, a standard oral care protocol for oral mucositis is still not available [14].
There are several activities that oral mucositis therapeutics should have. In the first place, anti-inflammatory, antimicrobial, and pro-regenerative activities are beneficial. Also, immunomodulatory effects are needed to overcome exaggerated immune response aggravating oral mucositis conditions. Many therapeutics have pain-reducing effects to prevent decreased food and beverage intake and worsening of patients’ general condition [1]. The natural component of healthy saliva, lysozyme, is among the compounds with several activities beneficial in the treatment of oral mucositis [15, 16]. Eminagić et al. [15] conducted an observational study and demonstrated the advantages of lysozyme-based compounds in the treatment of oral mucositis due to radio- or chemotherapy. Rasic et al. [16] showed that a spray containing lysozyme, cetylpyridinium, and lidocaine is effective and safe in the treatment of oral mucositis. Lysozyme plays a crucial role in innate immunity and has antimicrobial and immunomodulatory effects on the skin and mucous membranes in our body. It kills bacteria mainly by hydrolysis of peptidoglycans or by ionic interactions with the cell wall [17]. Lysozyme also interacts with nucleic acids and possesses antiviral properties [18]. Immunomodulatory effects are partly based on the production of peptidoglycan fragments stimulating immune response [17]. Lysozyme also shows anti-inflammatory effects [19] through interaction with complement and chemotaxis of activated leukocytes [20]. Its anti-nociceptive [19] and analgesic properties are described [21], and studies are showing the pro-regenerative and wound-healing properties of this enzyme [22]. Bianchi showed that lysozyme inhibits the conversion of arachidonic acid into prostaglandins while having no direct effect on the action of prostaglandins themselves [21]. Due to its natural origin and all the mentioned activities, lysozyme could bring added value to oral mucositis treatment protocols. Additional studies are needed to evaluate lysozyme-based medicines in the treatment of oral mucositis.
The aim of this study was to evaluate the effectiveness and safety of lysozyme-based spray compared to mouthwash preparation commonly used in the hospital pharmacy in reducing pain, erythema, and ulceration of the oral cavity and pharynx mucous membranes in patients undergoing head and neck radiotherapy.
A post-marketing, prospective randomized open-label study was conducted at the University Clinical Center of the Republic of Srpska, Banja Luka, Bosnia and Herzegovina from August 2020 until April 2022. The study was registered with the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina under the protocol number LCS-OM-01.
Adult patients with ulcerative inflammation of the oral cavity and pharynx mucous membranes were clinically assessed for oral mucositis according to the WHO Oral Toxicity Scale [7]. Patients were randomly divided into two groups: lysozyme group (using batches 0969, 1049, and 6024, Lysobact Complete Spray®, Bosnalijek d.d., Bosnia and Herzegovina, containing lysozyme hydrochloride 20.0 mg + cetylpyridinium chloride 1.5 mg + lidocaine hydrochloride 0.5 mg per 1 mL of solution) and control group (using compounded preparation, containing gentamicin sulfate 2.88 mg + dexamethasone 0.04 mg + lidocaine hydrochloride 10 mg per 1 mL of solution). For simple randomization, the R package “randomizr” (version 0.20.0) was used [23]. The study was open-label and both patients and clinicians were aware of applied therapy. Both groups applied therapies 3–6 times a day for 21 days, with minimum intervals of 2 hours between doses. No preventive therapies were applied before oral mucositis onset. During the study, patients used no other topical compounds.
Inclusion criteria were ongoing head and neck radiotherapy and age 18 years and older. Exclusion criteria were hypersensitivity to the active compounds and/or excipients of the drug, hypersensitivity to egg whites, pregnancy, and lactation. Withdrawal criteria were worsening of the underlying disease, development of serious side effects that require discontinuation of the therapy under investigation and/or discontinuation of oncology therapy, and the appearance of symptoms such as deeper oral ulcerations, extremely severe pain that requires the inclusion of additional drugs and the complete absence of the possibility of taking solid and liquid food.
Before the study started, a statistical plan was prepared and the sample size was calculated. No previous study compared the lysozyme-based spray and compounded preparation that were used in our study. The sample size was calculated for the proportions of patients with normal findings in each of the groups. A large effect size was expected based on the results published by Rasic et al. [16] that compared the same lysozyme-based spray with standardized bicarbonate-based preparations. The calculated minimum number of patients per group was 25. For the analysis, R: A Language and Environment for Statistical Computing [24] version 3.6.3. and the package “pwr” [25] (version 1.3-0) were used.
Data was collected on the baseline and three follow-up visits (7, 14, and 21 days after the baseline visit). The efficacy of therapy was evaluated by two approaches. One approach was evaluation by a medical doctor performing physical examination of lips, cheeks, tongue, and palate at each visit and recording results according to the WHO Oral Toxicity Scale [7]. The second approach was self-assessment and completion of a survey containing questions about pain presence and intensity when eating solid and soft food and speaking. Pain intensity was measured by a visual analog scale (VAS) from zero (no pain) to ten (the strongest possible pain). Therapy safety was monitored based on the frequency of occurrence and type of adverse events during the study.
The clinical trial was approved by the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina, number 08-07.5-6730-1/20 on July 20, 2020. The Helsinki Declaration from 1975 and its amendments from 1983 were followed in all procedures. Before any procedure started, each participant signed an informed consent form.
The usual descriptive statistical analysis (absolute and relative numbers) was applied. The normality of data distribution was evaluated by inspection of histograms, box plots, and Q-Q plots and analysis using the Shapiro-Wilk test. For normally distributed variable without outliers (the number of days from the start of radiotherapy to inclusion in the trial), Levene’s test for equality of variances was used to assess the similarity of variances, and then the t-test was used to compare groups. The Mann-Whitney U test was used to compare age, local findings on the lips, cheeks, tongue, and palate, visual assessment of pain intensity when eating solid food, eating soft food, and speaking. Chi-square or Fisher’s Exact test, depending on the number of units in the cells, was used to compare categorical variables between groups. The Friedman test was used to assess the difference in local findings on the lips, cheeks, tongue, and palate, as well as the visual assessment of the pain intensity when eating solid or soft food, and speaking at four visits. For comparison between the two measurement times, the Wilcoxon signed rank test with Bonferroni correction of the p-value was used (p < 0.008 was calculated as a statistically significant value). Cochran’s Q test was used to compare the presence of pain at the four measurements when eating solid or soft food or speaking. Before the analysis, the sample size was estimated, and for analyses where a small sample size was observed (n × k < 24, n representing the number of subjects and k representing the number of related groups) exact Cochran’s Q test was used while Cochran’s Q test was used for the others.
All tests were two-sided with p < 0.05 accepted as a statistically significant difference, except in the case of tests where the Bonferroni correction was applied. Statistical analysis was performed using the SPSS (Statistical Package for Social Sciences) program version 23.0 and using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria) version 4.2.2. Visualization was performed by using the “ggplot2” package [26] version 3.4.0.
The total number of participants was 56, of which 26 were in lysozyme and 30 in the control group. There was no difference between groups regarding age (U = 335, p = 0.370), sex (p = 0.200, Fisher’s Exact Test), and the number of days from the start of radiotherapy to inclusion in the trial with a mean value of 14 days [t (54) = 0.359, p = 0.721]. Also, there were similar characteristics of patients regarding the tumor site and Tumor, Node, Metastasis (TNM) classification (Table 1).
Baseline characteristics of participants
| Parameter | All participants(n = 56) | Lysozyme group(n = 26) | Control group(n = 30) | p-value lysozyme vs. control group |
|---|---|---|---|---|
| Age, years* | 65 (57–68) | 66 (59–70) | 64 (54–68) | 0.370 |
| Sex, male‡ | 50 (89) | 25 (96) | 25 (83) | 0.200 |
| Number of days from radiotherapy start to enrollment† | 14 (± 6) | 14 (± 7) | 14 (± 6) | 0.721 |
| Tumor site‡ | 0.400 | |||
| Oral cavity | 20 (35.7) | 7 (26.9) | 13 (43.3) | |
| Larynx | 19 (33.9) | 9 (34.6) | 10 (33.3) | |
| Pharynx | 12 (21.4) | 7 (26.9) | 5 (16.7) | |
| Larynx, pharynx | 4 (7.1) | 3 (11.5) | 1 (3.3) | |
| Nasal cavity | 1 (1.8) | 0 (0.0) | 1 (3.3) | |
| T stage‡ | > 0.900 | |||
| 0 | 2 (3.6) | 1 (3.8) | 1 (3.3) | |
| 1 | 1 (1.8) | 1 (3.8) | 0 (0.0) | |
| 2 | 13 (23.2) | 6 (23.1) | 7 (23.3) | |
| 3 | 17 (30.4) | 8 (30.8) | 9 (30.0) | |
| 4 | 8 (14.3) | 3 (11.5) | 5 (16.7) | |
| 4a | 10 (17.9) | 4 (15.4) | 6 (20.0) | |
| 4b | 4 (7.1) | 2 (7.7) | 2 (6.7) | |
| X | 1 (1.8) | 1 (3.8) | 0 (0.0) | |
| N category‡ | 0.200 | |||
| 0 | 18 (32.1) | 12 (46.2) | 6 (20.0) | |
| 1 | 5 (8.9) | 3 (11.5) | 2 (6.7) | |
| 2 | 10 (17.9) | 4 (15.4) | 6 (20.0) | |
| 2b | 11 (19.6) | 2 (7.7) | 9 (30.0) | |
| 2c | 3 (5.4) | 2 (7.7) | 1 (3.3) | |
| 3 | 8 (14.3) | 3 (11.5) | 5 (16.7) | |
| 3b | 1 (1.8) | 0 (0.0) | 1 (3.3) | |
| M category‡ | 0.700 | |||
| 0 | 53 (94.6) | 24 (92.3) | 29 (96.7) | |
| 1 | 2 (3.6) | 1 (3.8) | 1 (3.3) | |
| X | 1 (1.8) | 1 (3.8) | 0 (0.0) |
Data are presented as † mean (± standard deviation); * median (interquartile range, IQR), or as an ‡ absolute number (percentage of the total number of respondents in the subject group)
The efficacy parameters evaluated by medical doctors were similar between the groups. Both treatments decreased the number of patients with local findings after 21 days compared to the baseline (Table 2). Local findings at the palate were the most common, followed by local findings at cheeks and tongue. Local findings at lips were present in a small number of patients (Table 2). A Friedman test was performed to determine whether there were differences in local findings for the lips, cheeks, tongue, and palate over time for each group individually. No significant difference and no deterioration of symptoms within both groups was found.
Local findings at palate, cheeks, tongue, and lips according to the World Health Organization (WHO) Oral Toxicity Scale [7]. The lysozyme group was treated with a spray containing lysozyme hydrochloride + cetylpyridinium chloride + lidocaine, control group was treated with a compounded preparation containing gentamicin sulfate + dexamethasone + lidocaine
| Parameter | Baseline | After 7 days | After 14 days | After 21 days |
|---|---|---|---|---|
| Local findings—palate | ||||
| Lysozyme group (n = 26) | G0: 1 (4); G1: 22 (85); G2: 2 (8); G3: 1 (4) | G0: 1 (4); G1: 22 (85); G2: 2 (8); G3: 1 (4) | G0: 1 (4); G1: 23 (88); G2: 2 (8); G3: 0 (0) | G0: 3 (12); G1: 19 (73); G2: 3 (12); G3: 1 (4) |
| Control group (n = 30) | G0: 2 (7); G1: 23 (77); G2: 5 (17); G3: 0 (0) | G0: 1 (3); G1: 28 (93); G2: 1 (3); G3: 0 (0) | G0: 3 (10); G1: 23 (77); G2: 4 (13); G3: 0 (0) | G0: 2 (7); G1: 21 (70); G2: 5 (17); G3: 2 (7) |
| p-value | 0.868 | 0.367 | 0.980 | 0.363 |
| Local findings—cheeks | ||||
| Lysozyme group (n = 26) | G0: 19 (73); G1: 5 (19); G2: 1 (4); G3: 1 (4) | G0: 20 (77); G1: 5 (19); G2: 1 (4); G3: 0 (0) | G0: 21 (81); G1: 4 (15); G2: 1 (4); G3: 0 (0) | G0: 20 (77); G1: 3 (12); G2: 2 (8); G3: 1 (4) |
| Control group (n = 30) | G0: 21 (70); G1: 6 (20); G2: 3 (10); G3: 0 (0) | G0: 22 (73); G1: 7 (23); G2: 1 (3); G3: 0 (0) | G0: 22 (73); G1: 6 (20); G2: 2 (7); G3: 0 (0) | G0: 20 (67); G1: 4 (13); G2: 6 (20); G3: 0 (0) |
| p-value | 0.804 | 0.777 | 0.503 | 0.408 |
| Local findings—tongue | ||||
| Lysozyme group (n = 26) | G0: 13 (50); G1: 10 (38); G2: 2 (8); G3: 1 (4) | G0: 18 (69); G1: 7 (27); G2: 1 (4); G3: 0 (0) | G0: 15 (58); G1: 8 (31); G2: 3 (12); G3: 0 (0) | G0: 16 (62); G1: 6 (23); G2: 3 (12); G3: 1 (4) |
| Control group (n = 30) | G0: 19 (63); G1: 8 (27); G2: 3 (10); G3: 0 (0) | G0: 16 (53); G1: 13 (43); G2: 1 (3); G3: 0 (0) | G0: 16 (53); G1: 10 (33); G2: 4 (13); G3: 0 (0) | G0: 12 (40); G1: 14 (47); G2: 4 (13); G3: 0 (0) |
| p-value | 0.317 | 0.257 | 0.740 | 0.233 |
| Local findings—lips | ||||
| Lysozyme group (n = 26) | G0: 24 (92); G1: 1 (4); G2: 1 (4); G3: 0 (0) | G0: 25 (96); G1: 1 (4); G2: 0 (0); G3: 0 (0) | G0: 24 (92); G1: 1 (4); G2: 1 (4); G3: 0 (0) | G0: 24 (92); G1: 1 (4); G2: 0 (0); G3: 1 (4) |
| Control group (n = 30) | G0: 25 (83); G1: 4 (13); G2: 1 (3); G3: 0 (0) | G0: 26 (87); G1: 3 (10); G2: 1 (3); G3: 0 (0) | G0: 27 (90); G1: 2 (7); G2: 1 (3); G3: 0 (0) | G0: 27 (90); G1: 0 (0); G2: 3 (10); G3: 0 (0) |
| p-value | 0.338 | 0.213 | 0.778 | 0.765 |
Data are presented as an absolute number (percentage of the total number of respondents in the subject group). Grade 0 (G0): healthy; Grade 1 (G1): soreness/erythema; Grade 2 (G2): erythema, ulcers, can eat solids; Grade 3 (G3): ulcers, requires liquid diet only [7]
After 21 days, pain was present mainly when eating solid food, while a small number of patients experienced pain when eating soft food or speaking. A significantly lower pain intensity when eating solid food after 21 days was observed in the lysozyme group compared to the control group. Also, the median intensity of pain when eating soft food or speaking after 21 days was lower in lysozyme compared to the control group, but these differences were not statistically significant (Figure 1, Table 3).
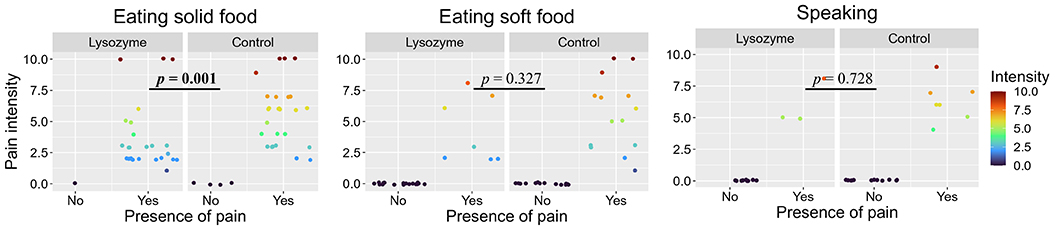
Presence and intensity of pain after 21 days of therapy with lysozyme-based spray (containing lysozyme hydrochloride 20.0 mg + cetylpyridinium chloride 1.5 mg + lidocaine hydrochloride 0.5 mg per 1 mL of solution) or control compounded preparation (containing gentamicin sulfate 2.88 mg + dexamethasone 0.04 mg + lidocaine hydrochloride 10 mg per 1 mL of solution). Bolded p-value is significant at the 0.05 level
Presence of pain and pain intensity [on visual analog scale (VAS) from 0: no pain to 10: the strongest possible pain] in participants experiencing presence of pain. The lysozyme group was treated with a spray containing lysozyme hydrochloride + cetylpyridinium chloride + lidocaine, control group was treated with a compounded preparation containing gentamicin sulfate + dexamethasone + lidocaine
| Visit | Parameter | All participants(n = 56) | Lysozyme group(n = 26) | Control group(n = 30) | p-value lysozyme vs. control group |
|---|---|---|---|---|---|
| Eating solid food | |||||
| Baseline | Presence of pain | 52 (93) | 23 (88) | 29 (97) | 0.328 |
| Pain intensity | 4.0 (3.0–5.3) | 4.0 (3.0–4.5) | 5.0 (3.0–6.0) | 0.181 | |
| After 7 days | Presence of pain | 52 (93) | 24 (92) | 28 (93) | > 0.900 |
| Pain intensity | 4.0 (3.0–5.3) | 4.0 (3.0–5.3) | 4.0 (4.0–5.3) | 0.509 | |
| After 14 days | Presence of pain | 53 (95) | 25 (96) | 28 (93) | > 0.900 |
| Pain intensity | 4.0 (3.0–6.0) | 3.0 (3.0–5.0) | 5.0 (3.0–6.5) | 0.062 | |
| After 21 days | Presence of pain | 51 (91) | 25 (96) | 26 (87) | 0.358 |
| Pain intensity | 4.0 (2.3–6.0) | 3.0 (2.0–4.0) | 6.0 (3.3–7.0) | 0.001 | |
| Eating soft food | |||||
| Baseline | Presence of pain | 25 (45) | 11 (42) | 14 (47) | > 0.900 |
| Pain intensity | 2.0 (2.0–4.0) | 2.0 (2.0–3.5) | 2.5 (2.0–5.0) | 0.440 | |
| After 7 days | Presence of pain | 28 (50) | 11 (42) | 17 (57) | 0.422 |
| Pain intensity | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 2.0 (2.0–4.0) | 0.434 | |
| After 14 days | Presence of pain | 23 (41) | 7 (27) | 16 (53) | 0.059 |
| Pain intensity | 4.0 (2.8–6.0) | 3.0 (2.5–7.0) | 4.0 (2.9–6.0) | 0.892 | |
| After 21 days | Presence of pain | 21 (38) | 7 (27) | 14 (47) | 0.170 |
| Pain intensity | 5.0 (3.0–7.0) | 3.0 (2.0–6.5) | 5.5 (3.0–7.0) | 0.327 | |
| During speech | |||||
| Baseline | Presence of pain | 9 (16) | 3 (12) | 6 (20) | 0.481 |
| Pain intensity | 4.0 (3.0–4.5) | 3.0 (2.5–4.0) | 4.0 (4.0–4.4) | 0.356 | |
| After 7 days | Presence of pain | 10 (18) | 3 (12) | 7 (23) | 0.310 |
| Pain intensity | 3.0 (2.0–4.8) | 2.0 (1.5–3.5) | 4.0 (2.0–5.0) | 0.345 | |
| After 14 days | Presence of pain | 7 (13) | 2 (8) | 5 (17) | 0.436 |
| Pain intensity | 6.0 (4.0–7.0) | 5.5 (4.8–6.3) | 6.0 (4.0–7.0) | 0.839 | |
| After 21 days | Presence of pain | 10 (18) | 3 (12) | 7 (23) | 0.310 |
| Pain intensity | 6.0 (5.0–7.0) | 5.0 (5.0–6.5) | 6.0 (5.5–7.0) | 0.728 | |
Data are presented as an absolute number (percentage of the total number of respondents in the subject group) or as a median (interquartile range, IQR)
Most of the subjects had palatal sensitivity recorded throughout the entire test period. In the control group, 21 days after the start of therapy, ulcerations developed in 2 subjects, while in the lysozyme group, one subject had a normal palate with no changes (Table 3). During the study, no adverse reactions to applied treatments were observed.
Both groups (lysozyme and control) achieved proper oral mucositis management with no significant deterioration of oral mucosa condition during 21 days of radiotherapy. The efficacy of both therapies was evident as a slight decrease in the oral mucositis grade at the palate, cheeks, tongue, or lips. The results were not statistically significant indicating mild effects towards healing but also activity against further development of oral mucositis. Also, the presence and intensity of pain did not significantly change during the follow-up. It is important to point out that in the lysozyme group, the percentage of patients with pain when eating solid food increased but the pain intensity decreased during the 21 days of follow-up, while the opposite results were seen in the control group. In the lysozyme group, the percentage of patients with pain during soft food eating decreased but the pain intensity increased from two to three, while in the control group, the percentage of patients with pain remained the same, but the pain intensity increased from three to six. While differences were not statistically significant, clinical significance should be considered. However, measurement of the minimum clinically important difference (MCID) is very challenging when different types and intensities of pain are analyzed [27]. The only statistically significant difference between groups was determined for decreased pain intensity when eating solid food after 21 days of follow-up in lysozyme compared to the control group. Although the control group received a higher dose of lidocaine compared to the lysozyme group, a better reduction of pain in the lysozyme group could be due to the direct effects of lysozyme. Hen egg white lysozyme effects on pain were investigated in mice where the alleviation of static mechanical pain was observed. Lysozyme caused epigenetic changes and influenced the NRF1-Parkin-TACAN signaling axis in sensory neurons [28]. Also, antimicrobial [29] and immu-nomodulatory [30] effects of lysozyme could lead to decreased pain due to inflammation. Additional studies are needed to confirm and explain in more detail the obtained results.
The form of a spray in the lysozyme group could have an advantage compared to mouthwash applied in the control group. When the oropharynx is the therapy target, spray has advantages over oral rinse. Patients need to be educated on appropriate rinsing techniques to achieve the maximum therapeutic potential of mouthwashes [31]. Very often, this education of patients is performed by nurses. The form of spray offers advantages due to easier application and less time needed for the education of patients [31].
This study had several limitations. Different forms of applied therapies (the lysozyme group received therapy in the form of mouth spray while the control group received therapy in the form of mouthwash) could lead to different therapy compliance. However, therapy compliance was not monitored. The small number of participants was based on the sample size calculation. Only one study could be used as a reference, and based on that study, the effect size was large [16]. However, the difference between groups in this study was not observed potentially due to a small sample size. The study was open-label which could lead to bias. Also, the study was conducted at only one clinical center. Another randomized, blinded, multicentric study on a larger number of patients should be performed with the same spray containing lysozyme or without lysozyme to confirm the role of lysozyme in oral mucositis treatment.
In conclusion, the effectiveness and safety of lysozyme-based spray for the treatment and prevention of deterioration of radiotherapy-induced oral mucositis was shown. The availability of new treatment options based on lysozyme, a natural enzybiotic present in the saliva of healthy subjects, could bring added value in treating oral mucositis and preventing its complications. However, a larger randomized, blinded study is needed to confirm our results.
WHO: World Health Organization
ZG, JR, and NG: Investigation, Writing—review & editing. AŠ: Conceptualization, Resources, Methodology, Supervision. MM: Conceptualization, Methodology, Supervision, Writing—original draft. ATA: Conceptualization, Writing—original draft. UG: Conceptualization, Data curation, Software, Formal analysis, Supervision, Visualization, Methodology, Writing—original draft. All authors approved the final version of the manuscript.
Aziz Šukalo, Meliha Mehić, Amna Tanović Avdić, and Una Glamočlija disclose the following relationships—employees of Bosnalijek d.d., a pharmaceutical company producing lysozyme-based products.
The study was registered with the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina under the protocol number LCS-OM-01. The clinical study was approved by the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina, number 08-07.5-6730-1/20 on July 20, 2020.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets generated for this study are available from the corresponding author upon reasonable request.
The authors declare that this study was supported by Bosnalijek d.d. (a company that is a producer of lysozyme-based products). Bosnalijek d.d. had a role in the design of the study; in the collection, analyses, and interpretation of data; in the writing of the manuscript, and in the decision to publish the results. The data and results of this study are not affected by the interest of the Bosnalijek d.d.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2476
Download: 40
Times Cited: 0
