Abstract
4-Aminobenzoic acid (PABA, para-aminobenzoic acid) exhibits multifaceted therapeutic potential in neuropsychiatric disorders through its roles in neurotransmitter modulation, anti-inflammatory action, and antioxidant defense. Experimental and clinical evidence demonstrates that PABA enhances serotonin and dopamine synthesis by activating key enzymes (e.g., tryptophan hydroxylase and tyrosine hydroxylase), thereby stabilizing mood and improving cognitive function. Mechanistically, PABA suppresses neuroinflammation by inhibiting NF-κB signaling and cytokine production (e.g., IL-1β, TNF-α) while scavenging reactive oxygen species (ROS) to mitigate oxidative stress and protect neuronal integrity. Clinical studies indicate that PABA may synergize with traditional antidepressants by targeting serotonin reuptake transporters (SERT) and monoamine oxidase (MAO), offering improved outcomes in major depressive disorder. Despite promising results, further research is needed to optimize dosing regimens, validate long-term safety, and explore pharmacogenomic interactions. Crucially, experimental validation through cellular and animal models is required to substantiate PABA’s proposed mechanisms, particularly its regulation of NF-κB signaling and enzyme activity in neurotransmitter synthesis. This review underscores PABA’s potential as a neuroprotective agent and calls for integrated strategies to translate mechanistic insights into clinical applications for complex neurological conditions.
Keywords
4-Aminobenzoic acid, neuropsychiatric disorders, molecular mechanisms, neurotransmitters, inflammatory responseIntroduction
In recent years, the study of 4-aminobenzoic acid, commonly referred to as para-aminobenzoic acid (PABA), has gained significant attention in the biomedical field, especially regarding its role in neuropsychiatric disorders. PABA is not only a byproduct of tryptophan metabolism but also plays an active role in various biological processes, such as neurotransmitter synthesis and antioxidant activity. This dual function highlights the need to explore its chemical properties, metabolic pathways, and potential therapeutic implications for neuropsychiatric conditions. PABA is recognized as a vital component in pharmaceuticals due to its structural flexibility, which allows for modifications at both the amino and carboxyl groups. This versatility has led to the creation of numerous derivatives that exhibit a wide range of biological activities, including anticancer, anti-inflammatory, and antimicrobial effects, making them promising candidates for therapeutic use in clinical settings [1, 2]. The metabolic pathway of PABA is closely associated with the shikimate pathway, which, while absent in humans, is essential for folate biosynthesis in various organisms, including malaria parasites. Research has shown that PABA serves as a precursor in the synthesis of crucial compounds like ubiquinone and aromatic amino acids, which are vital for cellular functions and energy metabolism [1, 3].
The significance of PABA is underscored by its role in synthesizing coenzyme Q, which is essential for mitochondrial respiration, thereby connecting it to energy production and overall cellular health. Research has also shed light on PABA’s neuroprotective properties, revealing its potential to reduce oxidative stress and inflammation—two major factors in neurodegenerative diseases. For example, PABA exhibits antioxidant capabilities that allow it to neutralize free radicals, thereby safeguarding neuronal cells from oxidative harm [1, 4]. Moreover, derivatives of PABA have shown encouraging outcomes in preclinical studies, suggesting they may enhance cognitive function and alleviate symptoms related to neuropsychiatric disorders [5]. Understanding the mechanisms through which PABA operates is vital for unlocking its therapeutic potential. Recent investigations have concentrated on how PABA interacts with various biological targets, including enzymes that play a role in neurotransmitter synthesis and pathways that regulate oxidative stress [1, 6].
The identification of interactions involving PABA not only sheds light on its pharmacological effects but also opens avenues for developing new therapeutic agents targeting neuropsychiatric conditions. Overall, the growing body of research highlights PABA’s diverse role in biological processes and its potential as a treatment option for neuropsychiatric disorders. To fully leverage its benefits in clinical applications, it is crucial to understand PABA’s chemical properties, metabolic pathways, and biological interactions. Future research should prioritize examining the safety and efficacy of PABA and its derivatives, with a particular focus on their mechanisms of action and therapeutic implications for neuropsychiatric health [1, 2]. Additionally, bridging the gap between theoretical mechanisms and experimental evidence is imperative. For instance, in vivo studies assessing PABA’s impact on NF-κB inhibition and ROS scavenging in neurodegenerative models would validate its therapeutic claims.
Chemical properties and physiological functions of 4-aminobenzoic acid
Chemical structure and biosynthesis
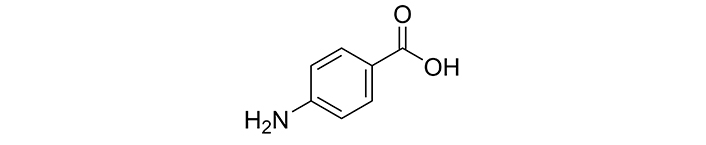
4-Aminobenzoic acid, especially its para isomer (PABA), features an aromatic structure that includes a benzene ring with an amino group and a carboxylic acid group attached (Figure 1). This distinctive structure enables a range of chemical reactions, making PABA a versatile compound in both synthetic and biological settings. The biosynthesis of PABA takes place via the shikimate pathway, which is essential for folate production in microorganisms and plants; however, humans cannot synthesize folate and must obtain it from their diet. PABA acts as a precursor in the formation of folic acid, which is vital for DNA synthesis and repair, and it also plays a role in the metabolism of various amino acids and other important compounds [7]. Recent research has underscored the potential of 4-aminobenzoic acid derivatives in creating new pharmaceuticals, particularly in addressing antibiotic resistance, highlighting their significance in the field of medicinal chemistry [1].
Overview of physiological functions
4-Aminobenzoic acid, commonly referred to as PABA, plays vital roles in several physiological processes. It is crucial for synthesizing folic acid, which is necessary for cell division and growth. PABA is also recognized for its antioxidant properties, which help protect cells from oxidative stress [1]. Moreover, it has been associated with modulating immune responses and maintaining skin health, particularly as a key component in sunscreens. However, the effectiveness of PABA in this capacity is debated, as there are concerns regarding its potential to produce harmful free radicals when exposed to UV light [8]. Additionally, research indicates that PABA and its derivatives have antimicrobial and anti-inflammatory properties, highlighting their potential as therapeutic agents for treating various conditions, including infections and chronic inflammatory diseases [9].
Role in the nervous system
4-Aminobenzoic acid, commonly known as PABA, has been shown to significantly influence neurotransmitter activity within the nervous system and may play a crucial role in neuroprotection. It is particularly involved in the synthesis of acetylcholine, which is essential for memory and learning. PABA enhances acetylcholine synthesis by upregulating choline acetyltransferase (ChAT) activity and increasing choline availability in synaptic terminals, thereby improving cognitive function [10–12]. Additionally, PABA may offer neuroprotective benefits against neurodegenerative diseases, likely due to its antioxidant properties that help reduce oxidative damage to neuronal cells [13]. Specifically, PABA activates the Nrf2 pathway to enhance glutathione synthesis and suppresses mitochondrial ROS production [14]. PABA synergizes with vitamin B6 to promote tetrahydrobiopterin (BH4) production, a critical cofactor for neurotransmitter synthesis, thereby enhancing neurological function [15, 16]. Therefore, the diverse roles of 4-aminobenzoic acid in the nervous system highlight its potential as a therapeutic target for treating neurodegenerative disorders.
The relationship between 4-aminobenzoic acid and neurotransmitters
The impact on 5-HT (serotonin) synthesis
4-Aminobenzoic acid (PABA) is important for the production and regulation of serotonin (5-hydroxytryptamine, 5-HT), a key neurotransmitter that affects various bodily functions, including mood, appetite, and sleep. Research indicates that PABA can impact the enzymes involved in serotonin production, which in turn influences the levels of 5-HT in the brain. For example, the synthesis of serotonin relies on tryptophan, a precursor that must be available for serotonin production. PABA may help increase the availability of tryptophan or affect the activity of tryptophan hydroxylase, the enzyme that converts tryptophan into 5-HT. Mechanistically, PABA allosterically binds to tryptophan hydroxylase, enhancing its catalytic efficiency [17]. Additionally, changes in PABA levels have been linked to variations in serotonin receptor expression, suggesting a complex relationship between PABA and the serotonergic system. This connection is especially significant in conditions like depression and anxiety, where serotonin regulation is often disrupted. Gaining a deeper understanding of how PABA affects 5-HT synthesis could lead to new treatment approaches for disorders related to serotonin [18, 19].
Regulation of the dopaminergic system
The dopaminergic system is essential for functions related to reward, motivation, and motor control, and it is influenced by 4-aminobenzoic acid (PABA). Research indicates that PABA can modulate the synthesis and release of dopamine, likely by affecting the availability of its precursors, particularly tyrosine. By promoting the conversion of tyrosine into L-DOPA, which is the direct precursor to dopamine, PABA may help elevate dopamine levels in the brain. Furthermore, PABA may also impact the expression of dopamine receptors, thereby influencing dopaminergic signaling pathways. This modulation is particularly significant in neuropsychiatric disorders where there is a clear dysregulation of dopamine, such as schizophrenia and attention-deficit hyperactivity disorder (ADHD). Additionally, studies suggest that PABA may interact with the neuroimmune system, potentially affecting dopamine transmission through inflammatory pathways. This relationship underscores the importance of PABA in maintaining a balanced dopaminergic system and highlights its potential as a therapeutic target for disorders associated with dopaminergic dysfunction [20, 21]. However, these hypotheses require empirical validation. Future studies should employ knockout models or pharmacological inhibitors to confirm PABA’s role in enzyme activation (e.g., tryptophan hydroxylase) and cytokine suppression (e.g., IL-1β, TNF-α) in neuroinflammatory contexts.
The role of 4-aminobenzoic acid in neurotransmitter imbalance
4-Aminobenzoic acid (PABA) has been linked to neurotransmitter imbalances, especially concerning serotonin and dopamine, which are crucial for mental health. When these neurotransmitters are not properly regulated, it can lead to various neuropsychiatric disorders. PABA appears to influence the synthesis and release of serotonin and dopamine, suggesting it might help protect against these imbalances. For example, during chronic stress or depression, when levels of serotonin and dopamine are often low, taking PABA supplements could potentially help restore balance by boosting the production of these neurotransmitters. Additionally, PABA acts as an antioxidant, which may enhance its protective effects on the brain by reducing oxidative stress that can worsen neurotransmitter dysregulation. Research has shown that PABA may improve cognitive functions and mood, highlighting its possible therapeutic benefits for conditions associated with neurotransmitter imbalances. However, further studies are needed to clarify how PABA affects neurotransmitter systems and to investigate its potential as a treatment option for related disorders [22, 23].
The association of 4-aminobenzoic acid with inflammatory responses
The role of neuroinflammation in neuropsychiatric disorders
Neuroinflammation is increasingly recognized as a crucial factor in the development of various neuropsychiatric disorders. This condition is marked by the activation of glial cells, such as microglia and astrocytes, which release pro-inflammatory cytokines and chemokines that can interfere with neuronal function and contribute to psychiatric symptoms. For example, traumatic brain injury (TBI) has been associated with neuroinflammation, which may play a role in the emergence of anxiety, depression, and other neuropsychiatric conditions following the injury. Research indicates that inflammatory processes can lead to secondary neuronal damage, worsening the mental health challenges faced by individuals with a history of TBI [24]. Additionally, neuroinflammatory responses are involved in autoimmune neuropsychiatric disorders like Sydenham chorea, where infections trigger autoimmune reactions that impact the basal ganglia, resulting in obsessive-compulsive behaviors and other psychiatric symptoms [25]. The complex interplay between neuroinflammation and neuropsychiatric disorders underscores the necessity for targeted therapeutic strategies that focus on the underlying inflammatory processes to enhance patient outcomes.
How 4-aminobenzoic acid modulates inflammatory mediators
4-Aminobenzoic acid (PABA) has demonstrated anti-inflammatory properties, likely due to its ability to influence various inflammatory mediators. Studies suggest that PABA can reduce the production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, which are typically elevated in inflammatory conditions [26]. Specifically, PABA may work by inhibiting myeloperoxidase (MPO), an enzyme linked to oxidative stress and inflammation. By reducing MPO activity, PABA helps lower the production of reactive oxygen species (ROS) and the resulting inflammatory responses, thus protecting tissues from potential damage [27]. This ability to modulate inflammatory mediators highlights PABA’s potential as an anti-inflammatory agent and emphasizes its therapeutic potential in treating chronic inflammatory conditions, such as autoimmune diseases and neuroinflammatory disorders.
Exploring the mechanisms of anti-inflammatory action
The anti-inflammatory mechanisms of PABA are complex and involve various pathways, both direct and indirect. One key mechanism is the inhibition of the NF-κB signaling pathway, which is crucial for the transcription of pro-inflammatory genes. By suppressing the activation of NF-κB, PABA can significantly lower the production of inflammatory cytokines and chemokines that lead to tissue inflammation and damage [28]. Additionally, PABA may boost the activity of antioxidant enzymes, which helps to decrease oxidative stress and its related inflammatory responses. This antioxidant effect is especially important in neuroinflammatory conditions, where oxidative stress can worsen neuronal damage and contribute to the advancement of neurodegenerative diseases [29]. Moreover, PABA treatment has been shown to influence immune cell function, particularly by promoting the polarization of macrophages towards an anti-inflammatory state, indicating a broader role in immunomodulation [1]. Together, these mechanisms underscore the potential of PABA as a therapeutic agent for managing inflammation-related disorders, highlighting the need for further research into its clinical applications.
Antioxidant capacity of 4-aminobenzoic acid
Relationship between oxidative stress and neuropsychiatric disorders
Oxidative stress has been increasingly recognized as a significant contributor to the pathophysiology of various neuropsychiatric disorders, including schizophrenia, major depressive disorder, and bipolar disorder. The brain is particularly vulnerable to oxidative damage due to its high metabolic activity and lipid-rich composition, leading to an imbalance between ROS production and the antioxidant defense mechanisms [30]. This imbalance can result in neuronal injury, inflammation, and apoptosis, which are commonly observed in neurodegenerative diseases. Evidence suggests that glial cells play a crucial role in modulating the oxidative environment, with purinergic signaling being implicated in the regulation of oxidative stress responses [31]. Furthermore, the activation of NADPH oxidase (NOX) has been identified as a major source of ROS in the brain, linking oxidative stress to neuroinflammation and neurotoxicity [30]. These findings underscore the importance of targeting oxidative stress pathways as potential therapeutic strategies for managing neuropsychiatric disorders.
Antioxidant mechanism of 4-aminobenzoic acid
4-Aminobenzoic acid (PABA) has garnered attention for its potential antioxidant properties, which may help protect cells from damage caused by oxidative stress. PABA functions as a radical scavenger, meaning it can effectively neutralize ROS and prevent lipid peroxidation, a harmful process that leads to cellular injury and inflammation [32]. This compound also influences the expression of key antioxidant enzymes, such as superoxide dismutase and catalase, which bolster the cellular antioxidant defense system and reduce oxidative damage [33]. Furthermore, PABA plays a role in the production of coenzymes that are essential for cellular metabolism, reinforcing its status as a potential antioxidant agent. Studies suggest that PABA can enhance mitochondrial function, which is vital for sustaining cellular energy levels and minimizing oxidative stress [34]. Therefore, the diverse antioxidant mechanisms of PABA may offer a promising strategy for alleviating oxidative stress in various neuropsychiatric disorders.
Analysis of related experimental studies
Experimental studies have shown that PABA has neuroprotective effects in various models of oxidative stress. For example, research on juçara fruit extracts has demonstrated that polyphenolic compounds, including PABA, can protect neuronal cell lines from glutamate-induced oxytosis [32]. These studies found that when PABA is used alongside other antioxidants, it significantly decreases oxidative damage and enhances cell viability. Additionally, investigations into biosynthesized nanoparticles, such as copper oxide and silver nanoparticles, in combination with PABA have indicated improved antioxidant activity and lower levels of ROS in cell cultures [33, 34]. Together, these findings suggest that PABA not only has its own antioxidant properties but also works synergistically with other antioxidants, making it a promising candidate for therapeutic applications aimed at treating neuropsychiatric disorders related to oxidative stress. However, further research is needed to clarify the specific mechanisms through which PABA provides neuroprotection and to assess its potential as a therapeutic agent in clinical settings.
The potential of 4-aminobenzoic acid in the treatment of neuropsychiatric disorders
Current clinical research progress
4-Aminobenzoic acid, especially in its various formulations, has been investigated for its potential therapeutic effects on neuropsychiatric disorders. Recent clinical studies have underscored its role in modulating neurotransmitter systems, which may help alleviate symptoms associated with conditions like depression and anxiety. For example, PABA enhances the efficacy of SSRIs by inhibiting SERT and MAO, leading to improved patient outcomes [35]. Furthermore, ongoing trials are exploring its neuroprotective properties, which could provide advantages in treating neurodegenerative diseases. The growing body of evidence indicates that 4-aminobenzoic acid might be useful as an adjunct therapy, particularly for patients who do not respond well to standard treatments. However, the variability in individual responses highlights the need for further research to clarify the mechanisms behind these effects and to establish standardized dosing regimens.
Related drug development dynamics
The landscape of drug development for 4-aminobenzoic acid and its derivatives is undergoing significant changes, with numerous pharmaceutical companies dedicating resources to explore its potential in treating neuropsychiatric disorders. Recent progress has been made in optimizing 4-aminobenzoic acid formulations to improve their bioavailability and therapeutic effectiveness. For instance, researchers are investigating innovative delivery systems, such as liposomal formulations, which could enhance the pharmacokinetics of 4-aminobenzoic acid, potentially leading to more effective treatment strategies for neuropsychiatric conditions [36]. Additionally, partnerships between academic institutions and industry are driving advancements in drug design, which may yield new compounds that preserve the beneficial properties of 4-aminobenzoic acid while reducing side effects. As researchers gain a deeper understanding of its pharmacological characteristics, there is also a growing interest in developing combination therapies that incorporate 4-aminobenzoic acid, aiming to tackle the complex nature of neuropsychiatric disorders.
Future research directions and challenges
Looking ahead, the future of 4-aminobenzoic acid in neuropsychiatric treatment appears promising, although it faces several challenges. A significant hurdle is the necessity for rigorous clinical trials to validate its efficacy and safety across various populations. Additionally, gaining insights into the pharmacogenomics related to 4-aminobenzoic acid treatment will be essential for predicting how different patients respond and for customizing therapies to meet individual needs. Future studies should also investigate the long-term effects of 4-aminobenzoic acid, especially regarding neuroplasticity and cognitive function [1]. As the medical field shifts towards personalized medicine, incorporating biomarkers into clinical practice could improve the accuracy of 4-aminobenzoic acid therapies. Overcoming these challenges will require a collaborative effort among neurologists, psychiatrists, pharmacologists, and researchers to fully harness the potential of 4-aminobenzoic acid in the treatment of neuropsychiatric disorders. Future research should prioritize experimental validation of PABA’s proposed mechanisms through integrated experimental approaches. Preclinical studies employing LPS-induced neuroinflammatory models should quantify dose-dependent effects on NF-κB signaling and cytokine profiles (IL-1β, TNF-α) using standardized assay protocols. The antioxidant mechanism warrants verification in transgenic models (e.g., NOX2-overexpressing mice) with real-time monitoring of ROS dynamics and mitochondrial membrane potential [37]. Clinical validation requires phase II trials examining PABA augmentation strategies with SSRIs/MAOIs, particularly in TRD populations showing inflammatory biomarkers [38, 39]. Parallel pharmacogenomic investigations should map genetic variants (e.g., COMT, BDNF polymorphisms) influencing PABA metabolism and treatment response [40, 41].
Conclusions
This review emphasizes the importance of 4-aminobenzoic acid in neuropsychiatric disorders, showcasing its diverse roles in modulating neurotransmitters, influencing inflammatory responses, and boosting antioxidant capacity. The growing body of evidence indicates that 4-aminobenzoic acid could be a promising therapeutic target, potentially leading to new strategies for managing neuropsychiatric conditions. Research reveals a wide array of findings regarding how 4-aminobenzoic acid functions; some studies highlight its neuroprotective properties, while others focus on its influence on synaptic plasticity and neuroinflammation. This variation in findings underscores the complexity of neuropsychiatric disorders and the need for a deeper understanding of how 4-aminobenzoic acid operates. It is crucial to reconcile these differing perspectives to create a well-rounded therapeutic approach that maximizes the advantages of 4-aminobenzoic acid. The authors propose that future studies should integrate AI to decode PABA’s pharmacodynamic network, yet caution against potential data biases. Future research should aim to clarify the specific molecular pathways through which 4-aminobenzoic acid affects the nervous system. Such studies could help determine the conditions under which 4-aminobenzoic acid shows its therapeutic potential and identify possible biomarkers for better patient classification. Furthermore, investigating the combined effects of 4-aminobenzoic acid with other pharmacological agents could improve its effectiveness and expand its use in clinical environments. In summary, while 4-aminobenzoic acid holds promise as a treatment for neuropsychiatric disorders, additional research is essential to confirm these findings and facilitate their application in clinical practice. By deepening our understanding of its mechanisms and uses, we may discover new treatment options, ultimately enhancing the quality of life for patients facing these complex and often debilitating conditions.
Abbreviations
| 5-HT: | 5-hydroxytryptamine |
| MAO: | monoamine oxidase |
| MPO: | myeloperoxidase |
| NOX: | nicotinamide adenine dinucleotide phosphate hydrogen oxidase |
| PABA: | para-aminobenzoic acid |
| ROS: | reactive oxygen species |
| SERT: | serotonin reuptake transporters |
| TBI: | traumatic brain injury |
Declarations
Author contributions
CS: Conceptualization, Methodology, Writing—original draft. SY: Validation. WW: Conceptualization, Investigation, Writing—review & editing, Project administration. CY: Validation. LT: Resources, Supervision, Funding acquisition. LY: Formal analysis, Resources, Data curation, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This study was funded by the Nanhai Xinxing Medical and Health Talent Platform Project of Hainan Province [NHXX-WJW-2023030] and Hainan Province Science and Technology Special Fund of China [ZDKJ2021034]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.