Affiliation:
1Faculty of Dentistry, University of Southern Santa Catarina, Palhoça 88137-270, Brazil
Email: delucamariana@gmail.com
ORCID: https://orcid.org/0000-0001-7995-4218
Affiliation:
1Faculty of Dentistry, University of Southern Santa Catarina, Palhoça 88137-270, Brazil
ORCID: https://orcid.org/0000-0001-9310-5642
Affiliation:
2Functional Genetics Laboratory, Department of General Biology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte 31270-901, Brazil
ORCID: https://orcid.org/0000-0003-4720-6746
Affiliation:
3Departament of Oral Patology and Surgery, Faculty of Dentistry, Federal University of Minas Gerais, Belo Horizonte 31270-901, Brazil
ORCID: https://orcid.org/0000-0002-5370-4867
Explor Drug Sci. 2025;3:100899 DOI: https://doi.org/10.37349/eds.2025.100899
Received: January 03, 2025 Accepted: February 27, 2025 Published: March 20, 2025
Academic Editor: Prasat Kittakoop, Chulabhorn Graduate Institute, Thailand
Aim: To evaluate the efficacy of experimental propolis varnish containing 15% green propolis in reducing Streptococcus mutans (SM) from saliva and dental biofilms.
Methods: Patients aged 8–11 years were recruited from a preventive program. After prophylaxis, propolis varnish was applied to collect saliva and biofilm samples at regular times (baseline/T0, 2nd day/T1, 3rd day/T2, 4th day/T3, 5th day/T4, 10th day/T5, 15th day/T6, and 30th day/T7). After each sampling, stimulated whole saliva was homogenized for 30 s, and tenfold serial dilutions in saline were plated on mitis-salivarius-bacitracin (MSB) agar and incubated at 37°C for 48 h. Colonies with typical morphology were counted, and the number of colony-forming units (CFU)/mL was compared via an independent sample t test. For quantitative real-time PCR (qPCR), the data were statistically analysed via one-way ANOVA with the Holm‒Sidak post hoc test.
Results: There was a significant difference in salivary SM levels between T0/T1 (baseline/2nd day) and T1/T7 (2nd day/30th day), but no difference was observed from the 3rd day onwards. The qPCR results also revealed a decrease in the number of SMs in the biofilm in the first days after varnish application.
Conclusions: A single application of green propolis varnish reduced SM in saliva and biofilms for three days. Whether these findings affect the incidence of caries needs to be confirmed through studies, which may improve the original formulation (International Clinical Trials Registry Platform registration code: NCT02052973).
Dental caries is a disease with a well-defined etiology that can be prevented and controlled [1]. Caries development depends on the stagnation of an organized biofilm on the dental surface, and its metabolic activity in the presence of sugar fuels disease progression [2, 3]. The predominance of bacteria with aciduric and acidogenic characteristics, which use sucrose as a substrate for the synthesis of exopolysaccharides, leads to the development of virulent biofilms on susceptible tooth surfaces [4, 5]. Oral streptococci are often associated with infections in various human sites and, along with other primary colonizers of human plaque, like Streptococcus sanguis and Actinomyces viscosus [6], Streptococcus mutans (SM) is considered the major pathogen of human dental caries and is often associated with the virulence of biofilms [7]. This bacterium is usually isolated from carious lesions [8, 9], and its levels are generally linked to both past caries experience and future caries activity [10, 11].
Antimicrobial treatments are generally employed to reduce the number of cariogenic bacteria in the oral cavity [12, 13]. Dental varnishes with antimicrobials as active ingredients incorporated into their formulations have been designed with the intention of adhering to the tooth surface and maintaining its activity for a longer period [14, 15]. The most common therapeutic agents are fluoride and chlorhexidine; however, in some countries, only fluoride varnishes are commercialized. There is little evidence that sufficient and bioavailable levels of fluoride in dental biofilms can significantly change the number or proportions of species found in this dynamic microbiome, not effectively addressing the infectious character of the disease [13, 16, 17].
Natural products have been tested as potential sources of new and active therapeutic agents [18]. Propolis, a bee product, has drawn interest for its safety and biological activity due to its polyphenolic compounds that exhibit proven antimicrobial activity, with potential antibiofilm and anticaries activities [17, 19–21]. Although this product has shown promising results in in vitro studies [22], only a few studies have been performed with the objective of evaluating its possible use in antibiofilm and anticaries chemotherapy via a clinical treatment protocol in vivo [7].
In the present study, we used propolis type 12, classified by number in a previous study, which corresponds to “green propolis”, which is found in southeastern Brazil [23, 24]. A previous study revealed that green propolis varnish was more effective in preventing early smooth-surface lesions under high cariogenic challenge than was the gold-standard fluoride varnish [25].
Owing to the paucity of studies in the dental literature, this study aimed to investigate the efficacy of an experimental varnish containing 15% propolis type 12 ethanolic extract on the reduction of SM in the saliva and biofilms of children over a 30-day period.
Propolis varnish was prepared as reported in a previous study at a concentration of 15% [26].
A clinical trial pilot study was conducted through convenience sampling among 11 children aged 8–11 years who were recruited from a preventive program of the pediatric dentistry clinic at the Federal University of Minas Gerais (UFMG). The inclusion criteria were as follows: (i) mixed dentition with at least eight permanent teeth; (ii) good general health condition [American Society of Anesthesiologists (ASA) I]; and (iii) not using mouthwashes or medications, especially antibiotics, until three weeks prior to the beginning of the study. Children with active caries lesions at the time of clinical examination were excluded.
Saliva samples were obtained from each participant prior to the application of propolis varnish to establish baseline SM levels. Volunteers were informed to refrain from oral hygiene procedures for 12 h prior to collection. A total volume of 360 µL of propolis varnish was subsequently applied to all the tooth surfaces. At each sampling, the subjects chewed a 1 cm piece of a sterilized latex tube to stimulate whole saliva, and the samples were collected in sterile bottles with no eating/drinking for two hours prior to the sampling. No transport medium was used, as culturing was performed within half an hour of sample collection [27]. Stimulated saliva was homogenized in a Thermolyne mixer (Barnsted International, Dubuque, IA, USA) for 30 s, and tenfold serial dilutions in saline were plated in duplicate on a Petri dish (Inlab, Diadema, SP, Brazil) containing mitis-salivarius-bacitracin (MSB) agar (Difco Laboratories, Detroit, MI, USA) via the drop-counting technique [28]. The plates were incubated under microaerophilic conditions (candle jar) at 37°C for 48 h. Only colonies with typical morphology were counted, and the number of colony-forming units (CFU)/mL of saliva was calculated by multiplying the colony count of each plate by its respective dilution factor [29]. The difference in the number of CFUs of SM at different time intervals, such as at baseline, after 1 day to the 5th day, on the 10th day, on the 15th day, and at the end of 30 days, was determined.
Bacterial DNA was extracted from the bacterial pellet of SM ATCC 25175 via the centrifugation of 10 mL of overnight culture and clinical biofilm samples via the use of a GeneJet Genomic DNA Purification Kit (Thermo Scientific) according to the manufacturer’s instructions. The amounts and quality of the extracted DNA were estimated via electrophoresis through a 1% agarose gel. To generate a standard curve for absolute quantification-based real-time PCR, DNA was extracted from a serially tenfold diluted bacterial culture.
The universal primer for 16S rRNA gene amplification was used to quantify total bacteria, and SM-specific primers were used to quantify the targeted bacteria in the samples. The sequence of the forward gtfB primer, Smut3368-F, was 5’-GCCTACAGCTCAGAGATGCTATTCT-3’, and the sequence of the reverse gtfB primer, Smut3481-R, was 5’-GCCATACACCACTCATGAATTGA-3’. The primer efficiencies were determined via a standard curve and were 100% [30].
The results, expressed as the fold difference (N) between the number of target gene copies and the number of 16S rRNA gene copies, were determined as follows:
Biofilm samples were obtained from the smooth buccal surface of the upper first molar (right or left) or from the smooth lingual surface of the lower molars (right or left). The samples were collected via sterile equipment and immediately placed in 40 µL of sterile phosphate-buffered saline (pH 7.2). All the samples were immediately transported to the laboratory on ice and stored at –20°C before further analysis.
The SM salivary level data were processed with SPSS 19.0 software. Comparisons between groups with a normal distribution were performed via the independent sample t test and the Wilcoxon test when the distribution was not normal (10th day). The level of significance considered for all tests was 5%. The results were also analysed for the reduction in the number of SM CFUs at baseline (T0) and at every other collection time (T1/2nd day; T2/3rd day; T3/4th day; T4/5th day; T5/10th day; T6/15th day; T7/30th day). The percentage SM reduction was calculated via the following formula:
Quantitative real-time PCR was performed on biofilm samples from patients in a follow-up scheme, and the results were subjected to one-way ANOVA with Holm‒Sidak post hoc statistical analysis.
No side effects were described by the children following the topical application of propolis varnish. Propolis varnish was adhered to until toothbrushing, which was performed after 12 h of varnish application, as recommended by the researcher. Patients complained about the product’s taste, characterizing it as “very bitter”, and a burning sensation immediately after application. However, no damage to the intraoral tissues was observed. No other specific complaints were reported.
Among the 11 participants in the study, 7 had a greater than 90% reduction in salivary SM relative to baseline on the 2nd day (T1). However, the CFU/mL counts started increasing on the 3rd day after varnish application in all patients.
The results of the means and standard deviations, as well as the minimum and maximum values of CFU/mL counts at all collection times, are shown in Table 1. There was a significant difference in CFU/mL counts only between T0/T1 (baseline/2nd day) and T1/T7 (2nd day/30th day). No differences were detected from the 3rd day to the 15th day.
Mutans streptococci colony-forming units in saliva [minimum/maximum values; mean; standard deviation (SD)] at different collection times
| Collection times | Min-Max | Mean | SD |
|---|---|---|---|
| Baseline/T0 | 4.6 × 105–2.0 × 106 | 1.2 × 106 a | 5.1 × 105 |
| 2nd day/T1 | 4.6 × 104–5.9 × 105 | 3.5 × 105 b | 1.7 × 105 |
| 3rd day/T2 | 6.2 × 104–1.4 × 106 | 6.9 × 105 a, b | 5.7 × 105 |
| 4th day/T3 | 6.2 × 104–1.4 × 106 | 7.0 × 105 a, b | 6.7 × 105 |
| 5th day/T4 | 9.3 × 104–3.2 × 106 | 1.4 × 106 a, b | 9.5 × 105 |
| 10th day/T5 | 3.1 × 104–7.9 × 106 | 2.3 × 106 a, b | 2.7 × 106 |
| 15th day/T6 | 1.4 × 106–2.4 × 106 | 1.9 × 106 a, b | 4.3 × 105 |
| 30th day/T7 | 8.1 × 105–3.2 × 106 | 1.9 × 106 a | 8.4 × 105 |
Means followed by different superscript letters indicate significant differences between the periods of saliva collection (p < 0.05). Repeated superscript letters indicate that the means were not significantly different (p > 0.05)
To confirm these results, we analysed SM colonization before and after propolis varnish treatment via a molecular and more accurate technique. In the first step, we analysed whether propolis varnish can interfere with the total bacterial load in biofilms. Our results revealed that after 2 days of propolis treatment, there was a significant reduction in the total number of bacteria colonizing the biofilm (Figure 1). Moreover, we conducted a second analysis regarding the prevalence of SM in biofilms. The results showed that in the initial days after propolis treatment, there was a great reduction in the presence of SM in biofilms from patients. SM DNA quantification revealed decreases of 44% and 82% on days 2 and 3 after propolis treatment, respectively. Moreover, this great reduction in the first days after propolis treatment was followed by a recovery of SM viability and colonization on day 4 (Figure 2).
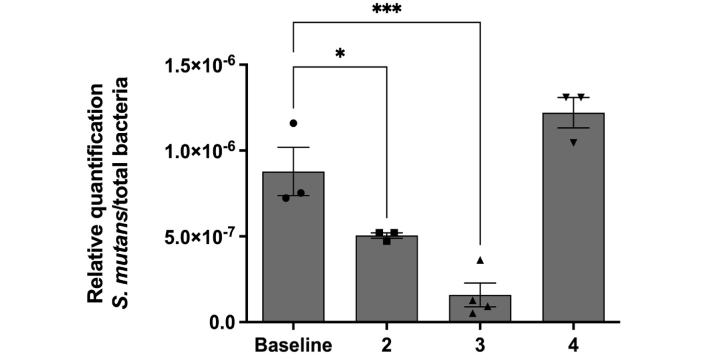
SM concentration profile normalized to the number of total bacteria identified via qPCR. Results were submitted to one-way ANOVA, with Holm‒Sidak posttest statistical analysis (* < 0.05; *** < 0.001). All data are presented as mean ± SEM. SM: Streptococcus mutans; qPCR: quantitative real-time PCR; SEM: standard error of the mean
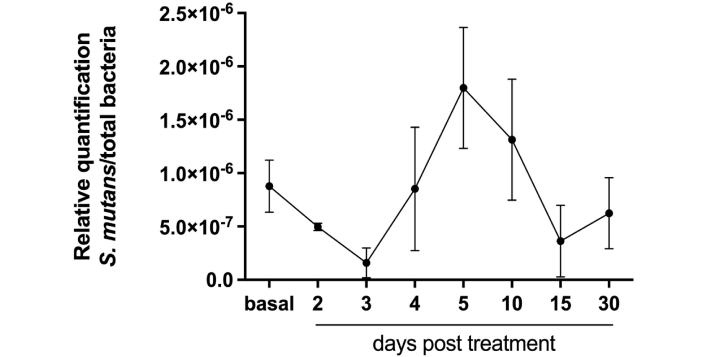
Statistical decrease in the amount of SM on days 2 and 3 after treatment. All data are presented as mean ± SEM. SM: Streptococcus mutans; SEM: standard error of the mean
This study aimed to evaluate the efficacy of green propolis varnish in reducing the cariogenic oral microbiota. After a single application of propolis varnish in children, salivary and biofilm levels were reduced for three days, confirming that its antimicrobial activity was maintained.
Strategies for managing dental lesions, both carious and non-carious, have developed in an era that aims to conserve dental tooth tissue in order to limit removal and preserve tooth structure [12, 21]. Nonrestorative or noninvasive caries treatment (fluoride- and nonfluoride-based interventions) are being employed to manage the caries disease process at the initial lesion level and minimize the loss of sound tooth structure [12].
Varnishes can be employed in pediatric dentistry because they are easy to apply and well accepted by children [13–15]. The main advantage of polymeric materials such as varnishes over gels is that they increase the contact time of the active compound on the tooth surface through a controlled or sustained system where a natural or synthetic polymer plays a crucial role [15]. Our formulation has chitosan as a polymeric base used in a wide range of dental applications, and previous in vitro studies have shown a variety of release times for the active ingredients contained in the formulation, whether synthetic or natural. In a study with chitosan and fluoride, fluoride was released for a few hours depending on the concentration of chitosan in the polymer [15]. When propolis was added to chitosan, it could be released for at least seven days, depending on its concentration. Although in vitro tests do not exactly correspond to in vivo performance, they are important for predicting the potential of a material and its probable clinical application. Through an in vitro study of propolis varnish, we hypothesized that a controlled release system using chitosan would maintain propolis release at a low level over time [26].
Clinical studies have demonstrated that the professional application of fluoride varnish prevents dental caries, and the amount of fluoride released from a varnish typically decreases within a few hours and continues to decrease for up to 24 h [14, 15]. In this clinical analysis, propolis release in saliva was not measured; only the SM amount in biofilms and saliva was measured. The results revealed that there was a significant reduction in this bacterium until the third day after application, leading us to conclude that this occurred due to the antimicrobial activity of propolis during these days.
Chlorexidine varnish, which has antimicrobial activity and substantivity, effectively decreases the microbial load in both buccal tissues and dental biofilms but has weak effects on the prevention of dental caries. Another formulation of varnish containing red propolis with antimicrobial properties was tested in a clinical trial [14]. The results revealed that the red propolis group developed dental caries, even if not in the teeth to which the product was applied, unlike the fluoride varnish group. Our research had no comparison groups, just preliminary results of a product in the development phase. However, the results of previous tests [25] revealed that the use of antimicrobial products in the prevention of tooth decay remains insufficient for disease prevention.
Furthermore, since individual antimicrobial resistance has emerged as a major worldwide health concern, research is being developed using natural products with inherent antibacterial action, like propolis, as substitute antimicrobials. Propolis’ antimicrobial effectiveness against oral bacteria has been thoroughly evaluated, and it has been found to be generally effective, making it a very appealing option for treating or preventing a variety of oral and dental disorders [20, 21].
Although the outcome was not the prevention of dental caries, it was the primary motivation for developing this product. Future research on the efficacy of new materials in the prevention of dental caries disease must consider its multifactorial aspects and not just its biological aspects. It is a biofilm-sucrose-dependent disease but has great social influence, and variables such as access to fluoridated water and dental services must be taken into consideration. Products that combine an antimicrobial substance with fluoride and that might have a low cost, combined with collective actions, can be allies in the prevention of dental caries in low-income communities.
The main limitations of this study are the small sample size and the absence of comparison groups. Future studies should expand the sample and compare the tested product with materials already available, following the study participants for a longer period, to verify whether the product is truly effective in preventing tooth decay.
In conclusion, according to initial research on the effectiveness of green propolis, the number of cariogenic bacteria decreased until the third day following application. It is important to conduct more research to refine the original composition and determine the appropriate duration of follow-up to confirm its efficacy in preventing dental caries.
CFU: colony-forming units
MSB: mitis-salivarius-bacitracin
qPCR: quantitative real-time PCR
SEM: standard error of the mean
SM: Streptococcus mutans
The authors would like to thank all patients who took part in this study; André Augusto Gomes Faraco and Juçara Ribeiro Franca for the propolis varnish manipulation; Miriam Pimenta Vale for the guidance and methodological insights; Suzane Gonçalves-Paixão, Elizete Maria Rita Pereira and Marieta Torres Abreu Assis for helping with the collections and laboratory work.
MPDL: Conceptualization, Investigation, Data curation, Writing—original draft. PSS: Writing—review & editing. FMS: Data curation, Writing—review & editing. VRS: Conceptualization, Investigation, Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
The present study was approved by the ethics committee of the Federal University of Minas Gerais (report no. 168.394). The study protocol was registered in the International Clinical Trials Registry Platform under registration code NCT02052973.
Informed consent to participate in the study was obtained from all participants and all the enrolled children signed an informed consent form, and their caregivers signed an informed consent form prior to participation.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study was supported by grants from CAPES and FAPEMIG. The CAPES grant was awarded to Mariana Passos De Luca as financial support for her PhD studies. The FAPEMIG grant was secured by Professor Vagner Santos, the PhD advisor, for laboratory maintenance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
