Affiliation:
Department of Endocrinology and Nutrition, Hospital General Universitario Gregorio Marañón, 28007 Madrid, Spain
Email: nachojimenezhernando@gmail.com
ORCID: https://orcid.org/0009-0006-5784-6308
Affiliation:
Department of Endocrinology and Nutrition, Hospital General Universitario Gregorio Marañón, 28007 Madrid, Spain
ORCID: https://orcid.org/0000-0003-4096-8115
Explor Endocr Metab Dis. 2024;1:234–243 DOI: https://doi.org/10.37349/eemd.2024.00019
Received: September 01, 2024 Accepted: November 04, 2024 Published: November 26, 2024
Academic Editor: Constantine A. Stratakis, Greece & ELPEN Research Institute, Greece
The clinical case presented describes a 44-year-old woman with a history of mucinous carcinoma in the right breast, who is diagnosed with a pituitary adenoma. Physical examination revealed signs of acromegaly, such as macroglossia and palmar hyperhidrosis. Hormonal tests were performed and showed elevated levels of insulin-like growth factor type 1 (IGF-1). The patient received GnRH analogues for breast cancer; and started medical treatment with somatostatin analogues for acromegaly, which provided improvement in symptoms. Pituitary bleeding was detected during hormone treatment, which caused the growth of the adenoma, causing it to contact the optic chiasm, although no symptoms of pituitary apoplexy were present. Transsphenoidal endoscopic surgery was performed to remove the adenoma, and the diagnosis of pituitary adenoma was confirmed by pathology. The importance of the evaluation of comorbidities in patients with acromegaly is discussed. Pituitary apoplexy as an uncommon complication of pituitary adenomas, which may be associated with the use of GnRH analogues, is addressed. Pre-surgical medical treatment in acromegaly is also discussed, highlighting the importance of a comprehensive assessment of prognostic factors and appropriate treatment selection to improve clinical outcomes. In conclusion, the importance of a multidisciplinary and personalized approach in the management of patients with GH-producing pituitary adenomas to improve quality of life and clinical outcomes is highlighted.
The primary objective of the article is to analyze and discuss the case of the patient diagnosed with acromegaly due to a pituitary adenoma, emphasizing diagnostic criteria, implications for treatment, and the management of associated comorbidities. The article references evolving diagnostic criteria recognizing that elevated insulin-like growth factor type 1 (IGF-1) levels can confirm acromegaly when clinical symptoms are present, reducing reliance on growth hormone (GH) stimulation tests. The importance of screening for comorbidities associated with acromegaly is noted, as patients may develop related conditions like obstructive sleep apnea, cardiovascular disease, and increased cancer risk. A focus on pituitary apoplexy, a serious complication that can arise with pituitary adenomas, emphasizes the need for careful monitoring, particularly in patients receiving gonadotropin-releasing hormone (GnRH) analogues, as in the presented case where pituitary bleeding occurred without acute symptoms.
Medical treatment using somatostatin analogues (SSA) is examined as a pre-surgical option to control symptoms and perhaps enhance surgical outcomes, especially in cases with significant disease burden. The relationship between increased levels of IGF-1 and heightened cancer risk, particularly breast cancer in women, is highlighted as well as the need for vigilance in monitoring such patients. The article discusses the significance of immunohistochemical markers (like E-cadherin and Ki67) in predicting tumor behavior and response to treatment, which may guide personalized medical decisions. The necessity of a multidisciplinary approach in treating patients with concurrent pituitary adenomas and cancer, catering to both the hormonal imbalance and oncological needs, is strongly advocated.
We present the case of a 44-year-old woman with a relevant medical history of having been diagnosed with mucinous carcinoma in the right breast, with surgical intervention performed in December 2022 followed by chemotherapy, radiotherapy, and adjuvant hormonal therapy with GnRH analogues.
In October 2022, the patient sought medical attention due to persistent dizziness despite antivertiginous treatment, without experiencing headaches. A brain magnetic resonance imaging (MRI) scan was requested, which revealed the presence of a 13 mm pituitary adenoma, subsequently confirmed on a pituitary MRI. On questioning, the patient had symptoms of enlarged acral parts and hyperhidrosis for 6–8 years, as well as an increase in shoe size in the last two years, from EU 38 to 41.
While the pituitary study was being performed, the patient continued with the management of her breast carcinoma. During hormonal treatment, the patient experienced pituitary bleeding detected on a follow-up MRI, with no associated stroke symptoms.
On physical examination, the patient was normotensive (blood pressure: 98/76 mmHg, heart rate: 73 bpm), weight 60 kg, and body mass index (BMI) 21.2 kg/m2. She presented macroglossia, mild mandibular prognathism, and palmar hyperhidrosis. No signs of hepatomegaly or splenomegaly were detected.
In the presurgical hormonal workup, elevated levels of IGF-1 were observed by more than twice the upper limit of normal (ULN). The rest of the hormonal axis was within normal parameters (Table 1). GH was not performed after 75 g oral glucose overload (OGTT 75 g). Complete blood count, complete biochemistry, glycemic, and lipid profile were also normal.
Hormonal results at diagnosis and during evolution follow-up
| Biochemical markers | At diagnosis | Units | Baseline values | At 48 h after surgery | 6 months post-surgery |
|---|---|---|---|---|---|
| IGF-1 (somatomedin C) | 618 | ng/mL | 93–224 | 337 | 212 |
| GH (somatotropin) | 20.5 | ng/mL | 0.1–5.0 | 3.62 | 0.27 |
| Prolactin | 25.9 | ng/mL | 5.2–26.5 | 1.5 | 7.5 |
| FSH | 74.4 | mUI/mL | 26.7–133.4 | 1.7 | 5.0 |
| LH | 21.8 | mUI/mL | 5.1–62 | 0.3 | 0.1 |
| TSH | 2.02 | µUI/mL | 0.35–4.94 | 0.68 | 2.07 |
| Free T4 | 1.0 | ng/dL | 0.7–1.5 | 1.2 | |
| Cortisol | 13.6 | µg/dL | 5–25 | 39.2 | 11.7 |
| ACTH | 25 | pg/mL | 7.2–63.3 | 23 | 20.8 |
Blank cell indicates that the measurement was not performed
Other complementary tests were requested to screen for acromegaly comorbidities, which included a colonoscopy without relevant findings, an electrocardiogram that showed sinus bradycardia without other alterations, normal bone densitometry, and abdominal and thyroid ultrasound scans without significant findings.
Initial MRI showed a 12 mm macroadenoma, which did not invade the cavernous sinus or contact the chiasm.
A complete neuro-ophthalmologic examination was performed, including visual field testing (automated static perimetry) and optical coherence tomography (OCT), which ruled out visual involvement.
The patient was referred to neurosurgery for surgical treatment. However, given the clinical manifestations secondary to acromegaly and the possibility of delay of surgery, it was decided to initiate medical treatment with SSA while awaiting surgery. Thus, she received two injections of lanreotide 90 mg monthly, which resulted in a remarkable clinical improvement, including the reduction of hyperhidrosis and hot flashes.
Regarding the breast carcinoma, the patient received parallel treatment with goserelin and exemestane. Coinciding with the administration of the first injection of GnRH analog, asymptomatic pituitary bleeding was detected in a follow-up MRI. The patient did not have headaches or symptoms suggestive of adrenal insufficiency. An enlargement of the macroadenoma as a consequence of the bleeding and contact with the chiasm was observed (Figure 1). Nevertheless, the patient was reevaluated ophthalmologically, and visual function remained preserved.
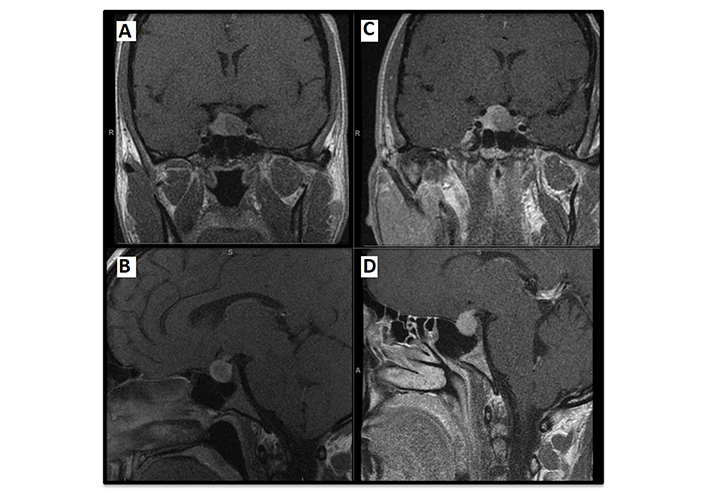
Magnetic resonance imaging. Pituitary MRI at diagnosis [coronal (A) and sagittal planes (B) respectively]: left macroadenoma of 13 × 11 mm [TV (transverse) × CC (craniocaudal)], with cystic components inside, without data of extension towards the cavernous sinuses (Knosp 0). Pre-surgical follow-up pituitary MRI [coronal (C) and sagittal planes (D) respectively]: left macroadenoma 11 × 13 × 14 mm [AP (anteroposterior) × TV × CC] which has increased with respect to previous control; hyper signal in basal T1 and in T2 in relation to hemorrhagic content; compression in optic chiasm and bulging of sella turcica, without signs of sinus invasion (Knosp 0). White letters in the images (S, A, R) indicate anatomical directions: superior, anterior, and right, respectively
Surgical treatment was performed by transsphenoidal endoscopic approach, without complications. Pathological analysis of the surgical specimen confirmed the presence of a pituitary adenoma. Immunohistochemistry (IHC) was diffuse cytoplasmic positive for GH, with a dense granular pattern, negative for other hormones, E-cadherin positive, p53 negative, and a Ki67 proliferation index of less than 3%.
The evolution in the diagnostic criteria for acromegaly has resulted in a significant change in clinical practice. Previously, performing a GH suppression test after a 75 g oral glucose (tolerance test OGTT) was imperative. However, in the latest consensus on diagnostic criteria for acromegaly in 2023, it has been recognized that the diagnosis can be confirmed with IGF-1 levels exceeding 1.3 times the upper limit of laboratory normal for the patient’s age group, provided they are accompanied by clinical manifestations of acromegaly [1].
In patients whose serum IGF-1 levels are increased but mildly [between 1.0 and 1.3 LSN (lower standardized normal)], it is recommended to confirm the diagnosis by measuring GH after a 75 g OGTT, establishing a GH nadir < 0.4 μg/L to exclude acromegaly in modern assays (< 1 in case of older assays). In addition, GH values are influenced by other factors as shown in Table 2. In the case of our patient, the presence of factors that could interfere in the determination of IGF-1 and GH was ruled out.
Factors influencing IGF-1 and GH concentrations [2]
| ↑ IGF-1 | ↓ IGF-1 | ↑ GH | ↓ GH |
|---|---|---|---|
| Oral contraceptives with estrogens or selective estrogen receptor modulators | Anorexia and malnutrition | Oral contraceptives with estrogens or selective estrogen receptor modulators | Late puberty |
| Use of testosterone | Renal and liver failure | Use of testosterone | Age > 60 years |
| Pregnancy | Poorly controlled diabetes | Pregnancy | Severe obesity |
| Severe obesity | Severe illness (e.g., sepsis or multiorgan failure) | Anorexia and malnutrition | |
| Late puberty | Renal and liver failure | ||
| Age > 60 years | Poorly controlled diabetes | ||
| Severe obesity | Severe illness (e.g., sepsis or multiorgan failure) | ||
| Severe obesity |
Once the biochemical diagnosis is confirmed, imaging tests are recommended to evaluate the size, appearance, and extent of the tumor, with MRI being the test of choice. A complete neuro-ophthalmologic examination including visual fields is essential when tumor proximity to the optic chiasm is detected and in all tumors larger than 1 cm.
In patients with acromegaly, it is essential to perform a thorough screening for possible associated comorbidities. This process includes various tests for colonic polyposis/colorectal carcinoma, obstructive sleep apnea, arterial hypertension, heart disease, diabetes, as well as thyroid nodules if treatment with SSA is required.
Another crucial aspect is the relationship between acromegaly and tumor pathology. A correlation has been observed between higher levels of IGF-1 and a higher incidence of cancer [3]. GH and the IGF-1 system have growth-promoting functions that regulate various cellular processes, including proliferation, differentiation, apoptosis, and angiogenesis [4]. Elevated levels of GH and IGF-1 may increase the incidence of cancer in patients with acromegaly due to their mitogenic and anti-apoptotic effects [5]. In contrast, the incidence of cancer in patients with GH deficiency or Laron syndrome is null [6]. Obesity is associated with increased IGF-1 concentrations, as well as insulin resistance and systemic inflammation, which may contribute to an increased risk of malignancies in the general population, including colorectal, breast, prostate, thyroid, and lung cancer [7].
In women without acromegaly, elevated levels of GH and IGF-1 have been associated with an increased risk of breast cancer [5]. The reported incidence of breast cancer in patients with acromegaly varies, although some studies have shown a significant increase compared to the general population [8–10]. Likewise, an association has been observed between higher IGF-1 levels and lower IGFBP3 with an increased risk of prostate cancer [11]. Patients with acromegaly also show a higher prevalence of prostate disorders, including those with central hypogonadism, suggesting a causal role of chronic GH and IGF-1 excess in these conditions [12, 13].
Pituitary apoplexy is characterized by hemorrhage and infarction of the pituitary tumor, being an uncommon complication of pituitary adenomas, with a reported incidence of 0.6% to 13%. This condition can be classified into two types according to its clinical manifestations and severity: fulminant pituitary apoplexy, also known as classic pituitary apoplexy, and silent, or subclinical pituitary apoplexy. The former is characterized by sudden severe headache, vomiting, and loss of vision or diplopia, while the latter is usually diagnosed by imaging tests or hormone measurements, as it has no obvious acute symptoms and can be detected during surgery. On MRI, pituitary apoplexy usually manifests with a hyperintense signal on T1 and a hypointense signal on T2, indicating the presence of hemorrhagic content, in addition to irregular enhancement. The apoplexy can occur spontaneously or precipitated by a trigger such as goserelin (a GnRH analogue) in our case. Cases of pituitary bleeding have been described with all FDA-approved GnRH analogues: goserelin, leuprolide, and triptorelin. These cases usually occur in patients with pre-existing pituitary adenomas, especially those larger than 10 mm, and mostly non-functioning, which are usually diagnosed late due to their silent nature. In women, cases of pituitary apoplexy related to the use of GnRH analogues have been documented also in the treatment of other conditions, such as endometriosis with heavy metrorrhagia or infertility and assisted reproduction. Although rare, cases of pituitary apoplexy have also been reported in patients with acromegaly and breast cancer receiving GnRH analogues [14].
The underlying pathophysiological mechanism is not yet fully elucidated, but several hypotheses have been proposed. It is suggested that acute stimulation of gonadotropic cells by GnRH analogues may cause an increase in tumor volume or peritumoral edema, precipitating compression of surrounding structures. In addition, the possibility of increased metabolic activity of the tumor leading to hypoxia and infarction has been raised. Furthermore, this phenomenon could be explained by the fact that GnRH analogues inhibit estrogen secretion. The latter have been closely related to GH metabolism and could induce hepatic resistance to GH so that by using analogues this resistance would decrease.
It is crucial to evaluate the need for surgical intervention in the presence of pituitary apoplexy. A multidisciplinary approach between Endocrinology, Neurology, Neurosurgery, Ophthalmology, and Radiology is required in order to distinguish between a simple pituitary bleed and a fulminant stroke, which requires surgical treatment preferably within the first seven days after the onset of symptoms. The neuro-ophthalmological examination will provide the diagnostic suspicion, which will be confirmed by a neuroimaging test, computed tomography, or pituitary magnetic resonance imaging. Patients without severe symptoms can be treated conservatively with close follow-up. In this case, surgery was performed but not urgently as the patient was asymptomatic and there was no neuro-ophthalmologic involvement.
Transsphenoidal pituitary surgery performed by an experienced surgeon is usually the treatment of choice in patients with acromegaly. In cases where surgery is not feasible or insufficient, medical treatment and radiotherapy are used as second- and third-line options [15]. Medical treatment prior to surgery can play an important role in the management of patients with complications such as sleep apnea or heart failure, which helps to reduce perioperative risk [16–19]. However, the response to treatment with SSA can vary considerably [20–24].
E-cadherin is a cell adhesion protein located in the cytoplasmic membrane and has been associated with tumor suppression [25]. Loss of E-cadherin expression has been associated with increased invasion and metastasis in several types of cancer, such as breast and lung cancer [26, 27]. Down-regulation of E-cadherin is considered a hallmark of EMT (epithelial-to-mesenchymal transition). However, studies on the association between loss of E-cadherin expression and aggressiveness of GH-producing tumors have yielded conflicting results [28–30]. Three types of somatotropinomas have been identified based on E-cadherin expression by antibodies against its intracellular domain: tumors with low or complete absence of expression (score 1), tumors with mild to moderate accumulation of E-cadherin in the membrane (score 2), and tumors with extensive membranous accumulation (score 3, more than 50% of cells). Tumors with high E-cadherin expression (score 3) tend to be smaller than those with low or medium expression [31].
According to the WHO classification of 2022 GH-secreting pituitary adenomas are classified as PIT1 lineage pituitary adenomas along with lactotroph adenomas and thyrotroph adenomas [32]. Adenomas that secrete both GH and prolactin tend to have a worse prognosis, with a lower likelihood of surgical remission and a greater need for adjuvant treatment compared to those that secrete exclusively GH [2, 33]. In pure GH-secreting pituitary adenomas, the cytoplasmic distribution of immunoreactive cytokeratin (CK) allows us to classify them into two types that have prognostic implications: sparsely granulated tumors, with a perinuclear and diffuse CK pattern; and densely granulated tumors, with drop-shaped CK and the presence of fibrous bodies greater than 70%. Sparsely granulated tumors tend to show a poor response to treatment with first-generation SSA [34–36]. Those GH-producing tumors that are hyperintense on T2-weighted images usually correspond to poorly granulated tumors on histological studies [37], and consequently, are associated with more aggressive behavior. Other predictors of response to first-generation SSA are shown in Table 3.
Predictors of response to first-generation somatostatin analogues
| Parameters | Higher response | Lower response | Ref. |
|---|---|---|---|
| E-cadherin | ↑ | ↓ | [31] |
| Ki67 | ↓ | ↑ | [38–40] |
| Age | Older age | Younger age | [30, 41–44] |
| Sex | Female | Male | [45–47] |
| Baseline GH and IGF-1 | Moderately elevated levels | High levels | [45] |
| T2 signal on MRI | Hypointensity | Hyperintensity | [37] |
| Tumor volume and invasion | ↓ | ↑ | [41] |
| Response to the short octreotide | Yes response (GH nadir < 3.5 ng/mL) | No response (GH nadir > 7 ng/mL) | [17] |
| Cytokeratin distribution pattern | Perinuclear distribution (densely granular) | Distribution in droplet; fibrous bodies > 70% (sparsely granulated) | [38] |
| SSTR-2 expression and SSTR-2/SSTR-5 ratio | ↑ | ↓ | [25] |
STTR: somatostatin receptor; AIP: aryl hydrocarbon receptor-interacting protein
In conclusion, the clinical case presented highlights the importance of a comprehensive evaluation of comorbidities and prognostic factors in patients with GH-producing pituitary adenomas. Early diagnosis, multidisciplinary management, and appropriate treatment selection are essential to improve clinical outcomes and the quality of life of patients. Advances in understanding the pathophysiology and predictive factors of therapeutic response may guide more personalized and effective therapeutic decisions.
GH: growth hormone
GnRH: gonadotropin-releasing hormone
IGF-1: insulin-like growth factor 1
MRI: magnetic resonance imaging
SSA: somatostatin analogues
IJH and LGF: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Validation.
Both authors declare that they have no conflicts of interest.
The study “Patient diagnosed with acromegaly and pituitary apoplexy after breast carcinoma treatment: challenges in diagnosis and management”, carried out by Dr. Ignacio Jiménez Hernando from the Department of Endocrinology and Nutrition Service of the Hospital General Universitario Gregorio Marañón, has been reviewed by the Ethics Committee of the Hospital General Universitario Gregorio Marañón (CEIm HGUGM), and CEIm HGUGM found no ethical impediment to the study’s publication and dissemination, as stated in Minute 19/2024 of October 21.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from the relevant participant.
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3686
Download: 298
Times Cited: 0
