Affiliation:
1Group of Study in Endocrinology and Metabolism, School of Arts, Sciences and Humanities, University of São Paulo, São Paulo 03828-000, Brazil
2Department of Experimental Pathophysiology, Faculty of Medicine, University of São Paulo, São Paulo 01246-903, Brazil
ORCID: https://orcid.org/0000-0002-8187-5495
Affiliation:
1Group of Study in Endocrinology and Metabolism, School of Arts, Sciences and Humanities, University of São Paulo, São Paulo 03828-000, Brazil
2Department of Experimental Pathophysiology, Faculty of Medicine, University of São Paulo, São Paulo 01246-903, Brazil
ORCID: https://orcid.org/0000-0002-7283-777X
Affiliation:
1Group of Study in Endocrinology and Metabolism, School of Arts, Sciences and Humanities, University of São Paulo, São Paulo 03828-000, Brazil
ORCID: https://orcid.org/0009-0003-8186-0909
Affiliation:
1Group of Study in Endocrinology and Metabolism, School of Arts, Sciences and Humanities, University of São Paulo, São Paulo 03828-000, Brazil
Email: fabiana.caf@usp.br
ORCID: https://orcid.org/0000-0002-8103-6923
Explor Endocr Metab Dis. 2025;2:101424 DOI: https://doi.org/10.37349/eemd.2025.101424
Received: November 23, 2024 Accepted: March 03, 2025 Published: March 13, 2025
Academic Editor: Tzong-Shyuan Lee, National Taiwan University, Taiwan, China
The article belongs to the special issue Metabolic Syndrome in Menopause
The accumulation of adipose tissue is associated with metabolic disorders, including insulin resistance, type 2 diabetes (T2D), dyslipidemia, metabolic syndrome, and cardiovascular diseases (CVD). Menopause might predispose women to increase body weight and adipose tissue, and decrease lean muscle mass. Furthermore, postmenopausal women display fat mass redistribution with greater accumulation in the visceral area mainly due to hormonal shifts that result in a higher testosterone/estradiol ratio. These effects are associated with a less favorable adipokine profile, dyslipidemia, insulin resistance, and cardiac dysfunction after menopause. Fat mass is determined by the balance between the storage of triacylglycerol (TAG) (lipogenesis) and the removal of stored TAG (lipolysis) in combination with the differentiation of new adipocytes (adipogenesis). Disturbances in adipose tissue dynamics lead to an increase in lipogenesis (hypertrophy) and/or in adipogenesis (hyperplasia) to accommodate excess energy intake. While large adipocytes are dysfunctional and have greater secretion of inflammatory adipocytokines, small adipocytes are healthier and associated with metabolic improvements. Different strategies can be used to prevent or reduce body weight gain and fat mass, as well as to maintain healthy adipose tissue; however, due to robust evidence, lifestyle interventions should be pillars in this process. This review provides a comprehensive summary of findings on the role of a balanced diet and physical exercise in improving body composition and promoting healthy adipose tissue in postmenopausal women.
Body fat expansion and distribution are modulated across the lifespan due to different factors such as aging [1], physical inactivity [2], poor diet [3], sarcopenia (lean muscle mass loss) [4], and hormonal status [5]. Obesity is more prevalent in females than in males due to fluctuations in sex hormones at different stages of reproductive life that can also determine the adipose tissue expansion [5]. Studies have demonstrated that the post-menopause period is associated with changes in body composition and visceral white adipose tissue (vWAT) accumulation, which is characterized by a central fat distribution [1, 6, 7]. According to Hales et al. [8], over 43% of postmenopausal women have obesity. In addition, healthy women transitioning into menopause increased total cholesterol and low-density lipoprotein (LDL) cholesterol, triglycerides, fasting glucose, insulin resistance, blood pressure, and the incidence of heart failure [9, 10].
The accumulation of adipose tissue is determined by the impairment of metabolic processes such as adipogenesis (preadipocyte differentiation into mature adipocytes), lipogenesis [triacylglycerol (TAG) biosynthesis and accumulation in the intracellular lipid droplet], lipolysis (TAG hydrolysis) and fatty acid oxidation [11], and is associated with metabolic disorders, including insulin resistance, type 2 diabetes (T2D), dyslipidemia, metabolic syndrome and metabolic dysfunction-associated steatotic liver disease (MASLD) [12, 13]. Large adipocytes are insulin resistant, proinflammatory, and more prone to apoptosis [14, 15]. In addition, endocrine dysfunction is observed in hypertrophied adipocytes [13, 16]. In contrast, small adipocytes are more sensitive to insulin and act as a potent reservoir for free fatty acids and TAG, preventing fat ectopic accumulation in the skeletal muscle, heart, and liver [14, 17].
Considering that menopause might predispose to increased obesity and cardiovascular diseases (CVD), it is crucial to target lifestyle interventions that can control body weight gain and promote healthy adipose tissue. Evidence on the effects of nutrition approach and physical exercise to prevent and decrease fat mass is consistent in the literature [18–21], and for this reason, they should be recommended for postmenopausal women. Other strategies can also be adopted, such as pharmacotherapy and bariatric surgery, however, the purpose of this review is to update the role of a balanced diet and physical exercise in improving body composition and promoting healthy adipose tissue in postmenopausal women.
Adipose tissue is composed of adipocytes, pre-adipocytes, fibroblasts, endothelial cells, and some immune cells such as macrophages, dendritic cells, and T cells [22]. There are two different adipose tissues named by their respective color: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is expressed in greater quantities and is classified according to its anatomical location into two major depots: subcutaneous WAT (sWAT) and vWAT [23]. sWAT is located just below the skin in the abdominal and gluteal-femoral regions, accounting for up to 80% of all fat mass in humans [15]. vWAT is found in the omental, mesenteric, retroperitoneal, gonadal, and cardiac regions [24].
Adipocytes from WAT are specialize in storing energy during periods of food abundance and supplying energy when there is an energy deficit. They store TAGs in a large droplet (unilocular) and have few mitochondria [15]. This monotonous role of storage and energy supply ended in the 1990s when the endocrine role of WAT was discovered. In fact, WAT synthesizes proteins called adipokines that can act in autocrine, paracrine, and endocrine manners, influencing health and disease conditions [15, 25–27]. Examples include leptin [28, 29], adiponectin [30, 31] fibroblast growth factor-21 (FGF21) [32, 33], interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein 1 (MCP-1) [15], which have effects on energy metabolism, thermogenesis, fat mass expansion, insulin sensitivity, and inflammatory response.
WAT depots’ specific differences give rise to clinical implications that contribute to determining cardiometabolic risk. Women have higher adiposity than men, but the adipose tissue accumulates mainly in the sWAT, which is associated with a lower risk of obesity-related complications [34]. Individuals with increased central adipose distribution (android) are more susceptible to developing T2D and cardiovascular complications, whereas those with increased peripheral adipose tissue distribution (gynoid) are more metabolically healthy [35, 36]. Differences between metabolically healthy and unhealthy obese were identified since the relative risk for incident T2D was higher in unhealthy obese [37].
BAT is found in greater quantities in the interscapular and supraclavicular regions, as well as along the spine, heart, and kidneys [38]. BAT is composed of brown adipocytes with TAGs stored in small and numerous droplets (multilocular), which provide a rapid and efficient energy supply [39]. It is also highly innervated by the sympathetic nervous system, vascularized, and rich in mitochondria, making it very efficient in controlling body temperature, i.e., thermogenesis [38–40].
The thermogenic capacity of BAT is due to the presence of uncoupling protein 1 (UCP1), which uncouples electron flow in adenosine triphosphate (ATP) synthesis, consuming energy and releasing it as heat [41]. To sustain all thermogenic activity, BAT consumes lipids [42] which makes it a potential target for the treatment of obesity, T2D, and metabolic syndrome [43]. BAT might also have a secretory role since the identification of several BAT-derived molecules, named batokines, have been found to act in a paracrine, autocrine, or endocrine manner. FGF21, IL-6, and neuregulin 4 are among the first batokines to be identified, which participate in physiological metabolism as extensively reviewed by Villarroya et al. [44].
A third type of adipocyte is called beige, which expresses mixed characteristics between white and brown adipocytes. The emergence of beige cells in the WAT is known as the browning of WAT, which can be induced by stimuli such as cold [45, 46], diet [47], and physical exercise [48]. Beige adipocytes express UCP1 and therefore also manifest thermogenic activity. The literature suggests that an increase in beige adipocytes can have protective effects against obesity, T2D, and metabolic dysfunction [49, 50].
Adipose tissue is a highly dynamic organ that can rapidly remodel through three processes: adipogenesis, lipogenesis, and lipolysis. Adipogenesis, which is the preadipocyte differentiation into mature adipocytes, results in an increase in the number of cells (cellular hyperplasia) [51–53]. This process involves the commitment of multipotent mesenchymal stem cells to the adipocyte lineage; the mitotic clonal expansion; and the terminal differentiation with the expression of transcriptional factors such as CCAAT/enhancer-binding proteins (C/EBPs) family and peroxisome proliferator-activated receptor-γ (PPARγ), and lipogenic genes such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) and adipocyte fatty acid binding protein (aP2) [54–56]. Differences in developmental origins of white, brown, and beige adipocytes have been extensively reviewed in the literature [55, 57, 58].
Adipogenesis is essential to renew adipose tissue and support adipose dynamics since approximately 10% of our body’s fat cells are regenerated each year [59]. It can influence metabolic outcomes since new adipocytes are smaller, more sensitive to insulin, and less inflamed [60, 61]. Thus, alternatives are being sought to target adipogenesis as a pathway to improve obesity, T2D, and metabolic syndrome [62, 63]. On the other hand, inhibitors of adipogenesis are a poor choice for amelioration of metabolic disease states because limiting fat cell expansion is associated with T2D [64].
The expansion of adipose tissue during development is also accomplished through increases in lipogenesis. In this process, fatty acids taken up from the circulation or synthesized de novo are most often directed to storage in the form of TAGs within the lipid droplet, causing adipocyte hypertrophy. Briefly, after free fatty acids are taken up by adipocytes, they are acylated with CoA, forming acyl-CoA by the action of acyl-CoA synthetase (ACS) [65]. Acyl-CoA, together with glycerol-3-phosphate, is esterified into TAGs by the enzymes glycerol-3-phosphate acyltransferase (GPAT) and diacylglycerol acyltransferase (DGAT) [66].
Adipocytes are also capable of synthesizing new lipids from circulating carbohydrates through a process known as de novo lipogenesis (DNL). In this process, mitochondrial citrate is exported to the cytosol, where ATP citrate lyase (ACL) and ACC drive malonyl-CoA production [67]. Malonyl-CoA is used as a source for TAG synthesis by the FAS [68]. Correspondingly, the excess metabolites that arise from the glycolytic pathway activate the pentose phosphate pathway, in which glucose-6-phosphate is oxidized by the enzyme glucose-6-phosphate dehydrogenase (G6PDH), generating the cofactor nicotinamide adenine dinucleotide phosphate (NADPH). This energy is required to fuel the fatty acid synthesis reactions that utilize malonyl-CoA to generate long-chain fatty acids for TAG synthesis [69]. DNL is a fundamental process that occurs in the liver, where lipids are stored and secreted by hepatocytes [70, 71]. A high supply of nutrients and DNL may contribute to the development of MASLD [71].
A third process that regulates adipocyte size is called lipolysis, which is characterized by the hydrolysis of TAGs for fatty acid liberation and oxidation [72]. Stimuli such as fasting, cold, and physical exercise induce lipolysis through the action of catecholamines on beta-adrenergic receptors, leading to an increase in cyclic AMP and activation of protein kinase A (PKA). PKA phosphorylates hormone-sensitive lipase (HSL) and perilipins, allowing the hydrolysis of triglycerides. Adipose triglyceride lipase (ATGL) and monoacylglycerol lipase (MAGL) also play important roles in TAG hydrolysis, with ATGL acting in the initial phase by cleaving the first fatty acid, and MAGL finishing the hydrolysis by cleaving the final fatty acid from diacylglycerol [73, 74].
The imbalance between the adipogenesis, lipogenesis, and lipolysis processes is directly associated with the expansion of adipose tissue, as occurs in obesity. Moreover, adipocyte size has important implications for health and disease since hypertrophic adipocytes are associated with impaired function and dysmetabolism, and decreased adipocyte size is associated with metabolic improvements [62]. Understanding how menopause can affect the remodeling of adipose tissue can help to establish strategies to maintain healthy adipose tissue and avoid negative effects on the health of postmenopausal women.
Menopause is associated with changes in body composition that are characterized by an increase in central fat mass and a decrease in lean muscle mass [75]. In a cross-sectional study of a representative population-based sample from Brazil, Donato et al. [76] found that postmenopausal women (53.3 ± 3.8 years old) had greater waist circumference (WC) and waist-to-hip ratio than premenopausal women (44.3 ± 3.6 years old), independent of age, body mass index (BMI), hormone contraceptive use, and hormone therapy. In addition, postmenopausal women had five times the chance of having central adiposity than premenopausal women, even after controlling for BMI and other contributing factors. Recently, Sun et al. [7] demonstrated that postmenopausal women (54.1 ± 2.8 years old) exhibited higher levels of fat mass, BMI, arm circumference, and WC compared with premenopausal women (32.4 ± 9.6 years old).
Some authors discuss that changes in body weight and adiposity are associated predominantly with increasing age without menopause influence [1, 5]. Davis et al. [5] showed in a review that the weight gain at midlife was about 0.5 kg annually due to age, while Sternfeld et al. [77] observed gaining an average of 0.7 kg per year, independent of age at baseline or menopause status. As recently discussed, factors such as decreased energy expenditure and physical inactivity are determinants of weight gain in midlife women [78].
Other authors elucidate that the increase in body fat might not be only age dependent [76, 79]. Since estrogen also plays an essential role in the health of muscle tissue by regulating protein synthesis, mitochondrial biogenesis, and function [80], the reduction in its concentration during menopause can cause sarcopenia synchronously with adipose tissue dysfunction [80]. The loss of fat-free mass leads to a drop in the amount of daily work, reducing caloric expenditure at rest, reducing the uptake and oxidation of glucose and fatty acids, and promoting body weight gain and adiposity [81]. Such changes also contribute to an increased risk of T2D, MASLD, and CVD [82]. In fact, Lovejoy et al. [6] demonstrated that menopause is associated with decreased energy expenditure and fat oxidation, which predispose postmenopausal women to obesity if lifestyle changes are not made.
Despite the discussion regarding the influence of age, there is a consensus in the literature that changes in fat mass distribution occur after menopause, with adipose tissue accumulating mostly in the visceral area [1, 83]. In a meta-analysis, it was shown that total leg fat percentage decreased by 0.17% per year, whereas fat mass increased in abdominal indexes, such as trunk fat percentage by 0.40% per year and WC (longitudinal) by 0.51 cm per year [1]. In another study, using radiologic imaging techniques to examine the effect of menopausal status on body composition, Toth et al. [79] showed that postmenopausal women (51 ± 4 years old) had 36% more trunk fat, 49% greater intra-abdominal fat area, and 22% greater subcutaneous abdominal fat area than premenopausal women (47 ± 3 years old), which revealed a preferential increase in vWAT independent of age and total fat mass.
It is important to elucidate that body weight and fat mass gains may occur before the onset of menopause. These responses were shown in a longitudinal study, since total body fat mass and vWAT increased significantly from 3–4 years prior to menopause compared with menopause onset and then remained relatively stable after 1–2 years following menopause [6]. In addition, circulating estradiol decreased and serum follicle-stimulating hormone (FSH) increased during the same prior time frame [6], corroborating the idea that the redistribution of fat mass in menopause is associated with hormonal shifts that result in a higher testosterone/estradiol ratio [84].
Estrogen deficiency in postmenopausal women changes the white adipocyte metabolism, and consequently the distribution of body fat mass. In fact, Rebuffé-Scrive et al. [85] showed that with higher circulating estrogen levels (premenopausal period), the preferential fat storage is in sWAT instead of vWAT since the first exhibit higher lipoprotein lipase activity and lower lipolytic activity. However, when the estrogen declines, the preferential fat storage in sWAT is lost and the lipolytic rate in vWAT is reduced, which may predispose to the accumulated fat in this depot.
Central fat mass accumulation postmenopause has important implications for the health of women. Cross-sectional and longitudinal studies showed that the waist-to-hip ratio and WC, both indicative of central obesity, were even stronger independent risk factors for insulin resistance, T2D, hyperlipidemia, and atherosclerosis, as compared with BMI [86, 87]. Additionally, a higher testosterone/estradiol ratio has also been associated with increased CVD in women [88], which explains, at least in part, the higher mortality rates associated with CVD in postmenopausal women compared with men of the same age [89].
Adipocytes from vWAT are less proliferative, replicative, and adipogenic [90], and more hypertrophic than adipocytes from sWAT [91]. The enlargement of adipocytes to adapt to increased fat storage produces an increase in the secretion of several inflammatory adipokines, such as leptin, IL-6, TNF-α, angiotensinogen, adipsin, and free fatty acids [13], while the levels of anti-inflammatory molecules, such as adiponectin, decrease [16, 92]. These changes in secretory pattern result in a low-grade systemic inflammation [93]. The larger the adipocyte, the greater the secretion of inflammatory adipokines, contributing to more inflamed, insulin resistant, and dysfunctional adipose tissue [15].
Postmenopausal women had elevated levels of proinflammatory cytokines than premenopausal women [94, 95]. Added to all known cardiometabolic damage caused by a less favorable adipokine pattern with higher secretion of proinflammatory relative to anti-inflammatory adipokines [96], it has been shown that high leptin concentration is associated with increased risk of breast cancer in postmenopausal women [97] and with changes in osteoblast/osteoclast ratio [98] that may contribute to the development of osteoporosis [99]. In addition, Thurston et al. [100] showed that early in the menopause transition, lower adiponectin and higher leptin were associated with elevated odds of reporting hot flashes, and that higher MCP-1 was related to increased night sweats. Interestingly, the higher incidence of heart failure in early menopause was not associated with serum adipokine levels [10].
Additionally, hypertrophied adipocytes from vWAT present greater lipolytic activity with non-esterified fatty acids (NEFAs) releasing directly to the portal vein, contributing to developing insulin resistance [13]. Increased levels of NEFAs, proinflammatory cytokines, and intermediary lipids, such as ceramides, in non-adipose tissues like the heart, kidney, and skeletal muscles, result in lipotoxicity and damage to insulin signaling [15]. The excess of NEFAs may augment the hepatic synthesis of TAGs, raising the risk of coronary disease associated with hypertriglyceridemia [101]. Also, the inability of the liver to deal with excess lipids elevates LDL and reduces high-density lipoprotein (HDL) levels, which combined with the chronic inflammatory process and oxidative stress, contribute to the development of endothelial dysfunction and atherosclerosis, increasing the cardiovascular risk [102].
The main effect of estradiol in the BAT is the activation of brown fat activity and the increase in body temperature, which is negatively associated with body weight. Centrally, estrogen enhances sympathetic nervous system-BAT signaling and controls BAT-mediated thermogenesis, while locally increasing thermogenesis through the expression of UCP1, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), and PPARγ [103, 104]. Thus, estrogen deficiency as observed in postmenopausal women may decrease thermogenesis and consequently, energy expenditure, leading to weight gain and obesity in the long term [105]. In addition, it has been shown that under an obese state, the thermogenic activity of BAT is reduced and the metabolic damage may be enhanced [106, 107]. Figure 1 summarizes the effects of estrogen deficiency on adipose tissue and the repercussions for the development of cardiometabolic diseases.
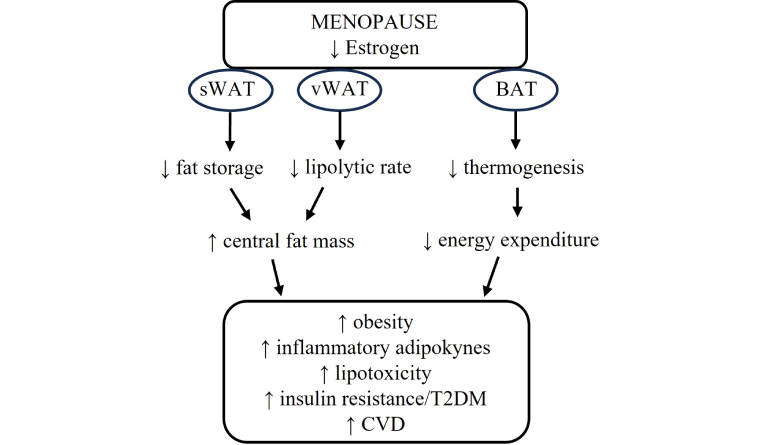
Repercussions of estrogen reduction for adipose tissue and cardiometabolic diseases after menopause. sWAT: subcutaneous white adipose tissue; vWAT: visceral white adipose tissue; BAT: brown adipose tissue; T2DM: type 2 diabetes mellitus; CVD: cardiovascular disease; ↑: increase; ↓: decrease
A particular aspect to be considered is menopausal hormone therapy (MHT), which is recommended for menopausal symptoms treatment of vasomotor and genitourinary [108]. MHT helps reduce the risk of breast, lung, and colorectal cancers, venous thromboembolism, atrial fibrillation, acute myocardial infarction, osteoporosis, and bone fractures [109]. Regarding the metabolic responses, MHT mitigates the increase in visceral adiposity during the menopausal transition [1, 110], improves adipokine/cytokine profile compared to women without MHT [111], and reduces total cholesterol and LDL levels [112]. However, it was observed that women under MHT presented lower vWAT compared to those who had discontinued MHT, which highlights the need for caution when the therapy is interrupted [113].
Considering the role of adipose tissue in developing cardiometabolic diseases and the fat mass remodeling associated with menopause, preventing or reducing body weight gain, and maintaining a healthy adipose tissue are determinants of postmenopausal women’s health. Different strategies can be used to assist during this period, however, due to robust evidence, lifestyle interventions with balanced diet and physical exercise are considered pillars in this process.
Body weight and fat mass gain after menopause are closely associated with unhealthy eating habits [3]. The consumption of a hypercaloric diet and a dietary pattern that includes ultra-processed foods (rich in fat, sugar, and salt) and reduced consumption of fruits, vegetables, and fiber are negative determinants of body composition in postmenopausal women [6, 92]. On the other hand, there are a variety of dietary approaches that recommend different foods and macronutrient consumption to improve health and body composition, however, there are no specific dietary guidelines to manage body weight and fat mass in menopause. So, what can be done to help promote a healthy weight and fat mass?
Less energy intake in combination with physical exercise has been recommended to control body weight since the reduction in energy intake in approximately 500 to 1,000 kcal·d−1 is effective for decreasing body weight by 1 to 2 lb·wk−1 [114]. An important dietary strategy to reduce energy intake is to decrease portion size. In fact, a recent systematic review and meta-analyses reported that smaller versus larger portions were associated with approximately 0.6 kg less body weight gain [20]. Furthermore, intermittent fasting and time-restricted feeding have also been used to cut calories, reduce body weight, and improve the inflammatory process with significant reductions in TNF-α and leptin levels, but no changes in IL-6 and adiponectin [115]. In addition, calorie restriction also leads to an increase in fatty acid oxidation and offers benefits to MASLD, as it can increase the amounts of protein and replace saturated fats with unsaturated fats, reducing the hepatic lipids content [116, 117].
Considering that a high-fat diet promotes elevated energy intake and body weight [118] and that a positive association between body fat mass (especially vWAT) and fat intake in both pre and postmenopausal women was found [7], reducing fat intake is a good plan to control body weight and fat mass. In addition, increasing protein intake and restricting carbohydrate intake are also important to enhance body weight and fat mass loss [19, 119], and should be targeted in dietary recommendations.
The benefits of a high-protein diet for postmenopausal women have also been studied, since with age, the need for protein consumption increases to preserve muscle mass, which tends to decrease [120]. High-protein diet is commonly used to promote anabolism and induce fat-free mass gain. Indeed, protein recommendations for postmenopausal women are higher than for the general population, and although gains in lean mass are not seen in some studies [121, 122], contributions such as improvement in body composition and preservation of lean mass in conditions of caloric deficit can be obtained with the consumption of high-protein diet [123, 124]. Also, it can induce greater satiety and promote weight loss when associated with physical exercise [125].
In a previous study, it was demonstrated that postmenopausal women who performed resistance exercise (RE) associated with higher habitual protein intake had lower body weight and BMI, and higher skeletal muscle mass compared to women who underwent a low-protein diet [125]. In fact, even when hypercaloric and hyper protein diets are consumed, although fat gain is like that of normal protein diets, improvements in lean mass are greater without impacting vWAT [126]. Thus, the International Society of Sports Nutrition recommends the consumption of a high-protein diet, as they have positive effects on body composition and maintenance of lean mass [127].
Some review studies have attempted to identify the best diet to alleviate the symptoms of menopause, among these, the Mediterranean diet. It is characterized by the consumption of a wide variety of foods, such as extra virgin olive oil, seeds, minimally processed whole grains, nuts, fruits, vegetables, dairy products, fish, and wine [128]. Mediterranean diet has shown protective effects against several chronic diseases, such as obesity, T2D, CVD, cancer, aging disorders, and overall mortality [129].
In postmenopausal women, there is evidence of the positive effects of the Mediterranean diet in reducing body weight [3]. In another study, women with obesity who consumed a 3-month Mediterranean diet showed improvement in the expression of genes involved in adipogenesis, angiogenesis, autophagy, and fatty acid metabolism in vWAT. These responses are associated with better oxidative and metabolic capacity of vWAT, which can reduce the size of white adipocytes [130]. Additionally, individuals at high risk of CVD showed a decrease in serum levels of pro-inflammatory adipokines resistin and visfatin and an increase in adiponectin after a period of Mediterranean diet. The study also showed that the Mediterranean diet reduced serum total cholesterol and LDL levels, and ceramides, a type of bioactive lipid associated with apoptosis. High ceramide levels can increase insulin resistance, inflammation, and cardiovascular complications [131].
The Mediterranean diet may also improve lean mass. Isanejad et al. [132] found an increase in lean mass in women aged 65–72 years who consumed foods characteristic of the Mediterranean diet. In a cross-sectional study with postmenopausal women, data assessed by dual-energy X-ray absorptiometry (DEXA) showed that greater adherence to the Mediterranean diet was associated with higher rates of appendicular lean mass [133]. Another cross-sectional study with women aged 18–79 years also reported the association between higher adherence to the Mediterranean diet with higher fat-free mass, suggesting that this diet is useful to prevent skeletal muscle loss. Furthermore, differences in fat-free mass were greater for women over 50 years compared to those under 50 years, revealing that the Mediterranean diet may be important in reducing the loss of skeletal muscle mass associated with aging [134].
As reported, part of the benefits in body composition offered by the Mediterranean diet is due to the presence of various foods rich in antioxidants that can help to protect against oxidative stress and its consequences, in addition to the presence of magnesium and other micronutrients that improve skeletal muscle function [3]. Thus, the Mediterranean diet pattern along with other healthy habits can improve body composition and prevent metabolic and CVD in the postmenopausal period [132, 133].
Another diet that has been studied to minimize menopause symptoms is the Dietary Approaches to Stop Hypertension (DASH) diet, which emphasizes the consumption of whole grains, fruits, vegetables, low-fat dairy products, nuts, and legumes, which are all rich in nutrients and fiber. In contrast, the DASH diet suggests decreasing the consumption of red and processed meats, sweetened beverages, and sodium, which helps to reduce the intake of saturated fats and sugars. This eating pattern is recommended by the United States Department of Agriculture for the general population [135].
Although the DASH diet was initially proposed to reduce hypertension and CVD risk, some studies have identified good results for postmenopausal women, indicating that the diet can be effective in improving menopausal symptoms, maintaining body weight, and preventing CVD [136–138]. These benefits are especially due to the elevated consumption of fruits and vegetables, which are rich in nutrients and fiber that improve digestive health and strengthen the immune system, and the reduced intake of red and processed meats, which are rich in saturated fats and harmful additives. Thus, this diet contributes to a healthier and more balanced nutrition and provides greater satiety and lower caloric consumption [137, 138].
In a previous systematic review and meta-analysis, it was demonstrated that the DASH diet improved CVD risk factors, including total cholesterol and LDL cholesterol, in adults with or without comorbidities, reduced oxidative stress and inflammation, and improved endothelial function, proving that the DASH diet is efficient for the prevention of atherosclerosis [139]. Additionally, as shown by Ferguson et al. [140], the DASH diet scores (assessed using the Dixon’s DASH diet index) were inversely associated with the visceral adiposity index (VAI), which is an equation that incorporates WC, BMI, circulating TAGs, and HDL data into sex-dependent equations as an estimation of visceral adiposity [141]. DASH diet was significantly associated with lower concentrations of leptin and C-peptide [142], and higher adiponectin levels [143]. In view of these benefits, the DASH diet can be useful to promote a systemic anti-inflammatory condition and to oppose the higher CVD risk associated with hormonal changes and central fat mass accumulation observed in postmenopausal women.
Despite the extensive body of research on different dietary patterns, the best diet for postmenopausal women is still debatable, with no clear superiority of one over the others. As described here, there are some diet options to maintain or to reduce body weight and fat mass as well as to get healthy adipose tissue. Furthermore, diets rich in fiber and low in carbohydrates with a high glycemic index have the potential to prevent insulin resistance and consequently T2D [116, 144]. Concomitantly, replacing the amounts of saturated fats with unsaturated fats reduces the LDL and increases HDL levels, improving the lipid profile. Thus, dietary changes can reduce cardiovascular risk in postmenopausal women, however, any expected benefit from diets depends on adherence and the specific condition of each individual.
Aerobic exercise (AE) refers to the type of repetitive and structured physical activity that requires the metabolic system to predominantly use oxygen to produce energy [145]. In turn, RE is any type of physical activity that employs the exercise of a muscle, or group of muscles, against external resistance with the goal of improving muscle strength, endurance, or power [146]. Both forms of physical exercise are recommended for the prevention and treatment of obesity, since AE has been shown to be effective in increasing daily caloric expenditure and substrate oxidation, and reducing body fat percentage, while RE increases daily caloric expenditure and promotes the maintenance of lean mass [147, 148].
According to the American College of Sports Medicine Consensus Statement about physical activity and excess body weight and adiposity for adults [81], a weekly caloric expenditure of 1,200–2,000 kcal should prevent body weight gain, while values between 2,000–3,360 kcal weekly can promote body weight loss. Considering that an energy deficit is necessary for body weight reduction, there is a dose-response relationship in the amount of caloric expenditure promoted by physical exercise and the expected body weight loss [148].
Although both AE and RE have the potential to improve body composition, systematic reviews with meta-analyses have shown that the magnitude of the effects is different in distinct parameters. While RE and high-intensity AE do not affect total body weight, moderate-intensity AE can reduce this variable. Part of this effect can be explained by the duration of the training session, which can promote greater energy expenditure and impact the caloric deficit. In addition, both high- and moderate-intensity AE led to a reduction in BMI, which again is not seen in RE possibly due to the increase in lean mass, hiding the results in BMI [21].
The influence of physical exercise on body weight has been extensively investigated and has often yielded contradictory results due to the heterogeneity of individual responses and the differences in types of exercises, intensities, and volumes. Thus, some researchers tried to find out which factors lead to these disparities and whether there is any estimate for weight loss [18]. As seen in previous studies, weight loss through RE or simply through an increase in the number of daily steps is unlikely. On the other hand, weight loss through AE from 0–2 kg is only likely if performed with a very high volume, precisely because of the impact of this variable on the session caloric expenditure [18].
In 2024, the American College of Sports Medicine published a statement showing that exercise-induced body weight reduction ranges from 0.5–3 kg. The work also reinforces that the weight loss response is dose-dependent on caloric expenditure and AE should be used to improve cardiovascular capacity and maximum oxygen consumption (VO2 max), while RE should increase strength, and muscular endurance and maintain lean mass to allow greater caloric expenditure in the session [81].
In addition to positive effects on body weight, physical exercise can reduce total body fat, visceral fat, and WC [21]. In a previous meta-analysis, it was shown that both high- and moderate-intensity AE promoted body weight reduction in women with obesity, but only moderate-intensity AE was able to reduce body fat percentage, while RE had no effect on any parameter, either body weight or fat percentage [149]. Although these results follow the expected direction, a recent meta-analysis demonstrated that high-intensity AE has a greater effect on vWAT than moderate-intensity AE and RE, which could impact cardiometabolic health markers [21]. In postmenopausal women, AE can block increases in body weight and BMI, improve the amount of lean mass and body fat percentage [150], and ameliorate markers of cardiovascular health [151, 152]. In addition, aerobic high-intensity interval training reduces body weight, total fat percentage, and visceral fat, but subgroup analyses showed no difference in any of these parameters in postmenopausal women [153].
It is important to notice that adaptation in the skeletal muscle oxidative phenotype is decisive for WAT responses to exercise because it can increase fatty acid and glucose oxidation [154]. With these effects, the liver receives fewer fatty acids, preventing the development of MASLD, improving the lipid profile, and reducing cardiovascular risk [102]. Concomitantly, exercise increases systemic insulin sensitivity and reduces blood glucose, which prevents insulin resistance and T2D. In addition, it improves the metabolic flexibility of the heart, reducing inflammation, oxidative stress, and fibrosis [155]. Figure 2 summarizes the effects of a balanced diet and physical exercise on body composition that are crucial for the reduction of cardiometabolic risk in postmenopausal women.
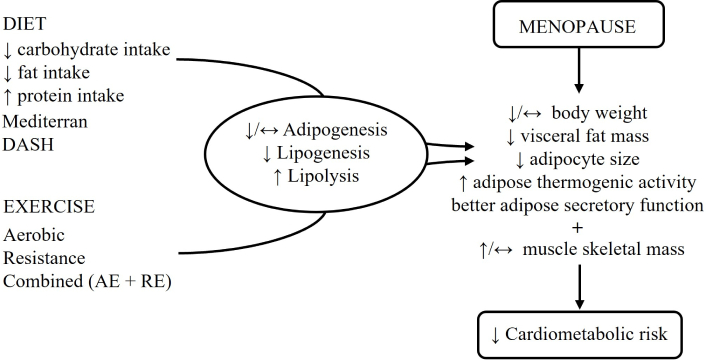
Effects of balanced diet and physical exercise on body composition and the reduction of cardiometabolic risk after menopause. DASH: Dietary Approaches to Stop Hypertension; AE: aerobic exercise; RE: resistance exercise; ↑: increase; ↓: decrease; ↔: unchange
Physical exercise modulates the adipose metabolic dynamic since its effects are mediated by changes in lipolytic and lipogenic activities that favor fat oxidation instead of fat storage, resulting in lower TAG content and adipocyte size [156, 157]. In a study with rats, AE significantly reduced the amount of sWAT and vWAT [158], as well as the size of adipocytes [159]. Part of this effect may be due to an increase in the expression and activity of lipolytic enzymes such as HSL and ATGL, better oxidative capacity associated with elevated expression of AMP-activated protein kinase (AMPK), and an improvement in mitochondrial dynamic [157, 160, 161]. AE can also reduce lipogenesis since the expression of ACC [157] and sterol regulatory element-binding protein 1c (SREBP1c) transcription factor were reduced in vWAT [162]. In previous studies conducted by our group, we demonstrated that the vWAT and sWAT remodeling induced by AE was associated with the prevention of obesity and insulin resistance in mice [157, 163], and contributed to preventing lipotoxicity in the kidney [164]. These results support the AE efficiency for maintaining metabolically healthy WAT, as well as for preventing comorbidities.
WAT adipogenesis was investigated in male and female mice submitted to voluntary exercise [165]. The authors found reduced adipocyte formation in sWAT and vWAT even in the absence of changes in body weight or total fat mass, suggesting that reduction in adipogenesis also may be an important exercise-induced mechanism of WAT remodeling. These results were obtained in lean mice; however, further study is needed to test if these responses are also observed in the condition of obesity.
Regarding the RE, the reduction in fat mass and adipocyte size in vWAT appears to be due to smaller lipogenesis and not to changes in lipolysis, since the expression of HSL enzyme did not change [166]. Furthermore, RE reduced the gene expression of ACC, PPARγ, and SREBP1c, which are involved in the lipogenesis process [166]. In postmenopausal women with obesity, RE did not change the expression of lipolytic enzymes such as HSL, ATGL, and MAGL in sWAT [167]. Similarly, no changes in the lipolytic enzymes ATGL and HSL were observed in animals with obesity that underwent RE, but the expression of stearoyl-CoA desaturase 1 (SCD1) was reduced, which suggests lower lipogenesis even in the absence of changes in lipogenic proteins such as ACC and FAS [168].
The effect of physical exercise on the thermogenic capacity of adipose tissue is still discussed in the literature. In the BAT, the thermogenic activity can increase, reduce, or remain unchanged after AE [169]. Fu et al. [170] showed that AE increased BAT deposition, restored the expression of UCP1 and AMPK, and promoted greater mitochondrial biogenesis in animals with obesity. In BAT of aged animals, both AE and RE increased the expression of AMPK, PGC1-α, and proteins involved in the electron transport chain [171]. On the other hand, Martinez-Tellez et al. [172] showed no effect of combined AE and RE on BAT volume and glucose uptake. Interesting that the adrenergic activation, metabolic production, and protein expression induced by AE can promote browning of WAT, with a metabolically favorable condition, more mitochondria, greater oxidative capacity, and less TAG [48]. In fact, our group showed that AE was able to increase the expression of UCP1 in sWAT, which is associated with metabolic improvement [163, 173]. Similarly, Otero-Díaz et al. [173] showed that AE increased the expression of brown and beige genes in abdominal sWAT of non-diabetic individuals with different BMI (normal, overweight, and obese).
Few studies have evaluated the isolated effect of RE on BAT. In fact, there is some evidence that this form of exercise can reduce BAT mass and that this reduction is due to a decrease in lipid droplets [174], but this result is not consistent [175, 176]. On the other hand, in elderly animals, RE prevented the reduction in BAT mass caused by age, which would directly affect energy expenditure throughout life, protecting against obesity [171]. Also, in relation to adaptations that increase energy expenditure, RE can stimulate the browning of WAT since it increases the gene and protein expression of UCP1 in both vWAT and sWAT [171, 174].
While increasing thermogenesis through higher activity of brown and beige adipocytes improves metabolic health, reducing the size of white adipocytes makes them healthier because they are more functional, capable of allowing better cellular communication, expressing fewer inflammatory substances, and secreting adipokines that are beneficial to metabolism [14, 17].
The benefits of physical exercise in the secretory function of adipocytes have been widely studied in the literature, and here we show some examples. Moderate-intensity AE increased circulating adiponectin in women [177] and animals [157], and high-intensity AE increased adiponectin gene expression in sWAT of humans [178] and animals [179]. Furthermore, RE increased the expression of adiponectin gene in the vWAT of type 1 diabetic mice [180], but reduced plasma concentration of adiponectin in postmenopausal women with vasomotor symptoms [181]. Increasing adiponectin contributes to improving insulin resistance by stimulating lipid oxidation and anti-inflammatory responses [182], however, not all studies showed the same changes in adiponectin concentration possibly due to the lack of training session intensity control [183], the practice of different exercise types [184], and lack of diet control and body composition analyses [181].
With respect to the adipokine leptin, Otero-Díaz et al. [173] showed that AE did not alter the expression of the leptin gene in sWAT. However, this response may vary according to the amount of fat mass, since in obese women there is a reduction in the expression of the leptin gene after AE [183]. In addition, lower serum leptin concentration was observed after AE [185]. Also, RE reduced leptin concentration after three different protocols (hypertrophy, muscular strength, and endurance) compared to baseline, but no differences were observed when compared to the control group possibly due to a lower energy expenditure of each session (300 kcal) [186]. In fact, when the energy expenditure of the session was higher (800 kcal), a decrease in serum leptin concentration was observed after RE [187].
FGF21 is another protein that has been investigated with physical exercise due to its involvement in glucose and fatty acid metabolism [188, 189]. AE enhances the oxidative machinery of sWAT and BAT by increasing the gene expression of FGF21, which will activate the AMPK pathway, stimulating lipolysis and β-oxidation [190]. Similar results were identified in RE since it increased FGF21 level in both vWAT and sWAT in animals with obesity, but only in the latter was observed an increase in PGC1-α protein expression [191]. Interestingly, when AE and RE were combined in the same training session, the increase in FGF21 tissue levels induced by RE was blocked [191]. In postmenopausal women with T2D, both AE and RE increased serum concentration of FGF21, which was associated with a reduction in fasting glucose, insulin, LDL, total cholesterol, and TAGs [192].
Although FGF21 is mostly secreted by the liver and BAT, it is shown in mice that high-intensity AE increases the expression of FGF21 in skeletal muscle [193]. In the same study, the authors found a positive correlation between the protein expression of FGF21 and the protein expression of UCP1 in sWAT, suggesting that the browning of sWAT induced by AE may be mediated, at least in part, by FGF21 produced in skeletal muscle [193]. Studies in humans support this result since high-intensity AE (85% of VO2 max) promoted greater systemic levels of FGF21 [194].
Physical exercise also modulates the secretion of inflammatory cytokines, which helps to reduce the chronic low-grade inflammation of adipose tissue typically observed in obesity. Félix-Soriano et al. [195] demonstrated that AE reduced proinflammatory adipokines such as TNF-α, IL-6, and macrophage-attracting proteins pro-inflammatory integrin alpha X (Cd11) and anti-inflammatory mannose receptor C type 1 (Cd206), suggesting a reduction in macrophage infiltration in the sWAT of aged obese mice. On the other hand, BAT was less responsive to AE with few changes in inflammation-related and fatty acid metabolism genes [195]. Similar effects were obtained with RE, since lower inflammation level was found in the vWAT of adult and elderly mice [196]. Other studies utilizing RE have shown decreased TNF-α protein levels in adipose tissue, creating a more functional and healthy adipose tissue that contributes to reducing body weight, and adipocyte area, and improves insulin resistance [197].
Although there are no meta-analyses studies on tissue inflammation outcomes in postmenopausal women, the effect of physical exercise on serum inflammatory markers has been investigated. Indeed, in a systematic review, it was demonstrated that moderate-intensity AE, RE, and combined exercises reduced C-reactive protein in postmenopausal women with overweight and obesity. However, the reduction in TNF-α levels occurred only after AE and RE, and the reduction in IL-6 and increase in adiponectin levels only after AE [198]. Reductions in IL-6, TNF-α, and C-reactive protein were also observed in postmenopausal women with obesity after 12 weeks of moderate-intensity AE, which were associated with lower BMI [199]. After 15 weeks of RE, postmenopausal women showed a reduction in the pro-inflammatory adipokines lipocalin-2 and resistin, however, adiponectin did not change possibly due to the characteristics of exercise performed [181].
The search for the most appropriate type of exercise to promote healthy adipose tissue and cardiometabolic benefits is advancing, however, there are no specific exercise guidelines for postmenopausal women. In any case, the evidence produced to date suggests that the combination of both AE and RE should be recommended to improve body composition and to achieve a healthy adipose tissue with smaller white adipocyte size, better brown/beige thermogenic activity, and adipose secretory function. It is important to keep in mind that when exercise is not prescribed appropriately, with adequate intensity, volume, and caloric expenditure, its potential becomes limited [200]. Thus, exercise prescription should be done by physical education professionals who will supervise the exercise session to optimize results with security and promote greater adherence.
In conclusion, studies on body weight and adipose tissue dynamics after menopause provide valuable insight into the complex mechanisms underlying adipose tissue function and the increase in the risk of cardiometabolic diseases. Lifestyle interventions are recommended since the combination of adequate dietary intake and physical exercise can reduce body weight, increase fat-free mass, and promote healthy adipose tissue with lesser accumulation of visceral fat, smaller adipocyte size, and better thermogenic activity and secretory function. Continued research in this field is important in advancing our understanding of the influence of lifestyle on adipose tissue after menopause and ultimately improving the prevention and treatment of related disorders.
ACC: acetyl-CoA carboxylase
AE: aerobic exercise
AMPK: AMP-activated protein kinase
ATGL: adipose triglyceride lipase
ATP: adenosine triphosphate
BAT: brown adipose tissue
BMI: body mass index
CVD: cardiovascular disease
DASH: Dietary Approaches to Stop Hypertension
DNL: de novo lipogenesis
FAS: fatty acid synthase
FGF21: fibroblast growth factor-21
HDL: high-density lipoprotein
HSL: hormone-sensitive lipase
IL-6: interleukin 6
LDL: low-density lipoprotein
MAGL: monoacylglycerol lipase
MASLD: metabolic-associated steatotic liver disease
NEFAs: non-esterified fatty acids
PGC1-α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha
PKA: protein kinase A
PPARγ: peroxisome proliferator-activated receptor–γ
RE: resistance exercise
SREBP1c: sterol regulatory element-binding protein 1c
sWAT: subcutaneous white adipose tissue
T2D: type 2 diabetes
TAG: triacylglycerol
TNF-α: tumor necrosis factor-alpha
UCP1: uncoupling protein 1
VO2 max: maximum oxygen consumption
vWAT: visceral white adipose tissue
WAT: white adipose tissue
WC: waist circumference
We thank Cynthia Walser for the careful review of the English in this manuscript.
FSE: Conceptualization, Writing—original draft, Writing—review & editing. BV, TLC, and NJRF: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study was supported by the São Paulo Research Foundation (FAPESP) to BV [2020/12005-0] and FSE [2020/15748-4]. TLC and NJRF held a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Butheinah A. Al-Sharafi, Samih A. Odhaib
Nagamani Gumpeny ... Sridhar R. Gumpeny
