Affiliation:
1Independent Scholar, Rovereto, 38068 Trento, Italy
Email: leokhanke@hotmail.com
ORCID: https://orcid.org/0009-0004-7863-4510
Affiliation:
3Department of Pharmacy, University of Salerno, 84084 Salerno, Italy
Email: paolamolettieri@gmail.com
Explor Foods Foodomics. 2023;1:244–257 DOI: https://doi.org/10.37349/eff.2023.00019
Received: July 10, 2023 Accepted: November 14, 2023 Published: December 28, 2023
Academic Editor: Marcello Iriti, Milan State University, Italy
The article belongs to the special issue Ketogenic Diet as Medical Nutrition Therapy
Cardiovascular disease (CVD) stands as the leading global cause of mortality, underscoring the critical need for practical tools to assess CVD risk at an early stage. An accessible approach involves the evaluation of bloodwork alongside simple anthropometric measurements. This narrative review seeks to establish the appropriateness of common parameters used in the outpatient setting in assessing the risk of developing CVD. These parameters encompass bloodwork values employed to characterize insulin resistance (IR) and dyslipidemia, as well as anthropometric measurements used to describe issues related to overweight and fat distribution. A particular emphasis is placed on understanding how Mediterranean and ketogenic diets influence these parameters. In the realm of bloodwork, findings indicate that the triglycerides (TG) to high-density lipoproteins (HDL) ratio serves as a valuable tool for assessing both IR and dyslipidemia. Less emphasis should be placed on total cholesterol and low-density lipoproteins (LDL) because the existing literature lacks consistency and fails to establish a clear, direct correlation between cholesterol levels, CVD, and mortality. On the other hand, numerous studies consistently demonstrate a direct correlation between CVD, mortality, and the levels of small-dense LDL (sdLDL), which represent the oxidized form of LDL. Regarding anthropometric parameters, the body mass index (BMI) falls short in value as it neglects to consider fat distribution and lean mass. More informative are anthropometric parameters that account for a single measure of fat mass and another for lean mass, such as the waist-height ratio (WHtR) or the waist-hip ratio (WHR). Both Mediterranean and ketogenic diets demonstrate improvements across major parameters used to evaluate CVD and mortality risk. The ketogenic diet, in particular, yields superior results in most aspects, except cholesterol levels. Further studies are recommended to refine dyslipidemia characterization and its connection to health outcomes.
The leading cause of death worldwide is cardiovascular disease (CVD), to the extent that discussions about cardiovascular risk are closely intertwined with discussions on mortality. There are numerous risk factors associated with CVD, which can be categorized into two main groups: those unaffected by dietary choices and those influenced by them. The former group comprises factors such as age, family history, physical inactivity, smoking habits, gender, and ethnicity. On the other hand, the key factors influenced by nutrition include insulin resistance (IR), dyslipidemia, and overweight.
In this review, we will explore the most commonly employed parameters for assessing CVD risk factors in outpatient settings. We will delve into their reliability and the extent to which they are impacted by Mediterranean and ketogenic diets.
An effective parameter should be straightforward to measure, offer valuable insights, exhibit high reproducibility and precision, and be cost-effective. Establishing a robust and unequivocal parameter for describing the risk of CVD and mortality is a complex endeavor, likely due to the multifactorial nature of CVD. We will evaluate the most prevalent blood chemistry tests employed to assess IR and dyslipidemia, as well as the commonly used anthropometric parameters, namely, body mass index (BMI), waist circumference (WC), waist-hip ratio (WHR), and waist-height ratio (WHtR).
We employed recent meta-analyses and other publications sourced from PubMed to establish a qualitative ranking of the most appropriate parameters for assessing cardiovascular risk through blood tests and anthropometric measurements.
According to data from the World Health Organization, in 2019, type 2 diabetes (T2D), which represents the advanced stage of IR, was responsible for 1.5 million fatalities, and in 2012, 2.2 million deaths were attributed to elevated blood sugar levels. Diabetic patients face a twofold increased risk of mortality, particularly due to elevated cardiovascular risk [1].
Hyperinsulinemia stands as an independent risk factor for mortality and is associated with a multitude of cardiovascular risk factors, including T2D, hypertension, impaired glucose tolerance, high triglycerides (TG), low levels of high-density lipoprotein (HDL) cholesterol, and central adiposity. Thus, it is imperative to possess effective tools for its early detection [2].
Direct methods for assessing IR, such as the hyperinsulinemic-euglycemic clamp (recognized as the most precise and gold standard for medical research), the glucose tolerance test (GTT), and the insulin suppression test, offer remarkable precision. However, they are hindered by their intricacy, high cost, time-intensive nature, and the need for specialized personnel. Consequently, these methods are not typically employed in outpatient clinical settings.
Indirect measurement techniques, while somewhat less precise, are practical and cost-effective, rendering them better suited for routine outpatient practice. The homeostatic model assessment (HOMA) for IR (HOMA-IR), derived from fasting blood glucose and insulin levels (glycemia × insulin/22.5), is a well-established and frequently used method. Studies by Bonora et al. [3] have demonstrated a strong correlation between measurements obtained through the euglycemic clamp and HOMA-estimated insulin sensitivity (r = –0.820, P < 0.0001), validating HOMA-IR as a reliable tool for assessing insulin sensitivity when only a fasting blood sample is available. The specific HOMA-IR cutoffs for identifying IR vary based on ethnicity and are determined locally [4].
Another reliable alternative is the quantitative insulin sensitivity check index (QUICKI), calculated as 1/[log (fasting insulin μU/mL) + log (fasting glucose mg/dL)] [5]. It exhibits a substantial correlation with glucose clamp studies (r = 0.78) and serves as an effective means of evaluating insulin sensitivity [6].
Hemoglobin A1c (HbA1c), a parameter frequently used in T2D diagnosis, also proves valuable for assessing IR [7]. HbA1c provides a representation of average blood sugar levels over the previous 2–3 months and can be evaluated independently or in conjunction with HOMA-IR. In the latter case, it has shown superior predictive capabilities for T2D development [8]. With a cutoff of 5.8% HbA1c (40 mmol/mol), the estimation of IR exhibits a sensitivity of 34% and a specificity of 80% [9].
Given the strong association between IR and elevated TG, the TG glucose index (TGI) has been proposed as a valuable parameter for assessing IR. TGI is calculated using the formula: ln [fasting TG (mg/dL) × fasting blood glucose (mg/dL)]/2 [10]. Some authors argue that this parameter, in addition to being more cost-effective as it doesn’t require costly insulin measurements, may even be more reliable than HOMA-IR. This increased reliability likely stems from its consideration of both glucotoxicity and dyslipidemia, which are pivotal in the development of IR [11]. A proposed cutoff of 4.5 for TGI has shown a sensitivity of 84% and specificity of 45% for diagnosing IR.
As reported by Aslan Çin et al. [12], the TG/HDL ratio is also an excellent parameter, on par with TGI, for evaluating IR and metabolic syndrome. Both of these parameters appear to exhibit higher sensitivity and specificity compared to HOMA.
In assessing IR in an outpatient setting, various parameters have been considered, all contributing significantly to estimating IR. TGI and TG/HDL ratios appear to be the most valuable, followed by QUICKI and HOMA (especially when combined with HbA1c). For more precise evaluations, a hyperinsulinemic-euglycemic clamp should be conducted in a hospital environment.
The efficacy of a ketogenic diet in improving IR is evident from multiple studies. As demonstrated in the meta-analysis by Choi et al. [13], a ketogenic diet resulted in a significant reduction in HbA1c of –0.5% (P < 0.001) in diabetic patients and –0.42% (P < 0.001) in the general population, along with a decrease in the HOMA index.
Similar beneficial effects have been observed in studies focusing on the Mediterranean diet. A meta-analysis by Kastorini et al. [14], which encompassed both randomized controlled trials (RCTs) and epidemiological studies, reported that adherence to the Mediterranean diet was associated with lower HOMA-IR levels compared to control diets, albeit with some heterogeneity in HOMA-IR levels.
Consistent outcomes were achieved in another meta-analysis comprising 10 RCT studies conducted by Moosavian et al. [15]. Among the articles considered, four reported a significant reduction in HOMA-IR, two reported a reduction in insulin levels, and one indicated reductions in fasting glucose and HbA1c.
Both the ketogenic and Mediterranean diets seem to effectively enhance insulin sensitivity and glycemic control, making them valuable tools for individuals with impaired insulin sensitivity or T2D seeking to regain control over their blood sugar levels.
When it comes to glycemic control, there is a growing consensus in favor of promoting the Mediterranean and ketogenic diets. However, assessing dyslipidemia presents a more contentious issue. Dyslipidemia, a key cardiovascular risk factor, in its acquired form, is typically defined as an elevation in TG or cholesterol, and/or a low level of HDL cholesterol.
It is worth noting that low-density lipoproteins (LDL) cholesterol is the primary lipoprotein assessed to evaluate the risk of developing CVD. However, the extent to which it truly correlates with CVD and overall mortality remains somewhat unclear.
The concern surrounding cholesterol dates back to 1913 when the Russian pathologist Nikolai Anitschkow conducted a controversial experiment. In this study [16], Anitschkow fed laboratory guinea pigs a diet consisting solely of pure cholesterol, which led to the development of atherosclerosis. It’s important to note that this experiment had limitations, as it didn’t account for the fact that rodents are not an ideal model for drawing conclusions about human metabolism, and pure cholesterol is not a substance typically found in a normal human diet.
Fast forward to the 1950s, and concerns regarding cholesterol intensified, largely due to the work of Ancel Keys [17]. His influential epidemiological publication, the “Seven Countries Study”, laid the foundation for contemporary dietary guidelines. This study revealed a direct correlation between the consumption of saturated fats in different populations across seven countries and the associated risk of CVD. The emerging connection between cholesterol levels and increased mortality from cardiovascular causes became a dominant paradigm. The notion that elevated cholesterol levels posed a significant risk became so deeply ingrained that subsequent studies often focused on describing the effects of specific interventions on secondary outcomes, such as cholesterol levels, assuming they would also impact primary outcomes like CVD and mortality. However, as it turns out, the relationship between cholesterol and these primary health outcomes is not as straightforward as once believed. RCTs that have explored the link between cholesterol and primary health outcomes, including mortality and CVD, present a different perspective.
Ancel Keys’ renowned study has sparked numerous controversies. Interestingly, the study initially encompassed not just 7 but 22 countries, and when all available data is considered, the purported connection between cholesterol and mortality does not materialize. Keys has faced allegations of selectively utilizing data to construct a narrative that supported his preconceived notion concerning the relationship between cholesterol and CVD. The debate on this issue has intensified in recent years, with meta-analyses conducted since 2014 revealing no direct correlation between serum cholesterol levels and mortality [18, 19].
Ancel Keys himself is credited with the authorship of one of the most significant double-blind studies ever conducted in the field of nutrition, focusing on the topic of cholesterol: the “Minnesota Coronary Experiment”, which took place between 1968 and 1973, involving 9,423 participants. In this RCT, mortality rates were compared between a group of participants with high cholesterol levels and another group that substituted dietary saturated fats with linoleic acid, resulting in a notable reduction in serum cholesterol levels. Surprisingly, the study’s findings contradicted the author’s expectations. It revealed that for every 30 mg/dL reduction in serum cholesterol, there was a 22% increase in mortality. The study remained concealed for years and was only published posthumously in 2016 after it was discovered in an archived location [20].
A similar fate befell the “Sydney Diet Heart Study”, conducted from 1966 to 1973 but only published in 2013. In this study, 221 out of 458 participants replaced dietary saturated fats with polyunsaturated vegetable oils, resulting in a reduction in serum cholesterol levels. However, this change also correlated with an increased rate of death from all causes and CVD [21].
Recent meta-analyses, such as the one by Harcombe et al. [19], demonstrate no direct link between cholesterol levels and mortality, suggesting a need for a reevaluation of dietary guidelines.
Furthermore, a recent epidemiological study led by Yi et al. [22], involving nearly 13 million people, and a meta-analysis incorporating data from approximately 30,000 individuals [23], analyzed the relationship between mortality and serum cholesterol. Both studies indicated that this association follows a U-shaped curve, with the lowest mortality occurring in patients with cholesterol values between 200 and 250 mg/dL. The risk of developing CVD was more pronounced in individuals with lower cholesterol values than those with higher values.
As the body of evidence continues to grow, it becomes increasingly clear that the quantity of serum cholesterol is not directly correlated with an increased mortality rate. Instead, numerous cross-sectional and prospective studies demonstrate a significant link between coronary heart disease and LDL particle size. The risk associated with LDL cholesterol appears to be more qualitative than quantitative, with the atherogenic potential of LDLs increasing when they transform into small-dense LDLs (sdLDLs), which possess a heightened atherogenic potential [24, 25].
As early as 1994, Young et al. [26] identified a crucial finding that the atherogenic nature is not inherent in healthy LDLs but in sdLDLs. This observation has been consistently supported by various studies, including the one conducted by Lamarche et al. [27]. This study revealed that unlike LDLs, sdLDLs act as an independent cardiovascular risk factor. They are associated with elevated TG, increased apolipoprotein B (apoB) levels, and decreased HDL cholesterol—a lipid profile resembling that of individuals who consume carbohydrate-rich diets [28]. For instance, the study by Griffin et al. [29] found that individuals with high sdLDLs, high TG, and low HDL had a significantly elevated risk of coronary heart disease and acute myocardial infarction. Furthermore, studies by Takeuchi et al. [30]. and Alique et al. [31]. highlight that the main risk factors contributing to sdLDL formation are glycation and oxidation. Notably, a damaged apoB-100 (or simply apoB) impedes LDL clearance from the bloodstream, ultimately enhancing the formation of highly atherogenic sdLDLs.
To assess the risk of sdLDL formation, various methodologies can be employed. Although ultracentrifugation, nuclear magnetic resonance spectroscopy, and gradient-gel electrophoresis offer precise sdLDL measurements, they are often impractical for routine clinical use due to their complexity and cost. Instead, readily available surrogate data can be utilized to estimate sdLDL levels effectively.
The most suitable parameters for estimating sdLDL quantity, as indicated by Hayashi et al. [32], are TG levels and apoB measurement. The rise in TG is associated with carbohydrate consumption, particularly simple carbohydrates that lead to glycosylation damage on LDLs. Since each LDL carries only one apoB molecule, its value reflects the number of LDL particles, thereby allowing for the calculation of their size. A low apoB value corresponds to a small number of larger LDLs, whereas a high apoB value indicates a greater quantity of small atherogenic LDLs.
Another reliable parameter for assessing LDL quality is the TG/HDL ratio, as observed in the study by King et al. [33]. This ratio is found to be an effective predictor for an atherogenic LDL phenotype, akin to TG measurement alone. A study conducted by da Luz et al. [34] investigated various lipid variables and concluded that the elevation in the TG to HDL ratio was the most potent predictor for extensive coronary heart disease among all variables examined.
The ketogenic diet has been linked in various studies to an increase in LDL cholesterol. However, concurrently, there has been an improvement in the parameters indicating a low sdLDL fraction. The meta-analysis conducted by Choi et al. [13] incorporated lipid panel measurements as part of the parameters considered in ketogenic diet interventions. The results indicate the following: a ketogenic diet led to an increase in HDL cholesterol and a significant reduction in TG levels: the best indicators for assessing cardiovascular risk. However, the meta-analysis also reveals an increase in total cholesterol and LDL cholesterol. Athletes adhering to a ketogenic diet experienced an even more pronounced increase in LDL cholesterol [35], attributed to the increased mobilization of fatty acids due to their higher energy requirements. While having an increase in total LDL, this article reported that the 20 study participants also had a decline in sdLDL.
The shifts observed in the lipid panel under both Mediterranean [36] and ketogenic [37] diets are the same changes seen with reduced average blood sugar levels: an increase in HDL, coupled with decreased TG levels and an improved ratio between them. These parameters collectively signify a favorable state of LDL health. If concerns persist about cholesterol values, it is advisable to conduct a direct assessment of the sdLDL fraction.
The reduction in LDL oxidation and sdLDL formation can be attributed, in part, to reduced sugar consumption, a major contributor to glycation. Another key factor promoting lipoprotein oxidation is the consumption of highly oxidative industrial polyunsaturated fats, such as palm oil [38] and heated vegetable fats [39]. In both Mediterranean and ketogenic diets, it should be considered crucial to avoid these inflammatory fats to prevent the formation of sdLDLs.
Being overweight is a well-recognized factor associated with CVD and overall mortality. However, the strength of this relationship varies significantly depending on how overweight is defined and measured.
When it comes to assessing overweight and body composition, various anthropometric measurements are commonly used in outpatient practice. These include the BMI, body circumferences such as WC, WHR, and WHtR, as well as the calculation of the percentage of fat mass and lean mass. These measurements are straightforward to obtain and do not necessitate expensive equipment or specialized skills. Yet, the question remains: how accurate are they in predicting mortality and CVD?
BMI is a biometric measurement expressed as the ratio between an individual’s weight and the square of their height (in kg/m2). It is commonly used to categorize individuals as underweight (BMI under 18.5), normal weight (BMI between 18.5 and 24.9), overweight (BMI between 25 and 29.9), or obese (BMI over 30). However, it is a rudimentary index that doesn’t consider various factors, including gender, the ratio of lean-to-fat mass, and bone structure. The data regarding BMI and its relationship to mortality is a subject of controversy and has undergone several revisions in recent years.
In 2013, Flegal et al. [40] published a meta-analysis that included 97 studies involving 2.88 million individuals, leading to surprising conclusions. Their data indicated that individuals with the lowest mortality were those categorized as overweight or with a BMI in the range of 25 to 34.9, including those with an I degree of obesity. However, this study faced criticism for potentially including frail individuals in the normal weight group, such as the elderly, heavy smokers, and people who had lost weight due to illness [41]. The authors defended the legitimacy of their results [42], which were consistent with other studies suggesting that overweight might be a protective factor [43–45], leading to the concept of the “obesity paradox”.
Less contentious results come from a large meta-analysis published in The Lancet in 2016, which examined 239 prospective studies involving more than 10 million patients [46]. In this analysis, the lowest mortality was associated with people with a BMI between 20 and 25. Notably, the risk of mortality increases gradually for BMIs above 25, with a more significant increase in mortality for BMIs below 20, indicating that underweight individuals face a higher mortality risk than overweight individuals. A similar analysis by Aune et al. [47], which included 230 cohort studies, confirmed these findings, with the lowest risk of death observed in individuals with a BMI between 20 and 24.
Another study by Kee et al. [48] highlighted that increased mortality in underweight individuals is associated with an elevated risk of death from all causes, while in those with a high BMI, the primary cause of death is CVD. Additionally, a prospective study by Lee et al. [49] suggested that increased mortality in individuals with a low BMI is linked to a low amount of lean mass.
A large 2017 Korean study by Kong et al. [50] yielded very similar mortality trends, affirming that the lowest mortality rate occurs with a BMI between 24 and 26, with risk escalating more significantly as BMI decreases rather than increases.
The meta-analysis conducted by Carmienke et al. [51], encompassing 18 studies and 693,739 patients observed over periods ranging from 5 to 24 years, concludes that BMI, when considered in isolation, inadequately predicts the risk of death and should be complemented by other anthropometric measures capable of estimating visceral fat, fat percentage, and lean mass. The analysis revealed that individuals with the highest mortality risk had low BMI and high WC or WHR, characteristics corresponding to lower lean body mass [51].
A low BMI appears to be closely associated with increased mortality, whereas a high BMI proves an unreliable indicator for describing the risk of CVD and death, as it fails to account for body composition and fat distribution, both of which are crucial factors in risk assessment [52].
These findings collectively help explain the “obesity paradox”, with overweight individuals often having a lower risk of mortality.
Body composition proves significantly more informative than BMI alone in evaluating the risk of CVD and mortality. This is evident in the observation that individuals within the same BMI categories, but with abdominal adiposity, face elevated risks of coronary heart disease and T2D [52]. Furthermore, visceral fat stands out as a reliable independent predictor of death and cardiovascular risk, both for men [53] and women [54, 55]. Accumulation of visceral fat strongly influences WC, which, even within the normal weight range, is linked to a higher mortality risk. However, WC is insufficient for distinguishing between fat and lean mass. To achieve a more precise evaluation, we require anthropometric parameters that account for body composition, comparing a parameter representing fat mass with one representing lean mass [52].
While multiple scan CT offers the most accurate assessment of fat and lean mass percentages [56], it is impractical for routine outpatient use due to its cost and time requirements. Ultrasound techniques, as explained by Professors Monaco and Castaldo [57], provide a reliable method for quantifying visceral fat, but they also have limitations in terms of cost, operator dependency, and the need for extensive practice.
In the absence of the precision offered by instrumental tests, the most practical and cost-effective tools for assessing fat distribution in outpatient settings are anthropometric parameters. As highlighted in Carmienke et al.’s meta-analysis [51], whereas the relationship between BMI and mortality follows a U-shaped curve, certain anthropometric parameters exhibit a linear increase in mortality risk, such as WC and WHR. WC directly correlates with visceral fat amount and its related metabolic profile, a connection shared with WHR. Among these parameters, the WHtR is proposed as the best choice because it considers height, a value more closely linked to the total body surface area of the individual.
The meta-analysis conducted by Jayedi et al. [52], involving 72 prospective studies, further emphasizes the association between central adiposity indices, including WC, WHR, and WHtR, and an elevated risk of mortality.
WC, being easily measured and strongly correlated with visceral fat, stands out as a more reliable indicator than BMI for assessing CVD and mortality risk, consistently yielding uniform results in studies.
For instance, the study by Koster et al. [58], following 245,533 participants over nine years, revealed that a large WC, corresponding to the fifth quintile of the sample, was associated with a 25% increase in mortality compared to the second quintile. Additionally, individuals with a normal BMI but an increased WC experienced a 20% higher mortality rate compared to those with equivalent BMI but a normal WC.
Jacobs et al. [59], analyzing over 100,000 subjects over nine years, found double the mortality rate in individuals with very high WC compared to those with normal WC. High WC has also been linked to elevated levels of inflammatory markers, IR, T2D, dyslipidemia, and coronary heart disease [60].
Similar results were reported by Cerhan et al. [61], who analyzed a sample of 650,000 adults and observed a reduction in life expectancy of 3 years in males and 5 years in females with high WC compared to those with a low one, with a more pronounced effect in young patients. This is particularly concerning given the increasing prevalence of overweight among children and adolescents.
A large Korean study conducted by Kim et al. [62], involving 23 million subjects tracked from 2009 to 2015, underscores the benefits of reduced WC across all weight categories and suggests its routine measurement, even for individuals with normal weight. This underscores the importance of the proportion between lean and fat mass and the distribution of fat.
Both the study by Welborn et al. [63] and the previously mentioned study by Carmienke et al. [51] indicate that WHR rises linearly with the risk of death, making it a superior parameter when compared to BMI and WC.
Yet, another anthropometric parameter, WHtR, demonstrates remarkable accuracy in predicting cardiovascular risk and mortality. In a meta-analysis by Ashwell et al. [64], WHtR emerges as a superior index compared to BMI and WC.
In 2005, Ashwell and Hsieh outlined six compelling reasons for WHtR’s prominence as the best anthropometric parameter [65]:
WHtR serves as an early and more relevant indicator of health risk than BMI, particularly due to its association with central obesity. It also compensates for height differences, making it suitable for diverse populations.
WHtR is cost-effective and simple to calculate, requiring neither a scale nor the patient to undress.
It permits uniform cutoffs for both men and women, with a value of 0.5 used for both sexes.
WHtR allows for the analysis of populations with varying height characteristics collectively.
The 0.5 WHtR cutoff is easy to remember and promote, often conveyed as “keep your WC below half your height”.
WHtR is applicable to both adults and children.
Recent meta-analyses examining the effects of ketogenic diets on WC consistently report positive outcomes. Dietary interventions, as per Yuan et al. [66], resulted in an approximately 9 cm reduction, while Castellana et al. [67] reported a reduction of 12.6 cm. Given the critical role of WC in assessing cardiovascular risk and mortality, these findings support the use of ketogenic dietary interventions for cardiovascular risk prevention.
In contrast, the effects of the Mediterranean diet on WC are less dramatic. Kastorini et al. [14] calculated an average reduction of just 0.42 cm from data on 500,000 people. According to the meta-analysis by Malakou et al. [68], there was a decrease of 1.64 cm, while Akhlaghi et al. [69], analyzing 13 studies, did not report any significant changes in WC.
As summarized in Figure 1, in the outpatient setting, the TG-HDL ratio emerges as the most effective parameter for CVD and mortality risk. This ratio aptly captures both IR and dyslipidemia, the primary drivers of increased mortality. When assessing IR, additional reliable parameters include the TGI, HOMA-IR, and QUICKI. However, the latter two are relatively more expensive due to the need for insulin measurements.
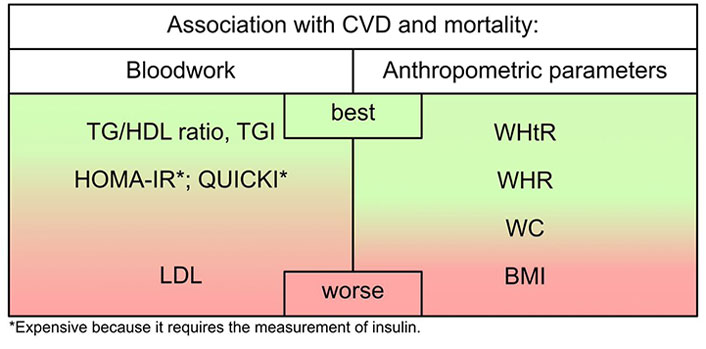
An evaluation of the efficacy of standard bloodwork panel outcomes and anthropometric parameters in delineating their link to CVD and mortality
Recent meta-analyses indicate that hypercholesterolemia, particularly the quantity of LDL cholesterol, lacks a direct association with CVD and mortality. Instead, there is a robust connection with sdLDL. A low TG-HDL ratio is linked to high-quality LDL, suggesting that concerns about the quantity of LDL cholesterol may be alleviated. If necessary, further investigations can be conducted to assess LDL quality, such as by evaluating apoB or directly measuring sdLDL.
BMI emerges as the least effective anthropometric parameter for evaluating CV and mortality risk due to its lack of consideration for fat distribution and lean mass. It exhibits a U-shaped relationship with mortality, offering limited value in risk assessment, except for individuals with underweight. The most versatile anthropometric parameter proved to be the WHtR, followed by the WHR. WC, while considering a single parameter, remains practical and should be a routine measurement.
Both Mediterranean and ketogenic diets have shown improvements in most cardiovascular risk parameters, with the exception of LDL cholesterol. It’s important to emphasize that LDL cholesterol has proven to be the least reliable indicator among all the parameters considered in describing CVD and mortality risk.
Further studies are needed to better evaluate the risk of developing CVD in relation to different lipid profiles and to reassess the guidelines regarding total cholesterol and LDL cholesterol.
apoB: apolipoprotein B
BMI: body mass index
CVD: cardiovascular disease
HDL: high-density lipoprotein
HOMA: homeostatic model assessment
HOMA-IR: homeostatic model assessment for insulin resistance
IR: insulin resistance
LDL: low-density lipoproteins
QUICKI: quantitative insulin sensitivity check index
RCT: randomized controlled trial
sdLDL: small-dense low-density lipoprotein
T2D: type 2 diabetes
TG: triglycerides
TGI: triglyceride glucose index
WC: waist circumference
WHR: waist-hip ratio
WHtR: waist-height ratio
PM: Supervision, Project administration. FP: Conceptualization, Data curation, Investigation, Methodology. LKH: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing—original draft, Writing—review and editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
The work is supported by Post Graduate University Course in “Diete e Terapie Nutrizionali Chetogeniche: Integratori e Nutraceutici (NutriKeto)”, from Dipartimento di Farmacia, University of Salerno. The University of Salerno contributed to the proposal and submission of the article, in addition, had no role in study design, data collection and analysis of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3464
Download: 37
Times Cited: 0
Giulia Izzo ... Mario Vitale
Paola Pellegrini ... Maria D’Elia
Xin Qi, Richard Tester
Büşra Atabilen, Yasemin Akdevelioğlu
Alejandro Borrego-Ruiz, Juan J. Borrego
