Affiliation:
Department of Dietetics and Applied Nutrition, Amity Medical School, Amity University Haryana, Gurgaon 122413, India
Email: lshrama@ggn.amity.edu
ORCID: https://orcid.org/0000-0002-4700-4792
Affiliation:
Department of Dietetics and Applied Nutrition, Amity Medical School, Amity University Haryana, Gurgaon 122413, India
ORCID: https://orcid.org/0000-0003-3264-4188
Explor Foods Foodomics. 2024;2:155–170 DOI: https://doi.org/10.37349/eff.2024.00031
Received: December 12, 2023 Accepted: February 05, 2024 Published: April 19, 2024
Academic Editor: Marcello Iriti, Milan State University, Italy
Infertility is a crucial and common health issue worldwide, that affects people both physiologically and psychosocially. The condition is described as the disease of the reproductive system of either male or female or both, incapability to instate a pregnancy after one year or more than twelve months of regular unprotected sexual intercourse or six months for women aged 35 years or more. Presently, the etiology of infertility is not well understood, many genetic factors, lifestyle factors, and environmental conditions such as stress work, oxidative stress, unbalanced nutrition, and unhealthy dietary patterns have been implicated to interfere with reproductive safety in both the sex. The nutritional factors are known to be amenable to normal and healthy reproductive function in both males and females. According to many studies, increased energy intake, dietary behavioral change, and low physical activity are responsible for epidemic disorders such as diabetes, heart disease, and obesity that affect reproductive health as well, and clear evidence indicates that there is a connection between inappropriate nutrition and sperm quality. Endocrinal disruption, occupational stress, and lifestyle behavior are positively linked with the pathophysiology of infecundity. Imbalance intake of both macro and micronutrients negatively affects normal reproductive function. Changes in eating behavior, and unhealthy dietary patterns such as a higher intake of food prepared with saturated and trans fats, spicy and salty foods, and a lower intake of antioxidants including fruit and vegetables are associated with reproductive life. This narrative review summarized that many studies with more consumption of fruit, vegetables, whole cereals, meat, poultry, skim milk, and seafood and less consumption of fried, spicy, salty, sugary, processed cereals and meats are linked with good sperm count.
Infertility is a crucial and common health issue worldwide, which affects people both physiologically and psychosocially [1]. So, infertility describes the disease of the reproductive system of incapability to instate a pregnancy after one year of regular unprotected sexual intercourse in either male or female or both [2], or the six months for women aged 35 years or more [3]. The disease affects up to 15% of the population globally where the male partner factors contribute approximately 40% of cases of infertility [4, 5]. Based on the reports by the World Health Organization, suggests that 186 million individuals and around 48 million couples are suffering from this disease globally [2].
Presently, the etiology of infertility is not well understood, many genetic factors, lifestyle factors, and environmental conditions such as stress work, oxidative stress, and unbalanced nutrition have been implicated to interfere with reproductive safety in both the sex. Oxidative stress may be defined as the imbalance in the production of free radicals and the ability of the body to neutralize their toxic effect through antioxidants [6]. The free radicals produced are also called reactive oxygen species (ROS). In the normal physiological process, ROS significantly regulates many processes in reproductive activity such as sperm development, acrosome reaction, hyperactivation, or fertilization but when elevated may cause cellular damage [7, 8]. Many pieces of evidence show that the antioxidants and dietary components play a crucial role in modulating spermatogenesis by minimizing the ROS concentration that is present in semen plasma and spermatozoa and boosting normal physiological function [9]. Besides the ROS, the abnormal body weight, energy supply, and nutrients all bring to bear a detrimental impact on spermatogenesis, ovulatory function, and implantation of the embryo.
A stressful life particularly hard-working stress may affect a large number of biological systems including the reproductive system. This type of stress may cause infertility where the symptoms are interconnected to anxiety and depression. These may produce psychological stress that may bring a change in the oocyte maturation in women [10]. Whereas in males stress may potentially affect semen parameters. The stress in the male may lower the luteinizing hormones (LH) and testosterone pulsing, which may cause lower sperm count and spermatogenesis [11, 12]. Many studies indicate that chronic and acute stress may cause amenorrhea and anovulation in women and reduce sperm motility, sperm count, and morphology of sperm in men [13, 14].
There is a fact that increasing stress and some mental disorders such as anorexia consequently alter the hypothalamic-pituitary-ovarian (HPO) axis. This alteration may cause to change in the reproductive hormonal milieu which in turn causes ovulatory dysfunction and infertility. Whereas in males the stress may cause coital and erectile dysfunction which leads to male infertility. The stress also affects the hypothalamic-pituitary-adrenal (HPA) axis. In this axis, corticotropin-releasing hormone or factor (CRF), cortisol, and adrenocorticotropin hormone (ACTH) play a crucial role. Several neurotransmitters such as oxytocin, epinephrine, norepinephrine, 3-endorphin, vasopressin, serotonin, and angiotensin II are known to respond the stress stimuli [15–17]. These are also called stress hormones because of their primary role in stress response. The increased CRF secretion is linked with the HPA axis. During a stress response, the catecholaminergic and serotoninergic systems act as markers for HPA activation and nullify stress response when interacting with some amines. The increased CRF activity in the brain may suppress the secretion of gonadotropin which in turn decreases gonadal function. In prolonged stress, there is a chronic increase in cortisol secretion that may lead to inhibiting the gonadotropin release [18]. There is still a lack of evidence that is there any direct effect of corticosteroids to suppress the gonadal function, so more research is needed to clarify this problem. How stress is linked with infertility, has been illustrated below (Figure 1).
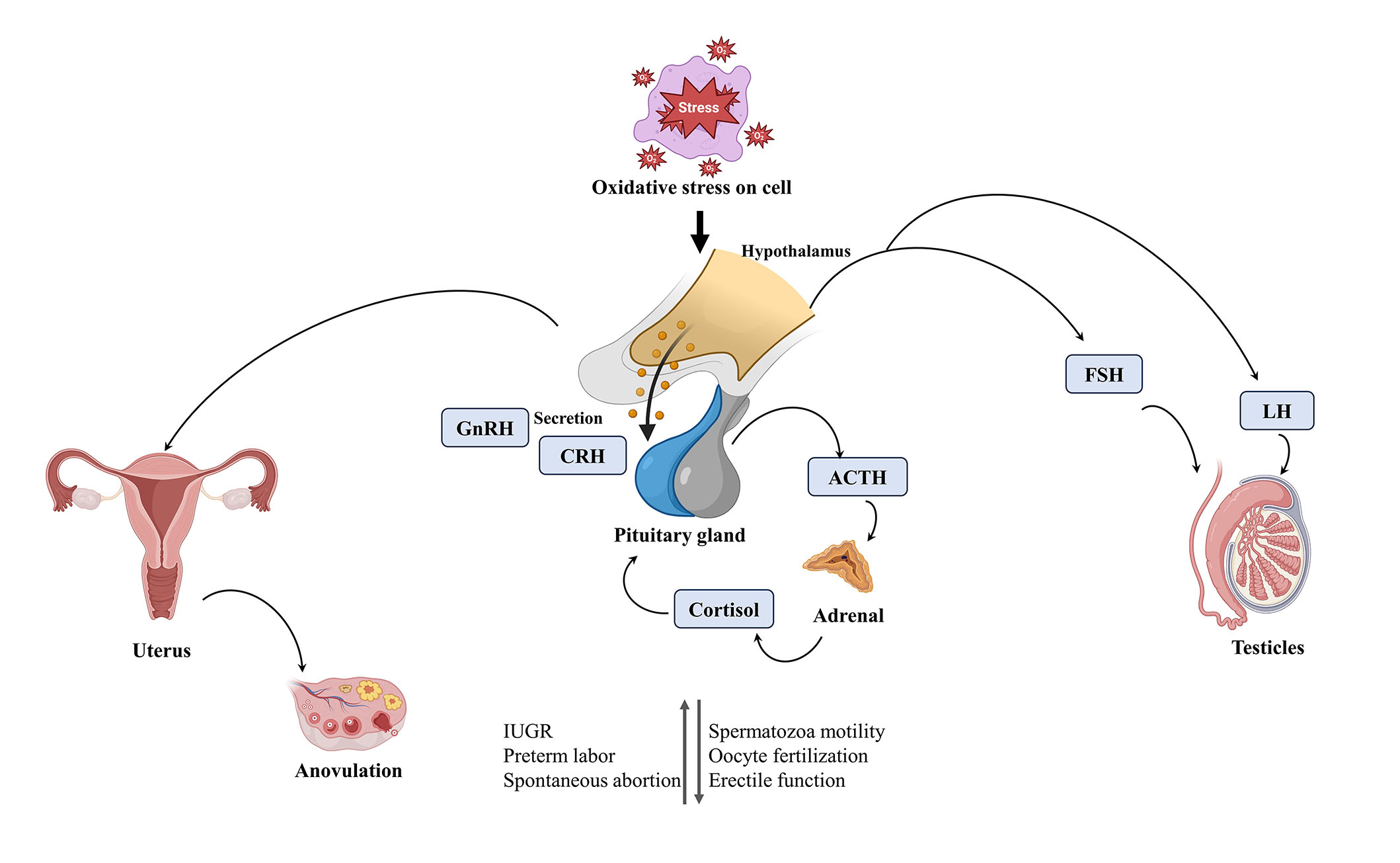
Indicating how stress is linked with infertility. GnRH: gonadotropin hormone-releasing hormone; CRH: corticotropin-releasing hormone; FSH: follicle-stimulating hormone; IUGR: intrauterine growth restriction. This figure was created in BioBender.com
The main aim of the current narrative review is to summarize the effect of psychological and physical stress on infertility (including both males and females) and the role of nutrition in combatting these problems.
Excessive emotional stress along with alteration in eating behavior, nutrition pattern, yoga, and physical exercise may cause prolonged anovulation. This is sometimes called hypothalamic amenorrhea. The increased cortisol levels are linked with amenorrhea and abnormal response to CRF in these women. Circadian changes have been also reported. In these women, the prolactin and cortisol responses are blunted in the afternoon meal [13, 19, 20].
Psychological distress is accredited as a contributing factor to infertility and is mostly found in infertile couples [21]. Women who have stressful lives and occupations mostly suffer from psychological amenorrhea. The circadian pattern of cortisol secretion and opioid tone changes have been noticed in such patients [22, 23].
Women suffering from anovulation and amenorrhea have noticed a close connection between stress and eating disorders. Both conditions reduce the LH-releasing hormone (LHRH) pulse generator, gonadal function, and gonadotropin secretion [24, 25]. Stress affects CRF, the aminergic system, and LHRH opioid peptides along with severe eating disorders such as bulimia and anorexia nervosa. These systems help to control reproduction, eating patterns, and autonomic functions, which shows the association of these disorders with infertility.
In women athletes, amenorrhea is mostly seen due to excessive exercise. They also suffer from several disorders such as oligomenorrhea, inappropriate luteal phase, anovulation, and delay in menarche onset [26, 27].
CRF, LHRH, and opioids are the brain hormones that are also found in the placenta. During stress responses, these hormones affect the secretion and function of the placental hormone as well. These changes may cause early pregnancy failure [13].
Many studies have been conducted to assess whether anxiety or depression is associated with infertility or not. One study shows that women having prolonged anxiety take longer to conceive and have more chances of miscarriage than women having lower anxiety [28]. One more study shows that women having a history of depression were more likely infertile than women who had no such history [29]. Additionally, one more study shows that in depression a higher level of LH is associated with infertility.
Stress may arise due to social or occupational pressure or in any way. Infertility itself is a stressful condition, associated with testing, diagnosis, failure in treatment, costs of the treatment, and social gossip [30]. Occupational stress is potentially linked with semen parameters. Stress may reduce testosterone pulsing and LH which in turn may reduce sperm quality and the rate of spermatogenesis [11, 12]. Whereas emotional stress is associated with oligospermia and semen quality [31].
As mentioned above, physical stress leads to reduced testosterone levels as the LH pulse diminishes, but it is not yet clear whether these effects are due to CRF/opioid system or ACTH/cortisol [32]. It is found that CRF and I-endorphin are stress peptides located in the testis and might play a paracrine role. Changes in these peptides may alter the endocrine and gametogenic factors of the testis [31]. Diet and exercise are also closely associated with stress-related infertility.
A study shows that acute stress is associated with impaired testicular function in the experimental rat. In some clinical study, the testicular tissues showed a higher level of glucocorticoids and displayed apoptosis of both Leydig and Gem cells [33, 34]. In humans, stress may be responsible for neuroendocrine, behavioral, and immune responses. Glucocorticoid receptors respond to glucocorticoids to regulate the physiology of Leydig and Sertoli cells. The nucleotide nuclear receptor subfamily 3 group C member 1 (NR3C1) polymorphism is linked with testicular function and sperm motility. Polymorphism of the glucocorticoid receptors could propound a response variability to stress [35, 36]. The emotional stress was negatively affecting the quality of semen. The overall result shows a decrease in 39% sperm concentration and 48% sperm motility on the day of the oocyte [37]. Furthermore, the study reveals that stressful life events, war, or environmental disasters do not affect sperm quality, but the stress burden associated with infertility.
Additionally, many more studies revealed that men undergoing infertility treatment have found low semen parameters during these treatments, but it is difficult to assess in such studies whether the stress is a cause or consequence of low semen quality [38, 39]. It is noticed that the stress increased after the diagnosis of infertility in males and failure of in vitro fertilization (IVF) treatment [30]. Another study shows that coping with stress may adversely affect fertility, which increases adrenergic activation that leads to more vasoconstriction in the testes [40].
According to the World Health Organization, being physically fit/active and having a nutritious/well-balanced diet are the key to a healthy lifestyle. This activity keeps a healthy body weight and reduces the severity of chronic disease [41]. There are several factors (such as modifiable and non-modifiable) that can affect fecundability and infecundity. So, the non-modifiable factors are the factors that can’t change such as genetics and age factors, whereas modifiable factors are unhealthy diet, dietary behavior, weight gain, excessive smoking, and alcohol consumption that remarkably affects the negative reproduction, but if improved or change the habits it might improve the reproductive health [42].
Among them, the nutritional factors are responsible for healthy reproductive function in both males and females. According to many studies, increased energy intake, dietary behavioral change, and low physical activity are responsible for epidemic disorders such as diabetes, heart disease, and obesity that affect reproductive health as well, and the results indicate that there is a connection between inappropriate nutrition and sperm quality [43–46]. Endocrinal disruption, occupational stress, and lifestyle behavior are positively linked with the pathophysiology of infecundity [43]. Imbalance intake of both macro and micronutrients negatively affects reproductive function [43, 44]. Changes in unhealthy dietary patterns and eating behavior, such as higher intake of food prepared with saturated and trans fats, spicy and salty foods, and lower consumption of antioxidants such as fruit and vegetables are associated with reproductive life. Many studies indicate that more consumption of fruit, vegetables, whole cereals, meat, poultry, skim milk, sea foods and less consumption of fried, spicy, salty, sugary, processed cereals and meats are linked with good sperm count [45, 46].
In many studies, it has been observed that healthy dietary patterns (such as the Mediterranean and Prudent diet) are positively connected with improved sperm quality. One study was conducted by using two dietary patterns (Western and Prudent dietary patterns) to analyze the factors associated with better sperm quality [47]. The Western pattern uses a high consumption of refined cereals, fast foods, high-energy drinks, and sugary foods along with red and processed meat, whereas in Prudent diet is associated with a high consumption of natural foods like high consumption of fruits, vegetables, and whole pulses and legumes, poultry, and fishes. The result showed that a Prudent diet was positively linked with progressively motile sperm [47]. Another study shows that a Prudent dietary pattern was linked with a decreased DNA fragmentation index (P = 0.5). High intake of such a diet is positively linked with increased sperm concentration and testosterone level and negatively linked with sperm chromatin structure [48]. A cross-sectional study was done among 225 men, and the result showed that the men with a lower tertile of Mediterranean diet score had a 2.6 times higher risk of sperm motility, sperm count, and abnormal sperm concentration than those with have higher tertile score [49]. The literature on the association between nutrients and dietary patterns on male fertility has been elaborately described in Table 1.
Literature on the association between nutrients and dietary patterns on male fertility
| Nutrient | Species | Studies | Dose and duration | Conclusions | References |
|---|---|---|---|---|---|
| Dietary pattern | |||||
| Mediterranean diet | Human | Cross-sectional study | 2 months | A healthy dietary pattern improved the semen quality, reproductive hormone level, and testicular volume of men. | [50] |
| Antioxidant nutrient | Case-control study | 2 years | The finding showed that adherence to antioxidant nutrients was inversely associated with asthenozoospermia. | [51] | |
| Healthy diet | Cross-sectional study | 3 years | The healthy diet intake improved the semen quality parameters. | [52] | |
| Proteins | |||||
| Soy product | Human | Prospective cohort study | 2004–2014 | Soy food intake was not linked with the clinical outcome of infertility. | [53] |
| Dietary fats | |||||
| Omega-3 fatty acid | Human | Cross-sectional study | 1 year | Trans fatty acids (TFA) are related to reduced fertility, whereas the omega-3 fatty acid was found protective against testicular volume. | [54] |
| Nut consumption | Randomized control trial | 14 weeks | The inclusion of nuts significantly improved the total sperm count and vitality, motility, and sperm morphology. | [55] | |
| Carbohydrate | |||||
| Low glycaemic food | Human | Cross-sectional study | 2008–2013 | The low glycaemic food might have a positive impact on total sperm motility, progressive motility, and normal sperm morphology. | [56] |
| Antioxidants | |||||
| Lycopene | Human | Randomized control trial | 25 mg/day for 12 weeks | The lycopene supplement improved the spermatogram and seminal oxidative stress. | [57] |
| 10 mg twice a day for 12 weeks | Lycopene helped to decrease the soluble receptor for advanced glycation end products (sRAGE). | [58] | |||
| Vitamin C | Human | Randomized control trial | 1 gm/day for 2 months | Vitamin C supplementation improved semen agglutination and increased viability. | [59] |
| 1 gm every other day for 6 months | The supplementation of vitamin C improved sperm concentration and motility. | [60] | |||
| Vitamin E | Albino Wistar rats | Randomized control trial | 500 mg/kg body weight, 3 times a week for 2 weeks | The testicular damage caused by the aluminum was diminished when supplemented with vitamin E. | [61] |
| Human | Clinical trial | 200 mg/day for three months | Supplementation of vitamin E decreased malondialdehyde levels and increased fertilization rates. | [62] | |
There is insufficient data available that may bring the connection between carbohydrate consumption and male reproductive potential. An observational study concluded that there was a positive link between semen quality and the consumption of fruits and cereals [63]. Additionally, a case-control study was carried out on 30 men suffering from lower sperm quality, which showed that the group has a higher intake of lipophilic foods (such as dairy and meat) was more likely to suffer from semen quality than the group having higher fruits, vegetables especially peach, apricot, and potatoes [46]. One more study shows that high carbohydrate consumption was linked with an increased prevalence of abnormal sperm count and motility [56]. One more study reported that the men who had a higher tertile of fruit and vegetable intake had a lower risk of asthenozoospermia [64].
High dietary protein intake and different dietary sources such as higher dairy and meat products may affect testosterone and LH and will cause male infertility. One study reported that the maternal beef intake during pregnancy may alter a man’s testicular development which adversely affects his reproductive capability, this is due to the presence of xenobiotics (an anabolic steroid) present in beef [65]. The result shows that the mother had higher beef consumption, her son was more prone to lower sperm concentration of 24.3%, and below 20 × 106/mL was three times higher than those whose mother consumed less beef [65]. A high intake of processed meat was linked with poor semen quality [63]. A similar result was revealed in a case-control study that the risk of asthenozoospermia was higher in the group that had the highest tertiles of processed meat [64]. One study reported that the men who adhered to traditional Dutch dietary patterns had higher sperm concentrations than those who stuck to a diet with high fish intake [66].
Another study shows that a high intake of plant-based protein such as soy protein on male infecundity [67]. Soy foods contain a high concentration of isoflavones. These phenolic compounds show estrogenic activity, which might adversely affect sperm parameters [67, 68]. One study shows that the consumption of varieties of soy-based products in the past three months showed that 99 male partners were suffering from infertility [67]. Higher consumption of soy foods leads to producing 41 million sperm/mL less than those who did not take such products [67].
Generally, fats are recommended for 30–40% of the total calorie requirement, for cell membrane formation, help in dissolving fat-soluble vitamins, and in hormonal function as well as perform many more functions. Higher or lower consumption of such nutrients may lead to infertility. All three types of fatty acids should be comprised of saturated, polyunsaturated, and monounsaturated from the above recommendation. Polyunsaturated fatty acids (PUFA) such as omega-3 and omega-6 fatty acids cannot be synthesized by the human body, so it has to be taken from foods to perform various physiological functions. These fatty acids help in the formation of the spermatozoa cell membrane [69]. A study shows that the patients suffering from asthenozoospermia had increased or decreased amounts of oleic acid and docosahexaenoic acid (DHA) in spermatozoa [70]. One more case study shows that in the fertile male, the serum and spermatozoa are found with a higher level of omega-3 fatty acids [71]. A clinical trial was conducted among 22 patients suffering from asthenozoospermia infertility. The supplementation of 600 IU of vitamin E and 465 mg of DHA was given for 12 weeks and showed a positive effect [72]. A randomized clinical trial was conducted among 119 men usually fed a Western-style diet. One group was fed 60 gm of nuts and the other did not. The result showed that the group fed a Western diet along with nuts, found improved motility, vitality, morphology, and sperm count [55]. So, the intake of omega-3 fatty acids helps to improve male fertility as it regulates testosterone hormones and reduces stress due to its antioxidant properties.
Many studies show that a low level of antioxidants may cause to increase in oxidative stress. This oxidative stress may cause lipid peroxidation to the sperm membrane, reduce the motility of sperm, damage the DNA of sperm, and many more complications [73]. According to a prospective cohort study conducted among 171 men couples. The result revealed that the men who were taking vitamin C and β-carotene had positive fertilization [74]. Similar findings were shown by the systemic review that antioxidant supplementation has a favorable effect on male fertility [75]. Another study shows that the supplementation of vitamin E, vitamin C, and coenzyme Q10 (CoQ10) effectively improves the semen parameters in infertile males [76].
Various studies have revealed that vitamin B12 directly affects the semen parameter by increasing sperm motility, and concentration and reducing DNA damage by ROS [77]. Vitamin E is known as a lipophilic chain-breaking antioxidant, which prevents the peroxidation of PUFA. Due to the high content of PUFA in germ cells and spermatozoa membrane, the risk of autooxidation is high which may hamper the reproductive function [78]. A randomized control trial of 330 men suffering from vitamin D deficiency and infertility, showed that 1,400 IU cholecalciferol and 500 mg/day for 150 days had a higher pregnancy but no difference in sperm count [79]. Another randomized trial was conducted on 179 infertile men for over 6 months, the result found that vitamin C supplementation had a positive effect on male infertility than clomiphene citrate [80].
Calcium is one of the most important minerals that help in the physiological process as a regulator in all living cells including sperm cells. An experiment showed that the addition of calcium ionophore (A23187) helps in the active transport of calcium ions from extracellular space to intracellular space, which induces the acrosomal reaction [81]. The deficiency of zinc may interfere with the spermatogenesis process, which may cause abnormalities in sperm morphology and negatively impact testosterone concentration. The supplementation of zinc plays an important role in male infertility [82]. Varicocele is a clinical condition in which the seminal parameter is reduced. Copper, zinc, and selenium are the essential trace elements that help in spermatogenesis and are also antioxidant agents that may reduce ROS. Selenium helps in sperm motility, morphology, and sperm concentration [83].
Many studies show that alcohol consumption adversely affects semen parameters. People having regularly consumption of alcohol seem to have worse semen quality than those who take it occasionally [84]. Another study shows that heavy intake of alcohol and smoking may affect sperm quality. The result shows that alcohol more drastically deteriorates sperm maturity and damages DNA than smoking [85].
A study was conducted in Spain among 485 women aged 20–45 years undergoing IVF [86]. Two dietary patterns Mediterranean diet and the Western diet type, and the result shows that the women who had a high intake of the Mediterranean diet had a lower odds ratio (0.56, 95% confidence interval 0.35–0.95) than those who adhered to the western diet of having fertility problems [86]. Another study shows that infertile women’s adherence to a “fertility diet” was linked with a lower risk of ovulatory disorders. The score obtained after the study was 0.34 (95% confidence interval 0.23–0.48, P < 0.001) [87]. The literature on the association between nutrients and dietary patterns on male fertility has been elaborately described in Table 2.
Literature on the association between nutrients and dietary patterns on female fertility
| Nutrient | Species | Studies | Dose and duration | Conclusion | Reference |
|---|---|---|---|---|---|
| Dietary pattern | |||||
| Mediterranean diet | Human | Cohort study | 3 years | The Mediterranean diet was linked with 2.7 times higher achieving of clinical pregnancy and live birth. | [88] |
| Case-cohort study | 9 months | The study showed that adherence to the Mediterranean-type diet may enhance women’s fertility. | [86] | ||
| Case-cohort study | - | The Mediterranean diet and lifestyle improved fertility. | [89] | ||
| Seafood | Human | Prospective cohort study | ≤ 1 year or until pregnancy | Seafood intake was linked with a higher sexual intercourse frequency and fecundity. | [90] |
| Protein | |||||
| Dairy food | Human | Prospective cohort study | 2005–2007 | The result of the study showed that there was a link between increased dairy foods and decreased estradiol concentration, and the yogurt and cream intake with the risk of sporadic anovulation. | [91] |
| Protein type and amount | 2004–2007 | The result showed that the higher dairy protein intake of more than 5.24% of energy was linked with lower antral follicle counts and accounts for infertility treatment in women. | [92] | ||
| Dietary fats | |||||
| TFA and omega-3 fatty acid | Human | Cohort study | 2011–2013 | The result showed that higher intake of TFA and low omega-3 fatty acid intake were linked with reduced fecundity. | [93] |
| Carbohydrate | |||||
| Whole grain | Human | Prospective cohort study | 2007–2014 | The result showed the higher intake of whole grain in the pre-treatment group was associated with a higher probability of live birth among IVF women. | [94] |
| Antioxidants | |||||
| Antioxidant’s supplementation | Human | Randomized controlled trials | - | The result showed low to very low evidence that intake of antioxidants may benefit sub-fertile women but also showed no risk of miscarriage, ectopic pregnancies, and multiple births. | [95] |
| Vitamin C and β-carotene | Observational | - | The couple’s intake of vitamin C and β-carotene was positively linked to fertilization rate but did not translate into higher pregnancy. | [74] | |
| Folic acid | Prospective cohort study | 2007–2011 | Folic acid supplementation was linked with increased fecundability. | [96] | |
-: not applicable
Very few studies show whether carbohydrate intake could have a prominent effect on female fertility. A cohort study was conducted among 17,544 women having no history of infertility in the last 8 years she was suffering from miss conceiving [87]. The study revealed that the prolonged intake of high glycemic food was linked with ovulatory disorder. Another study revealed that a higher intake of carbohydrates and high glycemic food is associated with hyperinsulinemia and obesity, which may cause a negative effect on glucose transporters type 4 (GLUT4) expression present over endometrium that leads to polycystic ovarian syndrome (PCOS) and may cause infertility in females [97]. A prospective study was conducted on 1,855 married, premenopausal women, during follow-up it was found that 438 women reported ovulatory infertility [98]. The study revealed that the total carbohydrate and dietary glycemic index was directly associated with ovulatory infertility among them. The carbohydrate intake risk ratio was 1.91 with a confidence interval of 1.27–3.02 at 95% and the risk ratio for the glycemic load was 1.92 with a confidence interval of 1.26–2.92 at 95% [98].
Protein is an essential macronutrient that performs lots of physiological and biological functions. The functions depend on the source and amount of the protein. One study shows that vegetable sources of proteins may lower the ovulatory infertility risk than those who consume animal sources of protein [99]. The result shows that ovulatory infertility among the animal protein intake group was 1.39 (confidence interval 1.01–1.90 at 95%, P for trend was 0.03) and for vegetable intake groups was 0.78 (confidence interval 0.54–1.12 at 95%, P for trend was 0.07) [99]. A prospective cohort study was conducted among 265 undergoing infertility treatment [92]. The result shows that high dairy protein intake was positively linked with lower ovarian antral follicle count. One study shows that a high protein diet (specifically animal protein) was negatively associated with a reduced level of testosterone level in women [100]. The hormone testosterone helps in the regulation of ovarian function and fertility in females, and abnormalities in androgen (testosterone) signaling may disturb androgen-related reproductive disorders, as it helps in ovarian follicular signaling [100].
Fat is another macronutrient that vitally affects female reproduction and fertility. One study suggests that high dietary fat intake may be linked with the menstrual cycle, reproductive hormones (LH), and embryo quality in assisted reproductive technology [101]. Fatty acids have also antioxidant and anti-inflammatory properties. Another study shows that omega-3 fatty acids undoubtedly affect fertility and it associated with steroidogenesis [102]. Another study shows that omega-3 fatty acid supplementation may reduce the follicle-stimulating hormones in normal healthy women, but it was not observed in obese women, it helps in reproductive lifespan [103]. One more study shows that low-fat dairy product consumption may lead to infertility in women due to anovulation, whereas high consumption of dairy products increases fertility in women [104]. The reason behind this phenomenon may be due to the high content of estrogen and fat-soluble vitamins in high-fat dairy products [104].
The reproductive capacity is influenced by oxidative stress and variation in DNA methylation. As earlier discussed, oxidative stress develops due to more free radical production and an imbalance in antioxidant protection. So, current clinical practices advise that some nutritional supplementation may help to reduce such imbalances and help to control oxidative stress as well as fertility. Antioxidants such as vitamin E, lipoic acid, vitamin C, and CoQ10 supplementation may improve the function of the whole detoxifying system of the body [105]. Many studies show that the intake of antioxidants induces reproductive capacity, but less intake may influence menstrual function [106]. One more study shows that regular intake of ascorbic acid (vitamin C) during pregnancy stimulates placental/trophoblastic steroidogenesis which improves the physiological condition of the body during gestation [107]. Another study shows that during abortion the level of this antioxidant was found to be lower [108]. Another study reported that inadequate or high dose consumption of such antioxidants may have an adverse effect also on the female reproductive system that may lead to infertility [95]. Additionally, many studies show that L-carnitine is an important antioxidant, and its supplementation may reduce many reproductive problems such as endometriosis, PCOS, or amenorrhea.
Many studies revealed that women going for IVF should have sufficient vitamin D levels (≥ 30 ng/mL) and also improve metabolic parameters in PCOS women. A high dose of vitamin D also reduces the chance of endometriosis [109]. Folic acid supplementation of 400 µg/day helps to improve the folic acid level and reduces homocysteine level in follicular fluid, which helps in better embryo quality, increases the chance of pregnancy, and reduces the ovulatory fertility risk [110]. Periconceptional supplementation of folic acid along with vitamin C, vitamin D, vitamin E, vitamin B6, DHA, and minerals has a positive impact on infertility treatment [111].
A randomized clinical trial was conducted among 74 infertile females. The result shows that copper deficiency may have a considerable role in female infertility [112]. Selenium affects thyroid gland function and acts as an antioxidant that helps in lowering oxidative stress. It induces the growth and maturation of oocytes [113]. Another important element is iodine. Iodine is an essential element for thyroid gland function and fertility. A study conducted among 501 women suffering from iodine deficiency found that pregnancy was delayed by 46% [114]. Magnesium is another important trace element that helps to regulate metabolic disorders and is associated with insulin sensitivity. Its abnormality may lead to PCOS in women [115]. Some studies show that a total blood Hg > 5.278 µg/L could drastically affect 157% of infertility [116].
Based on the current review most couples are experiencing infertility problems, and the situation is a much stressful and depressive period in their life. Stress is one of the important factors that adversely affect infertility in both sexes. Presently, the etiology of infertility is not well understood, many genetic factors, lifestyle factors, and environmental conditions such as stress work, oxidative stress, unbalanced nutrition, and unhealthy dietary patterns have been implicated to interfere with reproductive safety in both the sex. The nutritional factors are responsible for healthy reproductive function in both males and females. There is no doubt that diets play an effective role in the fertility of both sexes. The current review revealed that people having high consumption of processed cereals, high soy protein, high animal protein, fatty foods, and sugary foods negatively affect fertility whereas the Mediterranean dietary pattern, high dietary fiber, omega-3 fatty acids, vegetable protein, vitamins, and minerals have a direct impact on fertility.
ACTH: adrenocorticotropin hormone
CRF: corticotropin-releasing hormone or factor
DHA: docosahexaenoic acid
HPA: hypothalamic-pituitary-adrenal
IVF: in vitro fertilization
LH: luteinizing hormones
LHRH: luteinizing hormones releasing hormone
PCOS: polycystic ovarian syndrome
PUFA: polyunsaturated fatty acids
ROS: reactive oxygen species
TFA: trans fatty acids
LS: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing, Supervision. DS: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3189
Download: 64
Times Cited: 0
