Affiliation:
1Department of Food Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak 34469, Turkey
ORCID: https://orcid.org/0000-0002-8642-9160
Affiliation:
1Department of Food Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak 34469, Turkey
Affiliation:
2Department of Food Engineering, Faculty of Food Engineering, Sakarya University, Sakarya 54187, Turkey
ORCID: https://orcid.org/0000-0002-4540-3044
Affiliation:
1Department of Food Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak 34469, Turkey
ORCID: https://orcid.org/0000-0002-8422-0478
Affiliation:
1Department of Food Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak 34469, Turkey
Email: capanogl@itu.edu.tr
ORCID: https://orcid.org/0000-0003-0335-9433
Explor Foods Foodomics. 2024;2:252–274 DOI: https://doi.org/10.37349/eff.2024.00037
Received: December 26, 2023 Accepted: February 07, 2024 Published: June 20, 2024
Academic Editor: Andrea Gomez-Zavaglia, Center for Research and Development in Food Cryotechnology (CIDCA-CONICET), Argentina
The article belongs to the special issue Delivery of Hydrophobic Compounds in Food Systems
Encapsulation is a pivotal technique for protecting and enhancing the efficiency of sensitive natural bioactive substances, notably essential oils, vitamins, and phenolic compounds, widely used in foods and nutraceuticals. Critical considerations in selecting encapsulation agents encompass safety, release kinetics, stability, and cost-effectiveness. Yeast cells emerge as versatile carriers distinguished by their low cost, compatibility with biological systems, and eco-friendly degradation properties, accommodating both hydrophilic and hydrophobic bioactive agents. Various yeast strains, including Saccharomyces cerevisiae, Torulopsis lipofera, Cutaneotrichosporon curvatus, Yarrowia lipolytica, and Candida utilis, find utility in microencapsulation. Yeast cell encapsulation relies on the permeation of bioactive agents through yeast cell walls, predominantly composed of mannoproteins and polysaccharides. The encapsulation process includes passive or vacuum-infused diffusion of bioactive compounds inside yeast cells, precise droplet size control, and attractive forces to trap bioactive components within cellular structures. Yeast cells display versatility in various states, whether alive or dead, intact or plasmolyzed. In addition, the loading capacity of hydrophobic bioactives can be increased through chemical pretreatment techniques such as plasmolysis, autolysis, and enzyme hydrolysis, freeing up space within yeast cells by eliminating water-soluble components. In summary, yeast cell encapsulation presents a promising and sustainable technology with diverse applications within the food industry. Yeast cells enhance the stability and controlled release of bioactive compounds, magnifying the efficacy of natural hydrophobic bioactives like curcumin, essential oils, β-carotene, and vitamin D across various food products. This comprehensive review focuses on the encapsulation procedures, influential factors, characterization techniques, and applications, with a pronounced emphasis on hydrophobic materials.
Natural bioactive compounds are chemically unstable and easily broken down by environmental factors such as oxygen, light, moisture, and heat. In their pure and natural form, especially when applied in foods, they have relatively limited applications because of some unique characteristics, such as quick release, low solubility, poor bioavailability, and oxidative degradation [1]. Covering a bioactive component (core material) with a wall-like material, which is typically referred to as a shell, membrane, coating, carrier, matrix, or encapsulating material, is called encapsulation [2].
The fact that the core material is safe for oral intake and approved for use by the legislative bodies, including the Food and Drug Administration (FDA), is of great importance in food applications. The core material’s concentration, mechanism of release, stability, and cost are a few factors that can help in the selection of encapsulating agents. The purposes of encapsulation of the sensitive bioactive compounds (essential oils, vitamins, phenolic compounds, etc.) are (i) to preserve them from environmental factors or undesired interactions with some other ingredients; (ii) to control their release for a set amount of time in the desired matrix or environment; (iii) to increase their solubility; (iv) to ensure better bio-accessibility/bioavailability during the gastrointestinal digestion [2]. Fat-soluble vitamins, several categories of food colorants (especially carotenoids), antioxidants (such as phenolic compounds), essential oils, flavors, and aroma compounds are the key components of hydrophobic food bioactive chemicals and nutraceuticals [3]. Encapsulation systems, encapsulation techniques, and “generally recognized as safe” (GRAS) wall materials are crucial to create micro- or nano-scale particles or capsules for food applications. Microcapsules (1–1000 μm), submicron capsules (a few hundred nanometers to less than 1 μm), and nano-capsules (from 1 nm to a few hundred nanometers) are created through the encapsulation process [1].
Key ingredients in fermented goods like beverages, baked goods, and cereals are yeast cells. Chewing gum, cereals, sauces, and bakery and confectionery items can all contain yeast microcapsules. Microcapsules can be created as tablets or powders to protect the primary ingredients and release them upon hydration. Yeast cells are natural carriers for both hydrophilic and hydrophobic bioactive compounds [4]. Encapsulation in yeast cells is based on the penetration of the bioactive compound through the yeast cell wall, allowing the substance to stay inside the cells. Since mannoproteins and polysaccharides make up the majority of yeast cells, tiny molecules can freely diffuse through the cell envelope [5].
Yeast cell encapsulation is reasonably priced, biocompatible, and biodegradable. The ability of yeast cells to absorb significant amounts of hydrophobic compounds has long been recognized, and the process can be adjusted to produce cells that are hydrophobic-loaded or yeast-based capsules [6]. In reality, cell walls play more than just a mechanical function; they also serve as both controlled loading and release. Hydrophobic core material diffuses throughout the encapsulation process and becomes passively retained in the cell body, being released in response to the right stimulus [7]. The bioactive solution (aqueous or organic solvent solution) is mixed with the yeast cells (in the form of alive, wet or dried, plasmolyzed or non-plasmolyzed) or the aqueous suspension of yeast walls for several hours at a controlled temperature (typically 20°–60°C), to encapsulate bioactive compounds into the yeast cells or yeast walls. The stirrer speed allows for flexible control of the droplet size to achieve the appropriate diffusion. Hydrophobic interactions, hydrogen bonds, and van der Waals attractive forces are used to trap the bioactive inside the yeast cell. Bioactive-loaded yeast cells are dried using a fluidized bed dryer, spray dryer, or freeze dryer after being rinsed with water or an organic solvent to remove the non-encapsulated compounds in the final phase of the encapsulation process [8].
Yeast cells can be used alive or dead, whole, or broken down to just the cytoplasm, or even empty of all their original components (Figure 1). The low cost and biodegradability of this group of encapsulation technologies, which have thus far only targeted food, are their key selling features [7]. Some investigations indicate that only molecules with a molecular weight lower than 760 Da are capable of free entry into the yeast cell through diffusion mechanisms. Nonetheless, research by other authors suggests that compounds with a molecular weight of 20,000 and higher may be captured in yeast [9].
Saccharomyces cerevisiae is a well-known, suitable, and excellent container for the encapsulation of numerous bioactive substances [2]. The yeast cell walls of Saccharomyces cerevisiae are employed in the food and alcohol sectors as components of fermentation [10]. Torulopsis lipofera, Saccharomyces bayanus, Endomyces vernalis, and dairy yeasts such as Candida utilis and Kluyveromyces fragilis are other strains that have been employed for microencapsulation [2]. The oleaginous yeasts are capable of intracellular accumulation of significant amounts of lipids. The two oleaginous yeasts Cutaneotrichosporon curvatus and Yarrowia lipolytica are the most frequently used ones [7].
The loading of core materials could be increased, leading to better encapsulation yield, by executing a chemical pretreatment on yeast cells to empty the content of the cell. The three methods that are most frequently used are plasmolysis, autolysis, and enzyme hydrolysis. In the context of microencapsulation, plasmolysis is a pretreatment procedure used to extract the cell’s internal water. Meanwhile, some water-soluble components from the cells are also removed, making more room for the loading of the core material. These components include water-soluble proteins, nucleic acid, saccharides, and some enzymes. The process of a cell’s endogenous hydrolytic enzymes releasing a cytoplasmic molecule is known as autolysis [2].
Yeast cell encapsulation presents a promising and sustainable technology with vast potential across various applications within the food industry. This versatile approach not only enhances the stability of bioactive compounds but also affords precise control over their release, thereby serving as a valuable tool for enhancing the effectiveness and applicability of natural bioactive substances in diverse food products. This review provides recent insights into encapsulation processes in yeast cells, influential factors, characterization techniques, and applications, particularly focusing on hydrophobic materials such as curcumin, essential oils, β-carotene, and vitamin D.
Yeast cell, which has a 100–200 nm thick structure and 15–25% dry mass, is approximately 2–5 μm diameter [11, 12]. Yeast cell involves mitochondria, Golgi apparatus, peroxisome, vacuole, and nucleus in intracellular structure [13]. It also has a cell membrane (inner plasma membrane, < 10 nm thick) and rigid external cell wall, which is composed of polysaccharides like chitin and β-glucan (β-1,3-glucan and β-1,6-glucan) and glycoproteins (mannoproteins) [14, 15], as shown in Figure 2. The mannoproteins are responsible for the porosity and permeability of the cell wall and expand in deeper layers at the surface, shielding the glucose cans from outside threats and ensuring a hydrophilic environment, as the β-1,3-glucans and chitin are responsible for the mechanical rigidity and provide a strong cell wall and a sturdy structure [12, 16, 17]. Due to their rigid structure, oval shape, and inner plasma membrane structure, yeast cells are an excellent encapsulation material for substances. The plasma membrane, a phospholipid bilayer, provides selective permeability to compounds entering and leaving cells, serving as a barrier for permeating molecules and encapsulating them [16, 18, 19]. In addition, yeast cells can encapsulate hydrophilic and hydrophobic compounds due to their membrane functions being like a liposome and the phospholipids’ structure [20, 21]. Mannoproteins offer porosity during encapsulation [12, 22]. Thus, yeast cells permit molecules to readily diffuse depending on their molecular weight (up to 760 Da) and hydrophobicity [11, 23].
Yeast cells have several advantages for being used as encapsulation materials, including their selective permeability, bio-adhesive properties of the internal yeast cell membrane, thermostable properties during food processing (up to 250°C), oxidative stability, and encapsulation simplicity [19, 24–26]. Besides, yeast cells as carriers can protect encapsulated material from environmental stresses such as heat, light, oxygen, and moisture [14, 27]. For instance, Fu et al. [24] showed the thermostable effects of yeast cells against heat damage as an encapsulation material with krill oil encapsulation. In addition to this, the phospholipid proportion of the cell membrane was increased by 10% of krill oil-loaded yeast cells. Another study reported that carvacrol, a volatile active compound, was protected with decreasing volatility after loading yeast cell walls [28].
As a yeast encapsulation carrier, Saccharomyces cerevisiae [29–31], Saccharomyces bayanus, Saccharomyces pastorianus [32, 33], Yarrowia lipolytica [34, 35], Cryptococcus curvatus [36], and other types of yeasts (Candida utilis, Kluyveromyces fragilis, Torulopsis lipofera, Endomyces vernalis) [12] have been employed (Table 1). Among all these yeasts, Saccharomyces cerevisiae has been used as a predominant encapsulation carrier due to its abundance, easy-to-culture [37], high nutritional content [38], low cost [21], and acceptable color and flavor [39]. In addition, it is accepted as GRAS for consumption in various industries such as beverages, bread, and biomass production [40].
Yeast cells offer a cost-effective, simple, and straightforward encapsulation process [12, 24, 41]. The hydrophilic and hydrophobic compounds such as probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum) [42], oils [24, 30, 43], terpenes and flavors [20, 26, 35, 44, 45], phenolic compounds such as carvacrol, chlorogenic acid [28, 38, 39, 46–48], anthocyanins [32, 49], vitamins [33, 41], and bovine serum albumin [50] have been encapsulated successfully in yeast cells. Several processes have been investigated to improve encapsulation efficiency (EE), capacity, yields, and stability for yeast cell encapsulation. This section provides an overview of various pretreatment and encapsulation methods used in the yeast cell encapsulation process (Table 1 and Figure 3).
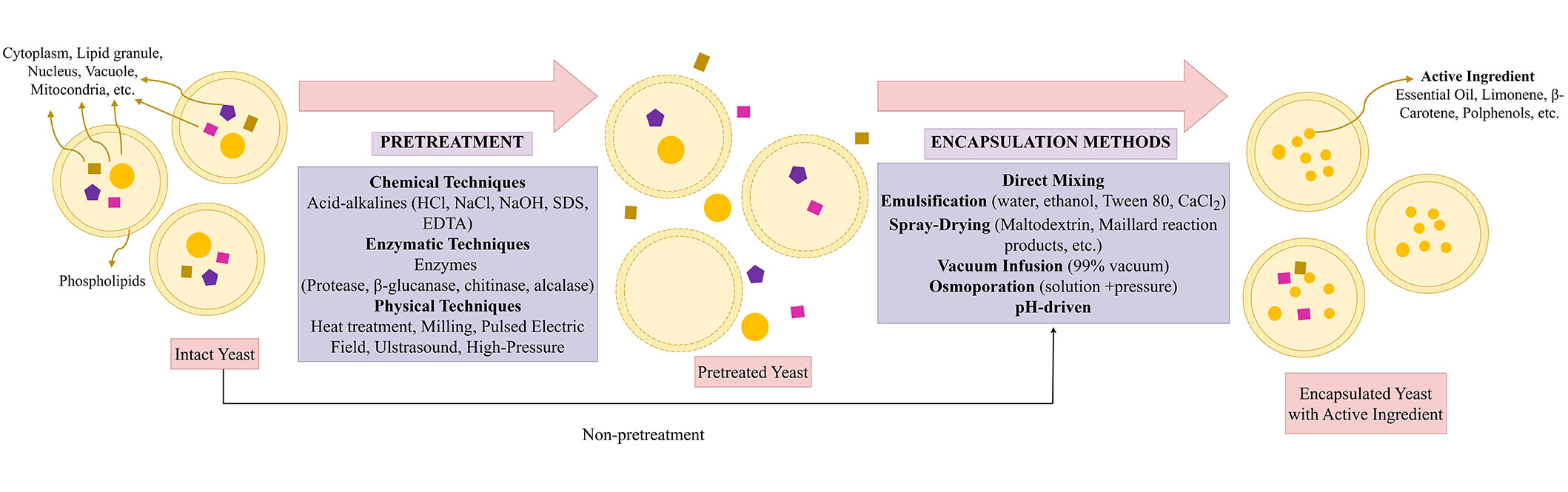
Yeast cell encapsulation process (pretreatments and encapsulation methods). SDS: sodium dodecyl sulfate; EDTA: ethylene diamine tetra acetic acid
Intact or pretreated yeast cells, yeast cell walls, or yeast glucan particles are selected depending on the compounds to be encapsulated and the encapsulation method. Intact yeast cells have beneficial effects on the compounds during encapsulation processes. However, some molecules cannot pass through the intact cells due to their molecular mass and high hydrophilicity. For instance, bovine serum albumin cannot penetrate intact yeast cells due to high molecular mass [50]. The poor permeability of intact yeast cells to some macromolecules restricts the range of applications for which they can be encapsulated, protected, or released [51]. Thus, cell pretreatments are implemented to enhance cellular structures’ porosity, permeability, and encapsulation capacity, protect the regrowing of cells, and inactivate endogenous enzymes, whereby molecules can enter the cell and diffusion can be allowed [18, 27, 52]. In this section, the pretreatments of yeast cells are classified into three different groups: chemical (acid-base hydrolysis [53]; autolysis [11] and plasmolysis [19, 20, 25, 32, 39], enzymatic (enzymatic hydrolysis) [25, 33], and physical [pulsed electric field (PEF), ultrasound, stirring, milling, heat treatment] pretreatments [34, 49].
Chemical pretreatments contain acid and alkaline hydrolysis to enhance the permeability of cell walls and to permit cytoplasmic leakage of cells by applying chemicals such as HCl, NaCl, NaOH, sulfuric acid, citric acid, β-mercaptoethanol, Tween 80, cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), dithiothreitol (DTT), and ethylene diamine tetra-acetic acid (EDTA) [35, 50, 54, 55]. Chemical pretreatments have resulted in autolysis and plasmolysis of yeast cells. Autolysis is hydrolyzing the macromolecules (cell wall polysaccharides, cellular proteins, DNA, RNA, etc.) by endogenous lytic enzymes (proteases, nucleases, glucanases, etc.) of yeast [56]. The plasma membrane structure of yeast cells was disrupted by activating enzymes with promoter chemicals such as ethanol, NaCl, diethyl ether, and sucrose [50]. Czerniak et al. [25] also evaluated that autolysis with ethyl acetate was an effective pretreatment for boosting membrane permeabilization and core material loading. Besides, Wang et al. [57] suggested that only the cell wall structure was affected concerning permeability and retained its shape after the treatment. The yeast cell can also plasmolyzed by chemical pretreatments. Plasmolysis is a modified autolysis process that causes direct cell membrane bursts and releases the internal cell contents by using inorganic salts or organic solvents such as ethyl acetate, NaCl, sucrose, glycerol, toluene, and ethanol [51, 56, 58]. Paramera et al. [21] suggest that when plasmolyzed and intact yeast cells were compared, there were no differences in encapsulation yield. However, Young et al. [38] have demonstrated significant differences between intact yeast cells and chemically pretreated cells in EE. Moreover, many studies have shown that plasmolyzed cells boost EE and facilitate the encapsulation process [11, 13, 23, 24, 31, 39, 41, 43, 59]. Besides, Kurek et al. [32] have shown that the color of anthocyanins loaded with plasmolyzed yeast cell walls was improved compared to non-plasmolyzed ones.
In conclusion, several studies have shown that chemical pretreatment leads to an improvement in EE, loading to cells, permeability of cell structure (by breaking down linkages in mannoproteins), and bio-accessibility after encapsulation [35, 54, 60]. This cell pretreatment has no detrimental effect, except for the high cost resulting from the removal of chemicals and the high-level generation of polluting waste materials [61].
Enzymatic pretreatments are processes in which cells are treated with enzymes such as proteases [25, 33, 35], β-glucanase, chitinase [25], and alcalase [58]. The presence of either endogenous or exogenous enzymes following their reaction with water is used to enhance enzymatic hydrolysis, which involves the breakdown of yeast cells or cell walls. Enzymatic hydrolysis with proteases significantly degraded the yeast cell walls and cell membranes (phospholipids), resulting in a decrease in RNA content, according to Marson et al. [33]. Thus, cell wall permeability and porosity were increased by proteases. Although Pham-Hoang et al. [35] suggested that proteases did not affect the EE of yeast cells, another study showed that EE and core material loading were boosted by pretreated cells with proteases, glucanase, and chitinase [25]. Also, these results were higher in combination with autolysis (ethyl acetate) [25]. Compared to the effects of autolysis, plasmolysis, and enzymatic hydrolysis on yeast cells in terms of EE, Takalloo et al. [58] concluded that enzymatic hydrolysis is the best pretreatment due to its fast, high recovery yield, no off-flavors, and no equipment requirement.
Physical pretreatments are direct exposure treatments to yeast cells such as PEF [11, 62], milling [42], high-pressure homogenization [11], ultrasonication (US) [29, 34], osmoporation [63], and heat treatment [35]. Before the encapsulation of oregano essential oil in yeast cell walls and cell membranes, the plasma membrane was disrupted (via electroporation) by PEF treatment. Thus, cell membrane permeability and porosity were increased [11]. However, high-pressure homogenization of cells resulted in significant cell damage; thus, the encapsulation load was reduced [11]. Although the physical techniques offer less waste chemical production than chemical and enzymatic techniques, physical pretreatment requires equipment and can damage cells’ intercellular structures.
As a result, all of the pretreatments can provide many advantages for EE and capacity by increasing cell wall porosity and permeability. However, they can exhibit detrimental effects on cells, such as high destruction. Therefore, yeast cell pretreatment must be designed for each specific process regarding the properties of compounds and pretreatments and their interactions.
Encapsulation of hydrophobic and hydrophilic compounds within the yeast cells can be performed through a process controlled without requiring cell viability [11]. This process is mainly by passive diffusion. Substances with a functional hydroxyl and polar group increase the fluidity of phospholipid membranes of cells, allowing easier passage for diffusing permeates. The hydroxyl group in the molecule permits their emulsification inside the densely packed hydrocarbon chains of the double phospholipid layer [52]. Therefore, hydrophilic compounds can directly and strongly interact within the yeast cells or intact yeast cells. However, hydrophobic compounds need oil-in-water (o/w) emulsion to be encapsulated in yeast cells. After the emulsion is formed, the compounds can move within the yeast cells by diffusing through the aqueous phase [51]. Hereby, hydrophobic interactions, van der Walls, and hydrogen bonds are formed between the core material and yeast cells. Compounds’ molecular size, shape, and hydrophobic substances’ solubility in yeast cells also affect the encapsulation process [52, 64]. Thus, hydrophobic and hydrophilic compounds are encapsulated within the prepared and intact yeast cells. For this purpose, direct mixing, emulsification, spray drying, osmoporation, vacuum infusion, vacuum pressure, ultrasound, and pH-driven encapsulation methods have been mainly used (Table 1 and Figure 3).
Emulsification is the most commonly used method in the encapsulation processes for hydrophobic compounds as core materials. Firstly, in the emulsification process, core materials, intact or pretreated yeast cells, or their particles are mixed with water or oil, alcohol, and Tween 80 to obtain an emulsion, followed by incubation under controlled temperature and stirring. Unlike emulsification, the cells are mixed directly with the compounds by adding water or not in the direct mixing method [28, 38]. Following the agitation process under certain conditions (up to 75℃ and several hours), the stirring speed is adjusted. After both emulsification and direct mixing, microcapsules are obtained via diffusion and separated from unencapsulated material by centrifugation. Finally, the encapsulated material is washed to remove residues and dried by freeze- and spray-drying [28, 32, 49, 52]. Oils, which are hydrophobic compounds, have been mainly loaded by emulsification methods after plasmolysis (NaCl), autolysis, and enzymatic hydrolysis of yeast cells [24, 25, 43]. In particular, essential oils have only been loaded in yeast cells by emulsification, as shown in Table 1 [11, 30, 59]. Oregano essential oil and terpenes (volatile and poorly water-soluble) were encapsulated in pretreated Saccharomyces cerevisiae cells and yeast cell wall particles, respectively, by diffusion [11, 46]. However, the diffusion requires time and energy costs in emulsification and direct mixing methods. Thus, several methods have been investigated to reduce process time. Young et al. [38] have compared the passive diffusion (24 h) and vacuum infusion methods (99% vacuum and 5 min). They concluded that the passive diffusion approach needed more time to encapsulate each component, and vacuum infusion is a quick procedure that requires no heating and takes a few minutes [38]. Also, curcumin was successfully encapsulated in hydrolyzed yeast cells and yeast cell wall particles in a short period using a vacuum infusion process [53, 65–67].
Encapsulation process by utilizing yeast cells as carriers
| Active ingredient | Yeast | Carrier | Pretreatment (for carriers) | Encapsulation method | References |
|---|---|---|---|---|---|
| Anthocyanins | Saccharomyces cerevisiae | Yeast cells | -Washing (water)-Heat treatment | Direct mixing | [49] |
| Anthocyanins | Saccharomyces cerevisiae, Saccharomyces pastorianus, Saccharomyces bayanus | Yeast cells | Plasmolysis (NaCl) | Direct mixing (water) | [32] |
| Ascorbic acid | Saccharomyces pastorianus | Hydrolyzed cells | -Heat treatment-Enzymatic hydrolysis (proteases) | Spray drying (with maltodextrin and Maillard reaction products) | [33] |
| Berberine | Saccharomyces cerevisiae | Yeast cells | ND | Emulsification (berberine, cells, water) | [23] |
| Bovine serum albumin | Saccharomyces cerevisiae | Yeast cells | Autolysis (NaCl, HCl, NaOH, CTAB, SDS, Tween-80, DTT, and EDTA) | -Emulsification (bovine serum albumin, cell, water)-Outer-layer chitosan coating | [50] |
| Black cumin seed oil | Saccharomyces cerevisiae | Yeast cells | Plasmolysis (NaCl) | Emulsification (deionized water, Tween 80) | [66] |
| Carvacrol | Saccharomyces cerevisiae | Yeast cell wall | Washing (water) | Direct mixing (water) | [28] |
| Curcumin | Saccharomyces cerevisiae | Yeast cells | ND | Osmoporation [water-glycerol solutions (iso-, 1.4 MPa) and hyper-osmotic (30.0 MPa)] | [47] |
| Curcumin | Brewer’s yeast | Yeast cells | -Washing (water)-Chemical treatment (NaOH) | pH-driven | [55] |
| Curcumin | Saccharomyces cerevisiae | Yeast cells and cell wall particles | Chemical treatment: -Ethanol for yeast cell-NaOH, HCl, acetone, isopropanol for cell wall particles | Vacuum infusion | [53] |
| Intact yeast cells and yeast cell wall particles | -Washing (water) for intact yeast-Chemical treatment (NaOH, HCl, isopropanol, acetone) | -Vacuum infusion-Emulsification | [65] | ||
| Curcumin, fisetin | Saccharomyces cerevisiae | Intact yeast cells and cell wall particles | -Washing (ethanol and ultrapure water) for intact yeast-Chemical treatment: NaOH, HCl, isopropanol, and acetone for yeast cell wall particles | -Ethanol concentration-Vacuum infusion-Vacuum pressure | [38] |
| D-limonene, ethyl hexanoate | Saccharomyces cerevisiae | β-Glucans extracted from drum-dried yeast cells | ND | Spray drying | [36, 45] |
| Essential oil (Mentha pulegium) | Saccharomyces cerevisiae | Yeast cells | Plasmolysis (NaCl) | Emulsification (oil, cells, water) | [59] |
| Essential oil (Oregano) | Saccharomyces cerevisiae | Yeast cells | -Chemical treatment: autolysis (NaOH)-Pulsed electric field-High-pressure homogenization | Emulsification (ethanol, oil, cells, deionized water) | [11] |
| Essential oil (Zataria multiflora Boiss) | Saccharomyces cerevisiae | Yeast cells | ND | Emulsification (oil, cells, distilled water) | [30] |
| Fisetin | Saccharomyces cerevisiae | Yeast cells | Osmoporation (water-glycerol solution, 1.4 MPa) | Osmoporation (water-glycerol solution, 1.4 MPa) | [63] |
| Fish oil | Saccharomyces cerevisiae | Cell walls | -Autolysis (ethyl acetate)-Enzymatic hydrolysis (β-glucanase, chitinase, protease) | Emulsification (ethanol, oil, cells, deionized water) | [25] |
| Flavors (Carboxylic acids, ethyl esters, lactones, alcohols, and ketones) | Yarrowia lipolytica | Yeast cells | -Heat treatment-β-mercaptoethanol treatment-Enzymatic hydrolysis (protease) | Direct mixing (water) | [35] |
| Flaxseed oil | Saccharomyces cerevisiae | Yeast cells or β-glucan | Plasmolysis (NaCl) | Emulsification (Tween 80, oil, cells, deionized water) | [43] |
| Gallic acid | Saccharomyces cerevisiae | Yeast cells | Plasmolysis (NaCl) | Emulsification (ethanol, acid, cells distilled water) | [31] |
| Ibuprofen and curcumin | Saccharomyces cerevisiae | Yeast glucan particles | Chemical treatment (NaOH) | Spray drying | [67] |
| Krill oil | Yeast | Yeast cells | Plasmolysis (NaCl) | Emulsification (Tween 80, oil, cells, deionized water) | [24] |
| Lactobacillus acidophilus and Bifidobacterium bifidum | Saccharomyces cerevisiae | Yeast cells | ND | -Emulsification (sodium alginate, probiotics, cells, Span 80, CaCl2, canola oil)-Triple layer (alginate-alginate-cell wall) | [42] |
| Limonene | Saccharomyces cerevisiae | Intact yeast cells | ND | Concentrated powder form-high-pressure spraying emulsion (Tween 80, limonene, cell, distilled water) | [44] |
| Phenolic compounds | Saccharomyces cerevisiae | Brewery waste yeasts | Washing (distilled water) | Spray drying | [48] |
| Taxifolin | Saccharomyces cerevisiae | Yeast cells | Micro-structuring (low-frequency ultrasonic cavitation) | Direct mixing | [29] |
| Vitamin D3 | Saccharomyces cerevisiae | Yeast cells | Plasmolysis (NaCl) | -Spray drying-Freeze drying | [41] |
| β-Carotene | Yarrowia lypolitica | Yeast cells | ND | -Ultrasound-Solvent (chloroform, ethanol, acetone, or hexane) | [34] |
CTAB: cetyltrimethylammonium bromide; SDS: sodium dodecyl sulfate; DTT: dithiothreitol; EDTA: ethylene diamine tetra acetic acid; ND: not detected or analyzed
Spray drying is a commonly used method following emulsification and direct mixing. The emulsion consisting of compounds, cells, and other substances is pumped into a heat chamber, and the atomization of solution particles occurs at high temperatures by a spray nozzle. Atomized droplets in a spray drier heat chamber evaporate rapidly. The dried end-product (microcapsules) is separated from the air at the appropriate outlet temperature. Although spray drying is a simple process, heat-sensitive molecules such as bioactive compounds and probiotics may degrade, and oils can be oxidized due to high temperatures (130°–220°C) during the drying process [13, 68]. However, hydrophobic (D-limonene) and hydrophilic (ethyl hexanoate) core materials were encapsulated in β-glucans extracted from drum-dried yeast cells and maltodextrin by the spray drying process [26, 45]. As a result, high oxidative stability of flavor compounds was obtained in the spray-dried yeast cells compared to maltodextrin powder. Besides, yeast cells had a better retention capacity in both flavor compounds. Also, Marson et al. [33] showed that high EE (101.90%) was obtained for ascorbic acid encapsulated in hydrolyzed Saccharomyces pastorianus cells and maltodextrin with Maillard reaction products by spray drying. Besides, vitamin D3 was successfully encapsulated by spray- and freeze-drying processes [41] According to the study, high EE (76.10%) was obtained in plasmolyzed yeast cells by spray-dried encapsulation.
Recently, novel methods such as concentrated powder form (CPF) with a high-pressure spraying and pH-driven method have been developed. CPF technology is a high-pressure spraying process that transforms a liquid emulsion into a powder product through adsorption, capillary forces, and agglomerates. By applying a CPF method, limonene-encapsulated yeast cells remained intact, and their EE was up to 85.9% [44]. They concluded that yeast cells formed a highly protective shell for limonene, and they were resistant to harsh washing with hexane. In another study, curcumin was encapsulated into yeast cells using a pH-driven method, resulting in a 5-fold increase in loading capacity and a 43.6-fold reduction in incubation time compared to the diffusion method, with 80.66% EE [55]. In addition, some studies showed that the EE was increased for curcumin and fisetin as core materials by the osmoporation technique [47, 63]. However, it is slow due to mass transport under high pressure. At the end of the encapsulation process in a few studies, microcapsules have been coated with hydroxypropyl methylcellulose, alginate, and chitosan to increase their oxidative stability [25, 42, 50].
Yeast cell microcapsule quality can be determined utilizing EE, oxidative or thermal stability, and in vitro release of core material [2]. EE was calculated as the proportion of successfully encapsulated bioactives [9]. In contrast, EE is the amount of bioactives that have been successfully encapsulated relative to the initial amount of bioactive compounds [69, 70]. When exposed to fluorescent/ultraviolet (UV) light or different ambient conditions, such as relative humidity at storage periods in laboratory conditions, the degradation state of the core material (hydrophobic compound) defines the stability of yeast microcapsules [71, 72]. Because several bioactive compounds are highly photosensitive and quickly oxidizable molecules, their positive effects are limited [71, 73]. Yeast cells as wall materials ensure better thermo- and oxidative stability of bioactive compounds compared to “standard” materials such as oligo/polysaccharides, maltodextrins, glucans or modified starches, or cyclodextrins [7].
In vitro release tests provide information on the release kinetics and diffusion mechanism of bioactive compounds in different buffer solutions against time [72]. Essentially, the proportion of hydrophobic compounds released as a function of incubation time from yeast carriers is calculated as a release profile [74]. Release studies in the gastrointestinal tract are among other studies that provide information about the bio-accessibility value of bioactive compounds. Due to their regulated release, protection from stomach conditions, and prolonged core release in the intestine medium, yeast cells may be known as excellent carriers for microencapsulating bioactives [72].
Particle size compares the sizes of various matrices, including solid or semisolid particles and liquid aerosols. Techniques for determining particle size include dynamic light scattering, polydispersity index, and surface charge (ζ-potential) [75]. The most crucial aspect of carrier systems is the particle size since it significantly affects the release of bioactive components and the stability of microparticles [76]. Larger yeast microcapsules contain more hydrophobic compounds due to greater loading [72]. Moreover, the ζ-potential represents the suspension stability potential under specified conditions [77]. The preferred method for examining how a molecule interacts with its surroundings is fluorescence lifetime monitoring. The average amount of time a fluorophore spends in the excited state following the absorption of an excitation photon is known as the fluorescence lifetime [73]. By using several analytical tools such as differential scanning calorimetry (DSC), X-ray diffraction (XRD) analysis, and Fourier transform-infrared (FTIR) studies, the encapsulation of hydrophobic compounds with yeast cells was assessed. XRD is used for the primary characterization of material characteristics such as crystal structure, crystallite size, and strain [75, 78]. Zade Ashkezary et al. [78] analyzed an X-ray pattern of native yeast microcapsules with a wide broad peak matching the amorphous state. The XRD pattern of the cholecalciferol microcapsules of yeast migrated slightly to the left and became more intense. The absence of the characteristic vitamin peaks, however, suggests that the cholecalciferol in yeast microcapsules was not in a crystalline condition. One of the most useful thermal analysis techniques for determining a substance’s thermal behavior is DSC [79]. Yeast microcarriers’ capacity to shield hydrophobic bioactive compounds from thermally induced degradation shows their thermal stability [80]. DSC can provide data on transition temperatures, degree of crystallization, melting, and heat capacity in addition to some thermodynamic factors [79]. DSC is a helpful method for observing the impact of various additives on the thermal characteristics of materials and is used to give qualitative details on the physicochemical condition of the core material in various matrices [81]. Dadkhodazade et al. [81] declared that the endothermic peak in plasmolyzed yeast microcapsules changed to a lower temperature in comparison to non-plasmolyzed one because plasmolysis has increased the fluidity of the yeast cell wall. Moreover, vitamin D incorporation did not affect the thermal behavior of yeast microcapsules. In another study, Normand et al. [20] recorded a shift in the melting peak of limonene-loaded microcapsules, indicating that limonene was inside the membrane and reducing the cohesive energy of the phospholipidic bilayer. Furthermore, it was stated that the broad endothermic peak, which had a maximum temperature of 227℃, was caused by the Mentha pulegium essential oil evaporating from the matrix [77]. da Silva Lima et al. [10] indicated that C-O vibrations related to the phenol group at 1300–1000 cm–1 as well as benzene (the aromatic ring) corresponded to stretching vibrations less than 860 cm–1 as a result of carvacrol being encapsulated. Dadkhodazade et al. [41] demonstrated that cholecalciferol-loaded yeast microcapsules had higher wave numbers of the OH peak because of an increase in hydrogen bonds, besides the intense and sharp -CH stretching peak. By fluorescent, confocal, atomic force, transmission electron, or scanning electron microscopy, the morphology characteristics of yeast capsules can be observed, and it can be observed that hydrophobic compounds are trapped inside the yeast cell [9, 10, 20, 34, 38, 44, 63, 65, 69–72, 77, 80, 82, 83].
Encapsulation of hydrophobic compounds into yeast cells is affected by many different factors. EE may change depending on the encapsulation conditions and the matrix into which the encapsulated material will be stored. Temperature, the ratio of core material and yeast cell, and the encapsulation method are some of the main factors affecting EE.
An increase in temperature can result in an increase in EE. Penetration is easier at temperatures higher than 35℃ due to the reason that phospholipids are in gel form under this temperature. This causes the cell membrane to transition towards the more fluid liquid crystalline structure, enhancing chemical penetration. Many studies observed that EE is the highest at temperatures ranging from 35°C to 50°C. For instance, the highest EE of fish oil in the yeast cell was studied at temperatures ranging from 4°C to 70°C. The highest EE was observed at 43.4°C [25]. In a similar study, the EE of the drug itraconazole into Saccharomyces cerevisiae was studied in five different temperature ranges, including 25°C, 30°–40°C, 40°–50°C, 50°–60°C, and 60°–70°C. The highest EE (64.6%) was observed at the temperature range of 40°–50°C [84]. The decrease in the EE was observed also in other studies [25, 45]. This decrease was associated with two reasons: the possible denaturation of proteins in yeast cells and the evaporation of the solvent used for the encapsulation process [84, 85].
The ratio of core material and yeast cells is another factor that affects the EE. This effect may vary depending on the core material. For instance, in a study that analyzed the encapsulation of manuka essential oil in yeast cells, the EE was increased with the increase of the core material up to the concentration of 14.24% [74]. However, an increase in the core material content results in a decrease in EE. For instance, the EE of cholecalciferol in yeast cells was studied at two different concentrations of the core material, including 100,000 IU/g and 500,000 IU/g yeast cells. Besides, different encapsulation and drying methods are analyzed. The higher EE was obtained in the lower concentration of core material in all circumstances. This result was associated with two possible reasons, including the lack of capacity in the yeast cell to carry the core material and the limitation of hydrogen bonding positions that connect the cholecalciferol to the yeast cell [41]. Similar results are obtained for the encapsulation of curcumin in yeast cells. The increase in the concentration resulted in a decrease in the EE, and this was associated with the saturation of yeast cells [55].
Many different methods, such as osmoporation, spray-drying, freeze-drying, coacervation, vacuum infusion, and pH-driven, have been used to encapsulate hydrophobic materials into yeast cells. For instance, spray-drying and freeze-drying methods were compared regarding their effect on the EE of cholecalciferol (vitamin D3) in yeast cells (Saccharomyces cerevisiae) [41]. As indicated in the results, there was no significant difference between the two methods loaded with the lower amount of cholecalciferol (100,000 IU/g yeast cell). However, the spray-drying method exhibited higher EE (up to 17.85%) when loaded with a higher amount of cholecalciferol (500,000 IU/g yeast cell). Another study compared the pH-driven and passive diffusion methods regarding EE for curcumin [55]. At the same core material and yeast ratio, the pH-driven method had an EE of 80.66% ± 2.63%, while passive diffusion had an EE of 16.05% ± 1.12%. In addition to the EE, the pH-driven approach lasted 33 min, whereas the passive diffusion method required 24 h. Curcumin was encapsulated in yeast cells via osmoporation in another study. The EE was 34.38% at optimum conditions [47].
Plasmolysis of yeast cells is also an important factor in EE. It is a method of pre-treatment that removes the interior water from the cell. Some enzymes (adenylate kinase and pyruvate kinase), organic solvents (acetone and ethyl acetate), and chemicals (glycerol, NaCl, sucrose, etc.) are used for the plasmolysis of yeast cells. Many studies indicated that plasmolysis enhances the EE by increasing the space for core material. For example, the plasmolysis of yeast cells before the encapsulation of vitamin D3 has an improving effect on the encapsulation yield by up to 33.55% [41]. Similarly, the encapsulation yield of anthocyanin from Hibiscus sabdariffa L. flowers was increased after the plasmolysis of yeast cells with 5% NaCl up to 18.21% [86]. In contrast to these findings, plasmolysis may negatively affect the EE. For instance, while the plasmolysis of Saccharomyces cerevisiae does not affect the EE of curcumin [21], it decreases the EE of fisetin [63]. The negative effect resulting from a decrease in EE can be associated with the damage that can occur during plasmolysis.
The recent studies on encapsulation of hydrophobic materials, i.e., curcumin, flavonoids, carotenoids, and essential oils, in yeast cells are presented in Table 2 and discussed in detail below.
Curcumin is a natural polyphenol found in turmeric that offers several biological advantages, including anti-inflammatory, anticarcinogenic, antioxidant, and neuroprotective properties [53, 87]. However, the limited water solubility and vulnerability of curcumin to degradation by factors such as UV-visible radiation, oxygen, heat, and alkaline pH make it challenging to use in the food and pharmaceutical sectors [47, 65].
Encapsulation is a valuable technique that can protect delicate compounds like curcumin by shielding them from oxidative agents. Recent studies have efficiently employed yeast cells as a core material for curcumin encapsulation. Curcumin has the capability to be enclosed within various forms of yeast, such as intact yeast cells, plasmolyzed yeast, and yeast cell wall particles. Young and Nitin [65] compared intact yeast cells, plasmolyzed yeast (yeast cell wall particles or yeast cell wall particles), and Pickering emulsions. Yeast cell wall particles exhibited superior thermal stability for curcumin, with retention rates of 91.8% at 70℃ and 99.7% at 90℃, outperforming intact cells and Pickering emulsions. This advantage was attributed to yeast cell wall particles lacking native subcellular structures susceptible to denaturation and curcumin release. Osmoporation enables encapsulation by temporarily permeabilizing the plasma membrane and stretching the cell wall. Conversely, intact yeast cells provided better oxidative stability for encapsulated curcumin. The study demonstrated that yeast cell wall particles acted as a more effective barrier against thermal degradation compared to intact yeast cells and Pickering emulsions, preserving bound curcumin by preventing further denaturation. In a study by de Medeiros et al. [47], various factors were examined, including yeast cell concentration, ethanol concentration, and curcumin concentration, to understand their impact on encapsulation. The optimization of the cells/curcumin ratio and the use of ethanol concentrations up to 60% resulted in enhanced EE. Furthermore, curcumin encapsulated within yeast cells demonstrated significantly improved photochemical stability, leading to a considerably extended lifespan when exposed to illuminated conditions.
Vacuum diffusion and passive diffusion represent distinct techniques for yeast encapsulation. Vacuum diffusion utilizes a vacuum to expedite the infusion of substances into yeast cells, offering speed and precise control but potentially affecting yeast viability. In contrast, passive diffusion relies on natural gradients for a more gradual and gentle encapsulation process, prioritizing yeast cell viability [38, 74]. Sentosa et al. [69] studied the influence of temperature on the encapsulation of temulawak extract and pure curcumin in Saccharomyces cerevisiae with passive diffusion. They found that the most efficient encapsulation occurred at 45℃ when cell membranes were in a liquid-crystalline state, enhancing intracellular transport. However, the encapsulation yield and efficiency were lower compared to pure curcumin, suggesting potential competition from essential oil within the temulawak extract. Using encapsulation, curcumin’s bioavailability can be increased, and its stability can be improved, making it more useful in various applications. In a study conducted by Young et al. [38], the researchers explored the use of vacuum infusion for co-encapsulating curcumin and fisetin into yeast cells, comparing it to the conventional diffusion-limited method. They discovered that these bioactive compounds, curcumin, and fisetin, were localized in separate cytoplasmic regions within yeast microcarriers. Furthermore, their research revealed that ethanol concentration and the chosen encapsulation method significantly influenced yeast encapsulation outcomes. Notably, vacuum infusion with 35% ethanol concentration achieved a high EE of 64%, outperforming the 18.6% achieved by passive diffusion. This superior performance was attributed to the rapid evaporation of the carrier solvent, particularly ethanol, which concentrated the bioactives in the solution and facilitated their swift partitioning into yeast cells.
Yeast glucan particles, derived from Saccharomyces cerevisiae cell walls, serve as advanced carriers for poorly soluble drugs. They also offer diverse bioactive benefits, including immune support, antioxidants, anti-inflammatory properties, antimicrobial capabilities, wound healing potential, cholesterol reduction, gut health promotion, and potential protection against cancer and radiation [67]. Plavcová et al. [88] found that while empty yeast glucan particles showed prooxidative activity, pharmaceutical composites displayed antioxidant effects. The glucan particles/curcumin composites notably reduced the activity of nuclear factor and activator protein 1 (NF-κB/AP1) and resulted in decreased cytokine secretion. Rotrekl et al. [89] conducted a study to test yeast glucan particles as carriers for curcumin and assess their potential in treating colitis in rats induced by dextran sulfate sodium. They measured anti-inflammatory effects through disease activity, analyzing gut tissue markers and enzymes. The curcumin-yeast glucan composites effectively reduced colitis symptoms and lowered pro-inflammatory factors more than pure yeast glucan or curcumin alone. In a study by Ruphuy et al. [67], they successfully encapsulated model drugs, ibuprofen, and curcumin, within glucan particles using spray drying. Various spray drying parameters were investigated to assess the impact of atomizing droplet size and initial solid content on EE. The results demonstrated that higher solid content and larger droplet sizes led to increased EEs. For instance, the EE of curcumin in glucan particles was significantly improved from 61.5% to 102% by increasing both the initial solid content and droplet size, mainly when using a two-fluid nozzle. A study indicated that yeast cell wall particles with reduced intracellular contents exhibited a quicker release of encapsulated curcumin in the intestine following gastric digestion [53]. This release was affirmed by multimodal imaging, revealing no significant changes in cellular structures, including cell walls.
Curcumin has significant antimicrobial effects against various strains of bacteria, including Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Escherichia coli, and Bacillus coagulans, inhibits biofilm formation, and reduces bacterial pathogenicity. Researchers have investigated the effect of curcumin-loaded active capsules on inactive pathogenic biofilms. Dou et al. [90] developed food-grade microcarriers for precise curcumin delivery to inactivate pathogenic bacterial biofilms using photodynamic treatment. They assessed three microcarrier types: live yeast cells (EE: 31%), deactivated yeast cells (EE: 37%), and yeast cell wall particles (EE: 18%). The differences in curcumin encapsulation are due to structural distinctions. Curcumin predominantly interacts hydrophobically and through hydrogen bonding with yeast cellular components like the phospholipid membrane, glucan, and proteins. In contrast, yeast cell wall particles, primarily composed of β-1,3-D-glucan and chitin, have porous microspheres with reduced curcumin retention due to the loss of cytoplasmic components. Notably, all formulations achieved bacterial inactivation exceeding 93% through photodynamic treatment, with yeast cell wall particles containing curcumin reaching over 99% inactivation. These formulations also effectively removed biofilms from plastic surfaces, highlighting the vital role of microcarrier binding affinity in biofilm elimination. Encapsulation techniques hold promising potential for boosting the efficacy and expanding the applications of curcumin, offering substantial benefits across the food, pharmaceutical, and healthcare sectors.
Encapsulation of hydrophobic materials in yeast cells
| Hydrophobic compound | Encapsulation process | Yeast | Effects of encapsulation | Reference |
|---|---|---|---|---|
| Pure curcumin and temulawak extract | Diffusion with an incubator shaker (35°–55°C, 18 h) and vacuum-drying | Saccharomyces cerevisiae | The highest encapsulation efficiency/yield is at 45°C, while the lowest is at 35°C. | [69] |
| Curcumin | Osmoporation | Saccharomyces cerevisiae | -Improvement of EE (60%);-Retained over 80% of antioxidant activity after exposure to high temperatures (150°C) and artificial light for 50 days;-Increase 5.7-fold in photochemical stability, with a half-life of 181 days under illumination conditions. | [47] |
| Pressure-facilitated infusion | Saccharomyces cerevisiae (Alive yeast cells, deactivated yeast cells, and yeast cell wall particles) | -EE: 18% for yeast cell wall particles, 31% alive yeast cells; 37% deactivated yeast cells;-99% bacterial inactivation of yeast cell wall particles with curcumin. | [89] | |
| Curcumin and fisetin | Dehydration, rehydration, vacuum infusion, or incubation | Saccharomyces cerevisiae (native yeast, yeast cell wall particles) | EE: 66.6% curcumin and 64% fisetin (35% ethanol). | [38] |
| Curcumin | Controlled evaporation | Saccharomyces cerevisiae (glucan particles) | EE: 88.5%. | [88] |
| Curcumin | Diffusion and freeze-drying | Chlorella Vulgaris | EE: 98%. | [87] |
| Curcumin and ibuprofen | Spray drying | Saccharomyces cerevisiae (yeast glucan particles) | EE: 102% curcumin; 64.3% ibuprofen. | [67] |
| Curcumin | Vacuum infusion | Saccharomyces cerevisiae (Intact yeast cells, plasmolyzed yeast, i.e., yeast cell wall particles) | EE: 99.7% (90°C for 1 min). | [65] |
| Mentha pulegium essential oil | Plasmolysis pretreatment, diffusion, and freeze-drying | Saccharomyces cerevisiae | EE: 36%. | [59] |
| Manuka essential oil | Vacuum infusion method | Saccharomyces cerevisiae | EE: 16.8 mg/g ± 1.8 mg/g (14.24% w/v the concentration of oil in the encapsulation solution). | [74] |
| Purslane seed oil | Pretreatments: Non-plasmolyzed; emulsification plasmolyzed, diffusion and freeze-drying; extra-coating with carboxymethylcellulose | Saccharomyces cerevisiae [non-plasmolyzed, plasmolyzed, and plasmolyzed carboxy methyl cellulose (CMC)-coated] | -EE: 53–65%;-The plasmolysis;-Treatment increased EE and decreased the peroxide value of oil;-The lowest oxidation rate plasmolyzed CMC-coated microcapsules. | [70] |
| Menhaden fish oil | Pretreatments: autolysis or enzymatic hydrolysis; emulsification, diffusion, and freeze-drying; extra-coating with hydroxypropyl methylcellulose | Saccharomyces cerevisiae | -Autolysis at 55°C, combined with 1.5% ethyl acetate pretreatment (EE: 90%);-Stable for 30 days under < 70% RH. | [25] |
| Black cumin seed oil | Plasmolysis pretreatment, diffusion, and freeze-drying | Saccharomyces cerevisiae | -EE: 59.97%, plasmolyzed loaded yeast capsules;-EE: 39.18%, non-plasmolyzed loaded yeast capsules. | [66] |
| Wheat germ oil | Alive, non-plasmolyzed, and plasmolyzed, freeze-drying | Saccharomyces cerevisiae | EE: 43.1%, plasmolyzed loaded yeast capsules. | [91] |
| Oil blends (rapeseed, camelina, black cumin, evening primrose, wheat germ, and rice bran oil) | Autolysis, microencapsulation by ultrasound process, and freeze-drying | Saccharomyces cerevisiae | EE: 8.25–13.98%. | [92] |
| Flavonoid: quercetin | Diffusion and freeze-drying | Yarrowia lipolytica W29 | Longer lifetime of the long-term quercetin population. | [73] |
| Flavonoid: fisetin | Osmotic dehydration, rehydration, and internalization | Saccharomyces cerevisiae | Improvement of EE (33%) and internalized fisetin content (1.199 mg) via osmoporation. | [63] |
| Flavonoid: fisetin | Sonoprocessing and either spray-drying or freeze-drying | Saccharomyces cerevisiae | Better EE, encapsulation yield, and antioxidant activity for spray-dried microcapsules. | [82] |
| Flavonoid: taxifolin | Ultrasonic micro-structuring and diffusion | Saccharomyces cerevisiae | -EE: 61.7% at 37°C compared to 28°C;-More than 1.5 times higher antioxidant activity and bioavailability after in vitro digestion via encapsulation. | [9] |
| Terpene: carvacrol | Diffusion and freeze-drying | Saccharomyces cerevisiae | -EE: 60%;-The highest larvicidal activity and lower volatility on Rhipicephalus microplus. | [10] |
| Terpene: limonene | Emulsification and high-pressure spraying process (concentrated powder form technology) | Saccharomyces cerevisiae | EE of concentrated powder form technology: 85.9%. | [44] |
| Terpene: limonene | Diffusion and spray-drying | Saccharomyces cerevisiae | -aw < 0.7: no limonene release;-Thermostable and resistant up to 263°C; the release of limonene started above 243°C. | [20] |
| Terpene: limonene, carvone, and linalool | Diffusion | Saccharomyces cerevisiae | The process of encapsulation was mainly passive diffusion with slightly active transportation. | [64] |
| Stilbenes: resveratrol | Diffusion and freeze-drying | Saccharomyces cerevisiae | -2–3 times higher the water solubility;-The slower photodecomposition;-Stronger antioxidant activity. | [71] |
| Carotenoids: β-carotene | Magnetic agitation | Yarrowia lipolytica | Optimum EE: 42.8 μg/g β-carotene with Yarrowia lipolytica cultured at pH 4.5, a medium volume equal to 115 mL, and agitation speed at 211 r/min. | [4] |
| Carotenoids: β-carotene | Ultrasound-assisted diffusion and freeze-drying | Yarrowia lipolytica W29 | Ultrasound treatment was four times higher than chloroform-mediated encapsulation. | [34] |
| Vitamin: cholecalciferol (vitamin D3) | Diffusion and spray-drying or freeze-drying | Saccharomyces cerevisiae | -Highest EE: plasmolyzed and spray-dried capsules, approximately 76%;-Diffusion mechanism: fickian mechanism;-Lower release rate in gastric conditions (up to 35.75%) than in intestinal conditions (up to 97.9%). | [72] |
| Vitamin: cholecalciferol (vitamin D3) | Diffusion and spray-drying or freeze-drying | Saccharomyces cerevisiae | -53% protection against UV and over 90% protection against thermal treatment (80°C, 1 h)-Over 90% encapsulated vitamin D3 in baked bread. | [81] |
EE: encapsulation efficiency; CMC: carboxy methyl cellulose; aw: water activity; RH: relative humidity; UV: ultraviolet
Process parameters (temperature, pH, and incubation time) control passive-diffusion protocols of yeast encapsulation, which are frequently inefficient, time-consuming, and damaging to heat-sensitive molecules [82]. de Andrade et al. [82] reported that EE, yield, and antioxidant activity of fisetin-loaded yeast capsules were higher for spray-drying than freeze-drying after so no processing. However, osmoporation eliminates barriers that adversely affect encapsulation with yeast and contains sensitive bioactive components. This technique involves rapidly dehydrating yeast cells by raising the medium’s osmotic pressure, followed by fast rehydration, which causes the active substance diluted in the solution to penetrate immediately [63]. de Câmara et al. [63] stated that osmoporation pretreatment enhances the EE of fisetin. At 30 MPa, the yeast cell membrane survived during osmoporation, and 84% of the treated yeast cells were still alive.
Kalinina et al. [9] reported that the ultrasonic micro-structuring procedure was required to increase the surface area contacting the solvent and equalize the particle size of the taxifolin in the solution, thus effectively improving the EE. The antioxidant activity and bioavailability of taxifolin were protected by yeast cell encapsulation after in vitro digestion, with a 22–24% loss. Mechanically, taxifolin is likely attached to the hydrophobic portions of the biopolymers found inside cellular structures and shielded by the cell wall, allowing for a delayed release of the substance. In another study, Shi et al. [71] emphasized the chemical alterations of resveratrol during encapsulation. As a result of higher resveratrol solubility and prolonged release, the yeast-encapsulated resveratrol also demonstrated better antioxidant activity, good stability, and constant release. Pham-Hoang et al. [73] also determined that the quercetin-to-cell ratio is another crucial metric, in addition to the significance of solubilization to prevent the presence of aggregates more significant than the pore size of the cell wall. The other half of the molecules interact with cell components that can protect them, whereas the other half are likely to be present in soluble or monomolecular forms. Compared to quercetin solutions, the long-term population in yeast microcapsules had a longer lifetime (about 27 nanoseconds) and was more concerned with 20% of the molecules.
Flavors are volatile molecules that suffer significant losses, especially in extreme conditions in the food industry. The encapsulation of the volatile molecules within a thermostable particle is a commonly used technique for minimizing taste loss during relevant industrial procedures. This issue can be resolved by encasing the flavor molecules in an empty yeast cell [20]. A volatile monoterpene called carvacrol is present in various essential oils. da Silva Lima et al. [10] emphasized that carvacrol encapsulated in yeast cells had better acaricide activity on Rhipicehaulus micrplus. Normand et al. [20] hypothesized that the yeast cell wall was stable up to 263℃ regardless of limonene content and lost its resistant ability to internal pressure, then broke or became permeable to gaseous flavor molecules. Limonene release was blocked below a water activity of approximately 0.7. An increasing limonene content with a longer encapsulation time was observed by Errenst et al. [44]. This correlation strongly indicated that the limonene was encapsulated inside the yeast cells and not only between the individual yeast cells due to the formation of agglomerates. The amount of water in yeast cells can control the encapsulation rate. Ciamponi et al. [64] clarified that the encapsulation process of limonene, carvone, and linalool was mainly a passive diffusion phenomenon with slightly active transportation, and the main influential factor of encapsulation kinetics was the solubility of the hydrophobic compound in the cell wall, which is inversely related to partition coefficient.
A yeast cell microencapsulation pharmaceutical strategy enhances compounds’ stability by creating a physical barrier, protecting the encapsulated molecules from environmental factors. Using yeast cell walls, this method encapsulated hydrophobic compounds like monoterpenes and essential oils [10].
Yeast cell microcarriers have been utilized for essential oil encapsulation, offering improved thermal stability. Liu et al. [74] found that yeast microcarriers enhanced the thermal stability of manuka essential oil by approximately 43%. Yeast cells hold significant potential as matrices for microencapsulating fish oil. Encapsulation method parameters of curcumin in yeast cells are also affected by several parameters such as retention time, process temperature, and drying step [59]. Various pretreatment methods, such as autolysis or enzymatic hydrolysis, can be employed to modify the properties of yeast capsules. Different pretreatment methods were evaluated for their impact on the suitability of yeast cells for microencapsulation [25]. Autolysis was found to be the most effective pretreatment method for yeast cells in microencapsulation, achieving an impressive efficiency of around 90%, and the addition of a hydroxypropyl methylcellulose coating further improved storage stability by enhancing the protective barrier and reducing oxygen diffusion [59]. In their study, Kavosi et al. [70] found that plasmolysis treatment significantly enhanced the EE and loading capacity of purslane seed oil using Saccharomyces cerevisiae cells. This enhancement was attributed to the entrapment of curcumin within yeast cells, either through adhesion to cytoplasmic cell membranes or interaction with cell wall components via hydrogen bonds. The encapsulation of oils in yeast cells proved to be an effective method for improving the in vitro stability of the oil [24]. This resulted in a prolonged release of the oil, coupled with increased bio-accessibility.
Natural pigments within the yellow-to-red color spectrum are called carotenoids [4]. Despite having a higher partition coefficient, larger molecules tend to crystallize and form supramolecular structures at relatively low concentrations. As supramolecular structures exceed the size restriction for loading, solubility restrictions appear to be the crucial factor [34]. Dang et al. [4] demonstrated the capacity of Yarrowia lipolytica to encapsulate large hydrophobic compound, β-carotene, and the culture environment and cultivation agitation had a significant impact on this EE Pham-Hoang et al. [34] also explained that the ultrasound treatment increased the encapsulation of β-carotene into Yarrowia lipolytica. When assessing the in vitro release of β-carotene in wheat germ oil encapsulated by alive, non-plasmolyzed, and plasmolyzed yeast cells, it was found that alive, non-plasmolyzed yeast cells exhibited a higher release rate under esophagus-stomach conditions. In contrast, plasmolyzed cells increased digestion in the duodenum and ileum [91].
One of the oil-soluble micronutrients is vitamin D. The proper action of vitamin D is necessary for immunological and nervous system function to be expected [72]. Dadkhodazade et al. [41] used plasmolysis with NaCl as a pretreatment before the encapsulation procedure of cholecalciferol, and this led to noticeably higher EE values because of the higher available space for core loading by ejecting water and even some water-soluble components from the cell, such as proteins, nucleic acid, and some enzymes. The mean particle size increased while the concentration of cholecalciferol content in the formulation’s EE declined. Also, in comparison to freeze-dried microcapsules, the spray-dried microcapsules were smaller and more uniform. Also, in another study, Dadkhodazade et al. [81] stated that the encapsulated vitamin D3 in yeast provides photochemical and thermal stability, and using the encapsulated form in bread protected it from baking temperatures. The free vitamin D3 remains at the rate of 34% in baked bread, while the encapsulated form remains above 90%.
Encapsulation of bioactive materials and micronutrients can be helpful to increase their efficiency by improving their stability in food products and the gastrointestinal system. In this regard, yeast cell encapsulation is an alternative method for encapsulating food ingredients and bioactive compounds. The technique has many advantages, including encapsulating hydrophobic and hydrophilic materials, being cost-effective, and simple to process. Furthermore, since yeast cells are biological materials, it is a suitable encapsulation method for consumers who prefer natural products. Many hydrophobic compounds, including curcumin, essential oils, phenolic compounds, carotenoids, and vitamin D, are encapsulated in the yeast cells, and even though encapsulation conditions/parameters may affect the process, encapsulation in yeast cells seems to be a promising method for encapsulating hydrophobic compounds. However, optimization of encapsulation methods and parameters is necessary to increase EE and stability, particularly for β-carotene and different kinds of essential oils. Consequently, further studies are needed to better understand the release and bioavailability of these hydrophobic materials encapsulated in yeast cells.
CPF: concentrated powder form
DSC: differential scanning calorimetry
EE: encapsulation efficiency
PEF: pulsed electric field
XRD: X-ray diffraction
DGK, ABB, GK, and BS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. EC: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Angela Daniela Carboni ... María Cecilia Puppo
Lucas O. Benitez ... Juan M. Castagnini
Luciana M. Julio ... Vanesa Y. Ixtaina
Juliana Ripari Garrido ... María Victoria Salinas
Lucía Cassani, Andrea Gomez-Zavaglia
