Affiliation:
Institute of Analytical and General Chemistry, Faculty of Engineering and Natural Sciences, Johannes Kepler University Linz, 4040 Linz, Austria
Email: bernhard.thalhamer@jku.at
ORCID: https://orcid.org/0000-0003-3589-3331
Affiliation:
Institute of Analytical and General Chemistry, Faculty of Engineering and Natural Sciences, Johannes Kepler University Linz, 4040 Linz, Austria
ORCID: https://orcid.org/0000-0002-0489-3534
Affiliation:
Institute of Analytical and General Chemistry, Faculty of Engineering and Natural Sciences, Johannes Kepler University Linz, 4040 Linz, Austria
Affiliation:
Institute of Analytical and General Chemistry, Faculty of Engineering and Natural Sciences, Johannes Kepler University Linz, 4040 Linz, Austria
ORCID: https://orcid.org/0000-0001-9139-4022
Affiliation:
Institute of Analytical and General Chemistry, Faculty of Engineering and Natural Sciences, Johannes Kepler University Linz, 4040 Linz, Austria
ORCID: https://orcid.org/0000-0001-6982-0558
Explor Foods Foodomics. 2024;2:460–470 DOI: https://doi.org/10.37349/eff.2024.00046
Received: January 19, 2024 Accepted: July 26, 2024 Published: August 16, 2024
Academic Editor: Jose Mendiola, Institute of Food Science Research (CIAL-CSIC), Spain
The article belongs to the special issue Food Authenticity and Emerging Challenges of Novel Food
Aim: Ashwagandha is a widely recognized medicinal plant in Ayurveda, a traditional Indian system of medicine. These extracts, which are concentrated forms of the root, contain specified withanolides (WLs) at a 5% median concentration on their packaging. Given the visual similarity between the capsule contents of these dietary supplements and authentic pulverized Ashwagandha root, there is a growing suspicion that these so-called extracts may be merely pulverized roots. To address these concerns, a procedure for evaluating Ashwagandha root extracts is presented that offers simplicity, cost-effectiveness, and the ability to provide a valid estimation.
Methods: The procedure incorporates microscopic investigations to facilitate the identification of plant fragments, which should be absent in properly prepared extracts. High-performance liquid chromatography (HPLC) with ultraviolet detection is employed to check whether the supplements are more than 10-fold concentrated compared to the powdered root, as claimed on the product labels.
Results: In the analyzed Ashwagandha root extracts, plant fragments and starch granules were detected, which could be attributed to the root in terms of size and shape. HPLC analysis of both root extracts and roots revealed nearly identical chromatograms with respect to peak patterns and signal intensity. Quantitative analysis indicated a WL content of approximately 0.15% in all tested Ashwagandha root extracts, considerably lower than the claimed 5% median content but consistent with published data for Ashwagandha root.
Conclusions: Notably, none of the 10 dietary supplements labeled as Ashwagandha root extracts fulfilled the manufacturers’ claims. These findings emphasize the need for practical and simple evaluation procedures, such as those proposed in this study. Such methods enable the evaluation of Ashwagandha root extracts without requiring the complex coupling of HPLC to mass spectrometry, making them accessible and feasible.
Ashwagandha [Withania somnifera (L.) Dunal, Solanaceae] is a widely recognized medicinal plant in Ayurveda, a traditional Indian system of medicine. It has gained considerable attention due to its diverse pharmacological properties, including anti-inflammatory, antioxidant, immunomodulatory, and adaptogenic properties. These therapeutic benefits are primarily attributed to bioactive compounds known as withanolides (WLs) and withanosides (WSs) [1–4]. To obtain a high concentration of these substances, Ashwagandha root extracts are produced, which are then utilized in the form of dry extracts, typically in capsules. A substantial number of Ashwagandha dietary supplements are explicitly labeled as root extracts, i.e., the concentrated form of the plant. Dietary supplements containing extracts are characterized by a higher quantity of bioactive compounds compared to natural plants, which is associated with increased efficacy and effectiveness of the supplements. However, in recent years, dietary supplements containing plant extracts have faced criticism for their quality [5–8]. The reliability of plant extracts in marketed dietary supplements is notably lower than that in medicinal products, particularly because the dietary supplement sector is less regulated. The intense competition in this specific market segment can lead manufacturers to opt for low-cost plant raw materials over expensive plant extracts to reduce production costs, resulting in compromised efficacy and quality.
As an additional quality marker, a median content of 5% WLs is typically indicated on products containing Ashwagandha root extract supplements. Although more than 40 different WLs have been identified to date [9], published analytical methods, primarily high-performance liquid chromatography-mass spectrometry (HPLC-MS) methods [10–13], generally determine only a small number of selected main WLs. However, the specific WLs included in these methods can differ considerably from study to study, complicating the comparison of results across different studies. Quantitative statements about total WL content [12, 14] are still approximations, albeit good ones, even though they are measured using sophisticated analytical equipment and have undergone extensive validation. Ultimately, the term “total withanolide content” found in several studies is not used precisely enough. The so-called total WL content is calculated by summing up the quantified individual WLs, thus not fully encompassing all WLs. In this context, it would be more accurate to refer to the summed content of the selected or observed main compounds. Another imprecision is that WSs are included in the “total” WL content. WLs are steroidal compounds, while WSs are glycosides of steroids, thus saponins. It would be more precise to explicitly mention that the determined content comprises the following two main groups: WLs and WSs. Research that specifically addresses WLs and WSs has led to a validated HPLC-MS method for a remarkable number of 11 different compounds [13], yet it unveils another challenge: only a few standard substances required for HPLC-MS analysis are commercially available. Consequently, four standard compounds (WS VII, viscosalactone B, 27-hydroxywithanone, dihydrowithaferin A) had to be isolated from the root, a process that requires considerable time due to their low concentration in the raw plant [11, 12, 15].
These challenges appear to contribute to the fact that determining WLs is not yet established in the routine mode for food safety control authorities. Implementing and maintaining a routine method for WLs is not only cost-intensive but also challenging if the total content is calculated as the sum of classically quantified individual components.
Therefore, addressing these limitations and developing a simple and feasible procedure for the qualitative and quantitative evaluation of Ashwagandha extracts in dietary supplements is crucial. Previous studies on the quality of commercially available Ashwagandha products differ in terms of product type, research questions, and investigated analytes from the work presented here, which specifically focuses on mono-herbal products that are explicitly labeled as root extracts and for which a total WL content is specified by the manufacturer. A previous study found significant differences in the content of selected WLs and WSs [16] in both mono- and poly-herbal products offered on the Indian market. In a recent study thin-layer chromatography and HPLC-UV were used to analyze phenolics and flavonoids in commercial Withania somnifera root from local markets and from their natural habitat [17]. The results of that study indicate adulteration or contamination by performing a comparative test with control samples and using gallic acid and quercetin as reference standards.
Ten different samples of commercially available dietary supplements labeled as Ashwagandha root extracts, which are according to legal classification over-the-counter products, were obtained randomly from drug stores, pharmacies, and online stores. Moreover, only mono-herbal Ashwagandha products were used but no poly-herbal preparations. To avoid potential conflicts, information about the manufacturers of products that do not meet their stated claims is deliberately omitted.
Ashwagandha root was procured from a pharmaceutical wholesaler (Kottas Pharma GmbH) who supplies Austrian pharmacies with medicinal plants. The specimen with the batch number W23201745, taken in accordance with GMP regulations, was checked and confirmed for identity by botanically trained experts. Another Ashwagandha root was obtained from Kräuterkontor (Berlin, Germany). Table 1 gives an overview of the investigated samples.
List of investigated Ashwagandha roots and Ashwagandha extract dietary supplements
| Sample code | Sample form | Sample content | Label claims WL% |
|---|---|---|---|
| A | Crushed | Ashwagandha root | - |
| B | Pulverized | Ashwagandha root | - |
| C | Capsule | Ashwagandha root extract | 5 |
| D | Capsule | Ashwagandha root extract | 5 |
| E | Capsule | Ashwagandha root extract | 5 |
| F | Capsule | Ashwagandha root extract | 5 |
| G | Capsule | Ashwagandha root extract | 5 |
| H | Capsule | Ashwagandha root extract | 5 |
| I | Capsule | Ashwagandha root extract | 5 |
| J | Capsule | Ashwagandha root extract | 5 |
| K | Capsule | Ashwagandha root extract | 2.5 |
| L | Capsule | Ashwagandha root extract | 2.5 |
-: no data. WL: withanolide
100 mg of each Ashwagandha dietary supplement was suspended in 15 mL of methanol and subjected to extraction at 70°C for 30 min in a closed vial using an ultrasonic bath. This procedure was repeated twice. The supernatants from these extractions were combined, and the total volume was adjusted to 50 mL in a volumetric flask. In summary, the samples (roots and dietary products labeled as root extracts) were extracted three times with methanol at elevated temperatures. In a fourth extraction only negligible amounts of the investigated compounds were detected, indicating that triple extraction is clearly sufficient. WS IV and withaferin A from Merck (Darmstadt, Germany) were obtained in the form of a stock solution in a concentration of 100 µg/mL methanol.
Water with a resistivity of 18 MΩ·cm was produced using a Milli-Q water purification system (Millipore). Analytical-grade acetonitrile and methanol were sourced from VWR (Fontenay-sous-Bois, France), ammonium formate from Sigma-Aldrich (St. Louis, MO, USA), and hydrochloric acid, phloroglucinol, iodine, potassium iodide, and WS IV and withaferin A from Merck (Darmstadt, Germany). Various types of starch were acquired from an Austrian pharmacy. Lugol’s solution, which was used for the microscopic staining of starch granules, was prepared with 2% iodine and 4% potassium iodide in water. The Wiesner reagent, employed for the microscopic staining of lignified cell parts, comprised 1% phloroglucinol in ethanol mixed with 12 M HCl (1:1, v:v).
An Agilent Series 1260 HPLC system equipped with a quaternary pump, a vacuum degasser, an autosampler, and a UV visible diode array detector was used for the analysis.
To identify WLs and WSs, the HPLC system was coupled with an Agilent 6520 quadrupole/time-of-flight mass spectrometer. Electrospray ionization in the positive ion mode was utilized. The ion source conditions were set as follows: drying gas temperature of 350°C, capillary voltage of 3750 V, nebulizer pressure of 45 psi, fragmentor voltage of 200 V, and drying gas flow of 10.5 L/min.
A Zeiss Axio Imager A1 microscope from Zeiss (Oberkochen, Germany) was utilized for the microscopic analysis with a Zeiss AxioCam MRc5 camera and Software AxioVision Release 4.7.2. Additionally, a Panthera Cloud light microscope from Motic (Xiamen, China) was employed for microscopic analysis using a built-in camera (Moticam X5 Plus) and Motic’s ImageOnDevice technology for real-time interaction.
The pulverized Ashwagandha root was placed on a microscope slide and moistened with a drop of water before covering it with a coverslip. To stain starch granules, a small drop of Lugol’s solution was carefully placed on the slide at one side at the edge of the coverslip to enable diffusion into the specimen. This interaction with starch granules results in a distinctive dark blue coloration, indicating their presence. This coloration occurs due to the reaction of iodine with the spiral structures of amylose and amylopectin in the starch. To enhance the visibility and contrast of the delicate structures of the starch grains, a diluted form of Lugol’s solution was also used to achieve a lighter blue coloration. To assess the effect of water and heat on starch grains (i.e., gelatinization), a small amount of the pulverized Ashwagandha root was placed on a microscope slide and exposed to 70°C in a drying cabinet for approximately 30 s. To test for the presence of lignified cells (i.e., xylem elements), observed particles were stained red with Wiesner reagent, a reaction between the aldehyde groups in lignin and phloroglucinol catalyzed by hydrochloric acid.
The chosen UV detection wavelength was 227 nm. Chromatographic separation was achieved using a YMC-Triart C18 column (100 × 3 mm ID, 3 μm particle size) supplied by YMC (Kyoto, Japan). The injection volume was set at 5 μL. The mobile phase [13] comprised acetonitrile (solvent A) and water (solvent B), with a gradient program starting at 25% solvent A and 75% solvent B. This ratio shifted to 55% solvent A and 45% solvent B at 10.0 min, then to 95% solvent A and 5% solvent B at 13.0 min, which was maintained until 16 min. The conditions returned to their initial conditions at 16.1 min, completing the run within a total time of 20 min. The flow rate was maintained at 0.5 mL/min.
The chromatographic conditions for HPLC-MS, including the column, run time, flow rate, and acetonitrile gradient, were identical to those of the HPLC-UV method, with the sole modification of replacing 5% of solvent B with a 10 mM ammonium formate solution in water containing 0.1% formic acid (solvent C).
Regarding the specificity of the method, the individual WSs and WLs were identified using high-resolution mass spectrometry. For linearity 10 different concentrations in the range of 0.2–10 µg/mL for WS IV and withaferin A were analyzed. The precision of the method was tested by a triplicate determination of a dietary supplement labeled as Ashwagandha root extract. The limit of quantitation was set as the concentration corresponding to one-fifth of the lowest standard of the calibration for WS IV and one-tenth for withaferin A.
Microscopic examinations were conducted to compare dietary supplements of Ashwagandha root extracts with pulverized authentic Ashwagandha root. These comparisons revealed a substantial issue at first glance: plant fragments were visible in the dietary supplements, which contradicts the expectation that properly prepared extracts should be devoid of plant parts. The supplements were labeled explicitly as extracts and not as mixtures of extract and plant material, creating a discrepancy between the observed presence of plant fragments and the product labeling as “extract”. Figure 1 shows the microscopic identification of the xylem fragments described and depicted in prior literature [18, 19]. These lignified xylem parts were stained red with Wiesner reagent (Figure 1, right image).

Microscopy of xylem parts identified in a dietary supplement (sample K) labeled as Ashwagandha root extract. Transmitted light microscopy of xylem parts unstained (left) and stained with phloroglucinol (right), magnified 500×
Xylem parts were found in all examined Ashwagandha root extracts. A remarkably large xylem fragment of about 800 µm is shown in Figure S1 in the supplementary data. Even small xylem fragments are clearly identified due to their characteristic shape, see Figure S2 (supplementary data).
The starch found in the root appears as roundish to oval single grains, as well as in conglomerates of two, three, or, more rarely, four grains [18]. The fissure shapes emanating from the mostly centric hila exhibit varying shapes, ranging from longitudinal to asymmetrical stellate in both single grains and conglomerates. Figure 2 shows the starch grain aggregates, each comprising two grains, stained with diluted Lugol’s solution. The starch grains of authentic Ashwagandha roots measure up to approximately 30 µm, as shown in Figure 2.
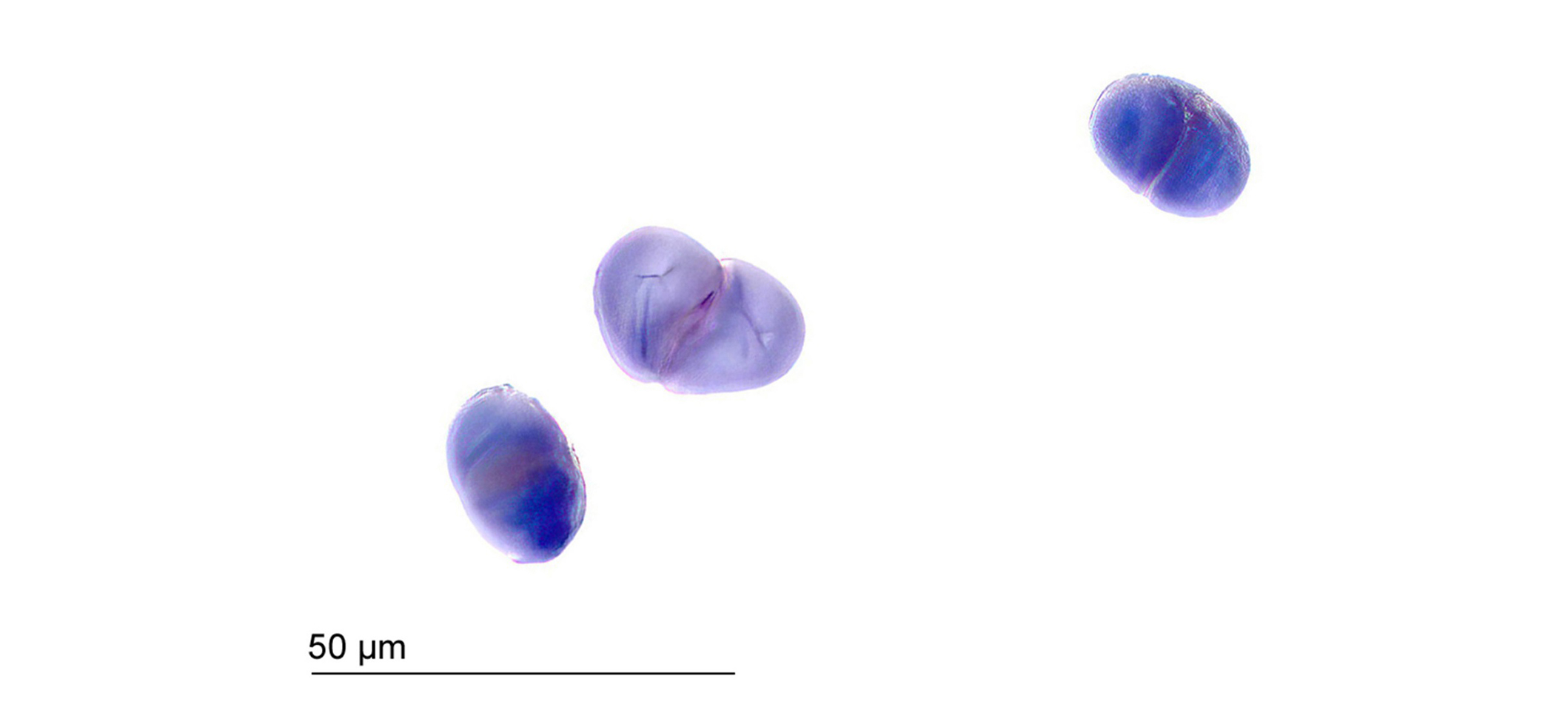
Starch aggregates composed of two starch grains stained with diluted Lugol’s solution of Ashwagandha root (500×), sample A
Characteristic starch grains as described above were not found in any of the analyzed dietary supplements. Instead, in certain samples, the starch grains measured up to 50 µm, approximately double the size of those in pulverized Ashwagandha root. Their shape resembled that of deformed, gelatinized starch grains, with a typical bulge at the edge, as shown in Figure 3. The deformation of starch granules, which often results from thermal or pressure effects, is a recognized phenomenon.
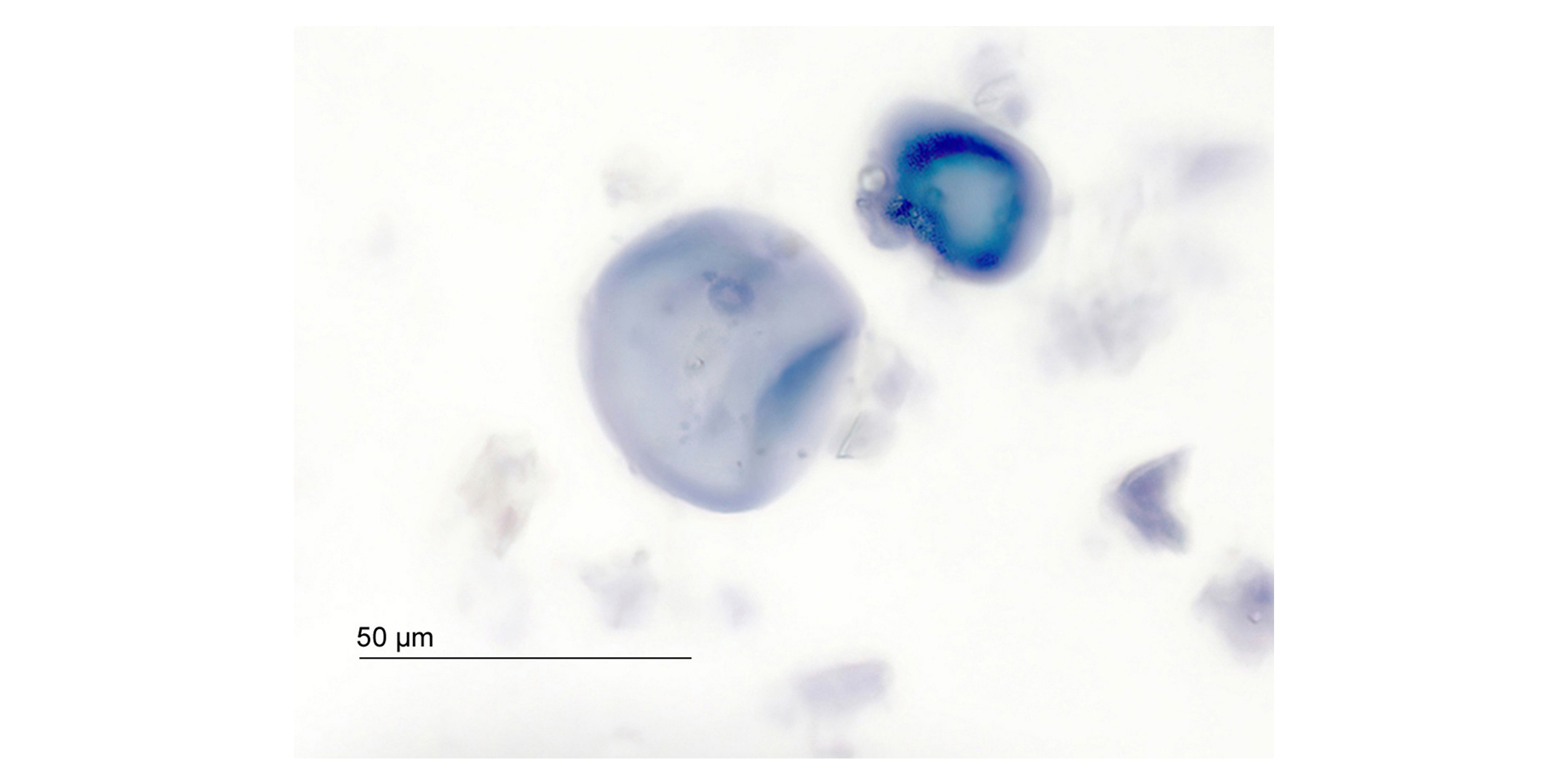
Deformed gelatinized starch grains stained with diluted Lugol’s solution in a dietary supplement labeled as Ashwagandha root extract (500×), sample K
The gelatinization of starch grains was investigated using authentic roots. The resulting gelatinized starch grains bore a striking resemblance in shape and size to those found in some analyzed capsule contents labeled as Ashwagandha root extract.
It should be noted that starch (from wheat, rice, and maize) may be used as an excipient in preparing these capsule-based Ashwagandha extracts. Therefore, careful attention to the shape and size of starch grains is crucial when identifying the presence of starch using Lugol’s solution.
HPLC-UV is a pivotal analytical method that is widely acclaimed for its versatility and broad applicability in areas such as food control. In the present case, specificity was confirmed by unambiguous detection of both the molecular ion [M+H]+ and the ammonium adduct [M+NH4]+. Concerning linearity, the calibration curve for withaferin A yielded an equation of y = 100.39x – 0.9197 with a coefficient of determination R2 of 0.9999. For WS IV, the equation was y = 36.375x + 0.3797 with an R2 of 0.9999. The precision from a triplicate determination showed a relative standard deviation of 2.1 % for withaferin A and 4.6% for WS IV. The limit of quantitation was 0.04 µg/mL for WS IV and 0.02 µg/mL for withaferin A. The developed chromatographic method was used for comparison between Ashwagandha root extracts and authentic Ashwagandha root.
The integration of HPLC with high-resolution mass spectrometry was solely for the accurate peak identification of principal WLs and WSs throughout the HPLC-UV method’s development. The principal signals were identified by high-resolution mass spectrometry and correlated with their respective UV signals. Table 2 organizes the individual compounds by their retention times.
List of individual compounds, including withanosides (WSs) and withanolides (WLs), based on their chromatographic retention time (RT)
| Peak | RT (min) | Name | Sum formula | Type |
|---|---|---|---|---|
| 1 | 5.53 | WS IV | C40H62O15 | WS |
| 2 | 6.18 | WS VII | C40H62O15 | WS |
| 3 | 8.06 | Dihydrowithaferin A | C28H40O6 | WL |
| 4 | 8.65 | WS V | C40H62O14 | WS |
| 5 | 9.05 | Withaferin A | C28H38O6 | WL |
| 6 | 9.74 | 12-Deoxywithastramonolide | C28H38O6 | WL |
| 7 | 10.02 | Withanone | C28H38O6 | WL |
| 8 | 10.52 | WL A | C28H38O6 | WL |
| 9 | 11.21 | WL B isomer | C28H38O5 | WL |
| 10 | 11.83 | WL B isomer | C28H38O5 | WL |
| 11 | 12.30 | WL B isomer | C28H38O5 | WL |
| 12 | 13.84 | WL B | C28H38O5 | WL |
Figure 4 shows a chromatographic comparison between the dietary supplements labeled as Ashwagandha root extracts and the authentic Ashwagandha root used as a reference. The chromatograms of the dietary supplements and the authentic root are almost identical in terms of peak pattern and signal intensity. The concentration of WLs in the dietary supplements agrees with that of the purchased Ashwagandha root. This finding corroborates the microscopic observations, indicating that the capsules tested do not contain a properly prepared concentrated Ashwagandha extract. It may be an interesting question if leaf material has been added to the dietary supplements labeled as root extracts. For this purpose, the signal intensities of withaferin A and WL A were compared. As can be seen from Figure 4, withaferin A exhibits a signal intensity similar to WL A. However, a previous study investigating a WL-rich fraction prepared from roots and leaves showed a significantly different peak pattern in chromatography. The chromatogram of this rich fraction from roots and leaves demonstrates a signal ratio of Withaferin A to WL A exceeding 10 times [20]. Therefore, it is not expectable that the samples used in our study are contaminated or adulterated with leaves.
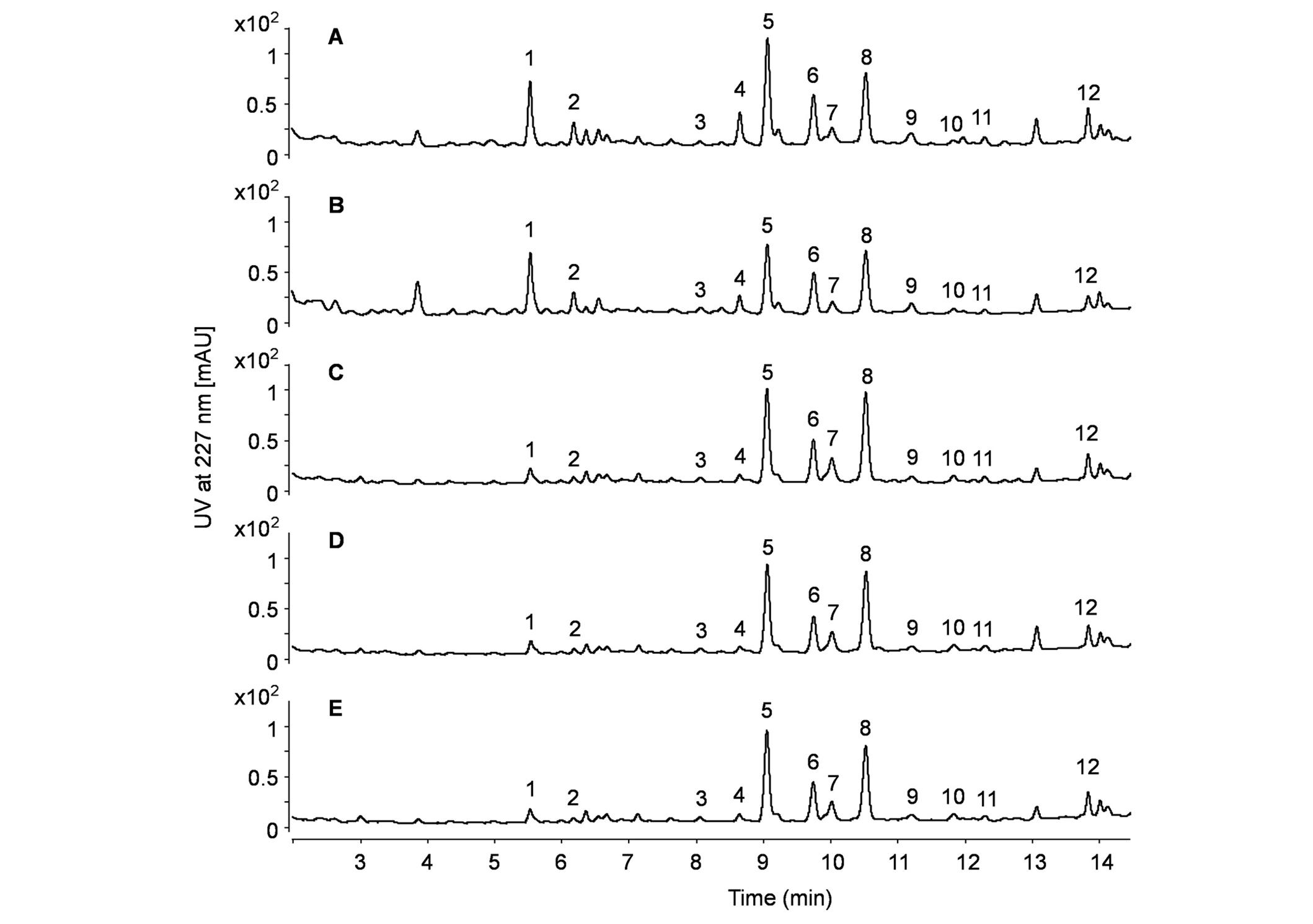
Chromatographic comparison between authentic Ashwagandha roots and dietary supplements labeled as Ashwagandha root extracts. A: Crushed Ashwagandha root; B: pulverized Ashwagandha root; C–E: dietary supplements containing Ashwagandha extracts. Peaks 1–12 are detailed in Table 2
WS IV and withaferin A were selected as standard substances for quantifying the principal components of WSs and WLs, respectively. Using the response of a standard substance for the quantitative characterization of a group of ingredients is a common approach for secondary plant metabolites [21, 22]. The UV response of WS IV facilitated the quantification of WS IV (peak 1), WS VII (peak 2), and WS V (peak 4). The quantities of WSs determined in this way were summed and reported as the WS content in percentage. Similarly, the response of withaferin A was employed to estimate the content of dihydrowithaferin A (peak 3), withaferin A (peak 5), 12-deoxywithastramonolide (peak 6), withanone (peak 7), WL A (peak 8), WL B (peak 12), and its isomers (peaks 9, 10, 11). The cumulative results of the WLs quantified using this approach were also summed and expressed as the WL content in percentage.
Table 3 lists the content for both summed WSs and WLs of all coded samples. The detected main WL content in all tested Ashwagandha root extracts was on average 0.13%, and therefore is considerably lower than the claimed 5% median content, yet it agrees with our measurements of Ashwagandha root and corroborates published data on this matter.
Content for all summed WSs and WLs in Ashwagandha root and dietary supplements labeled as Ashwagandha root extracts. In the case of capsules, the percentages refer to the capsule content
| Sample code | Label claims WL% | WS% measured | WL% measured | WL% + WS% |
|---|---|---|---|---|
| A | - | 0.10 | 0.11 | 0.21 |
| B | - | 0.08 | 0.09 | 0.17 |
| C | 5 | 0.02 | 0.11 | 0.14 |
| D | 5 | 0.02 | 0.10 | 0.12 |
| E | 5 | 0.02 | 0.10 | 0.12 |
| F | 5 | 0.03 | 0.11 | 0.14 |
| G | 5 | 0.02 | 0.10 | 0.12 |
| H | 5 | 0.03 | 0.11 | 0.14 |
| I | 5 | 0.01 | 0.10 | 0.11 |
| J | 5 | 0.02 | 0.09 | 0.11 |
| K | 2.5 | 0.03 | 0.10 | 0.13 |
| L | 2.5 | 0.02 | 0.11 | 0.13 |
WS: withanoside; WL: withanolide. -: no data
The presence of only 0.13% of the main WLs in all tested Ashwagandha root extracts, which is considerably lower than the stipulated 5% median content, aligns with published data regarding Ashwagandha root.
The microscopic detection of plant fragments in dietary supplements labeled as Ashwagandha root extracts prompts several concerns. One possibility is that the plant material was intended as a filler but was not appropriately disclosed on the supplement packaging. Alternatively, there could have been a mix-up in the manufacturing process, where the pulverized root was used instead of the extract. Another reason may be a highly inadequate extraction procedure which could explain the presence of xylem fragments and the much too low concentrations of the main ingredients.
Given that multiple batches of some supplements exhibited this issue, a systematic processing error may be at play. For instance, supplement manufacturers might be sourcing from a central wholesaler whose “extract” is used without proper verification.
Regardless of the underlying reason, plant fragments raise serious concerns about the proper preparation and the integrity of the claimed concentrations. HPLC-UV analyses further validate these concerns, demonstrating that the Ashwagandha root extracts in dietary supplements do not meet the manufacturers’ claimed median WL content of 5%. The concentration of selected main WLs detected falls within the range typical of the herbal raw material, suggesting that the efficacy of the tested supplements is similar to that of the raw plant rather than an enhanced extract.
The pressing issue is the universal failure of tested dietary supplements labeled as containing Ashwagandha root extract to live up to their claims. The discovery that none of the samples from 10 different manufacturers contained the advertised pure Ashwagandha extracts signals a dire need for regulatory oversight and quality testing in the food industry. The reasons behind this widespread lack of compliance could be manifold, but a notable factor might be the intrinsic limitations of current testing methodologies. Determining the total WL content, even with advanced techniques, yields only approximate values. Consequently, adequate testing protocols may not have been established or enforced. Furthermore, there has been a decline in the application of simple pharmacognostic tests like microscopy, likely due to the emphasis on sophisticated instrumental analysis in recent decades.
The testing methods introduced in this study, employing simple and accessible tools such as microscopes and HPLC-UV, demonstrate that it is feasible to conduct a valid evaluation of dietary supplements containing Ashwagandha root extracts. These proposed methodologies not only negate the need for the labor-intensive isolation of standard compounds but also considerably reduce the costs associated with advanced analytical equipment. In addition, the quantitative estimates produced by these methods align with published data on the WL and WS content in Ashwagandha root, using more expensive and sophisticated equipment. The comparison of these results with those obtained from HPLC-MS analyses demonstrates the reliability of the procedure presented herein. This comparison highlights the advantages of the proposed approach in terms of cost efficiency and simplicity without compromising performance.
Overall, this approach represents a notable advancement in the evaluation of natural products by introducing a straightforward and rapid quality check for Ashwagandha root extracts in dietary supplements. This procedure offers a reliable estimate of the main WL content.
HPLC: high-performance liquid chromatography
MS: mass spectrometry
UV: ultraviolet
WLs: withanolides
WSs: withanosides
The supplementary figures for this article are available at: https://www.explorationpub.com/uploads/Article/file/101046_sup_1.pdf.
The microscopic images were taken at the Institute of Polymer Science at the Johannes Kepler University, Linz, Austria. Our thanks go to Prof. Dr. Sabine Hild.
BT: Conceptualization, Writing—original draft, Writing—review & editing, Investigation, Supervision. MH: Investigation, Validation, Writing—review & editing. BS: Investigation. CK: Writing—review & editing. WB: Writing—review & editing. All authors read and approved the submitted version.
Not applicable.
Not applicable.
Not applicable.
Data can be provided for any qualified researchers on reasonable request.
The authors declare that they have no conflicts of interest.
Not applicable.
© The Authors 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Anushi Madushani Wijethunga, Chijioke Emenike
Yanmei Zhu ... Haiyan Fu
Adindu O. Onyeodili ... Raquel P.F. Guiné
