Abstract
Aim:
The aim of this study is to investigate the polyphenol composition and distribution in the core, flesh, and peel of 20 apple varieties from China and its relation with browning characteristics of apple slices in the drying process.
Methods:
In this paper, the prominent phenolic compounds, which was determined by photo diode array-high-performance liquid chromatography (PDA-HPLC), and the chromatic value [coherent infrared energy (CIE) L*, a*, b*] were correlation analysised.
Results:
The results showed that apple core, flesh, and peel were characterized by phloridzin, chlorogenic acid, quercetin, and related derivatives respectively. The 20 apple varieties showed a significant difference (* P < 0.05) in browning variation in the drying process. The browning at the initial stage was mainly L* declined, which was induced by polyphenols enzymatic oxidation. While the browning was characterized by b* and a* value increment at the end of the drying process, where the Maillard reaction was the dominant factor.
Conclusions:
The correlation distance between the main phenolic compounds in apple core, flesh, and peel with the average chromatic L*, a*, and b* values varied at different stages of the drying process.
Keywords
Apple, polyphenols distribution, drying process, browning, correlation distanceIntroduction
Apple (Malus domestica) is an important dietary polyphenols source. Polyphenols are not only responsible for most of the antioxidant activities of the fruit but also contribute to the sensory characters of apple products. Apple polyphenols are prevalent antioxidants featured by their electron-rich phenolic groups that are susceptible to being oxidized and chelated with the metal ion. Therefore, apple is deemed as a healthy fruit that could be interfered with oxidation-related degenerative diseases, like cardiovascular disease, diabetes, cancer, and so forth [1, 2]. Besides, apple polyphenols are also active in food processing. Except for polyphenol enzymatic browning-induced color deterioration in fresh-cut apple and apple juice, apple polyphenols interacting with the Maillard reaction could also contribute to the browning deterioration. It was reported that catechin/epicatechin and phlorizin elevated the redness value (a*) and retarded 5-hydroxymethyl furfural (5-HMF) formation, whereas chlorogenic acid boosted the yellowness value (b*) and accelerated 5-HMF formation in D-fructose/L-lysine solution, which was explained by catechin interact with lysine, fructose or both at the para position of its B ring to form red-colored adducts, while simultaneously inhibiting 5-HMF formation [3, 4].
Apple polyphenols include phenolic acids, flavonols, hydrochalcones, anthocyanins, and procyanidin. It involves 25 phenolic acids, 15 flavonols, 5 hydrochalcones, 21 anthocyanins, 14 procyanidins, and so forth [5, 6], whereras polyphenols had different space distributions in apple fruits. Generally, peel had higher total phenolic content and more diverse polyphenol composition than that in flesh [7]. Moreover, the polyphenol composition and content distributed in the peel, flesh, and core of apple fruit were different among different cultivars [6, 8, 9].
Nowadays, there are more than 200 apple cultivars bred for different utilizations, like dessert, ornamental, cider, and culinary apples [10]. Dried apple is one of the important products because they are easy to store, transport and are widely used as an ingredient in food industry, whereas apple cultivars that are suitable for drying processing have a specific requirement for browning. Browning is an important color deterioration in apple drying process, which is induced by several reasons. Polyphenols play roles in enzymatic oxidation and Maillard reaction, and affect the browning accumulation on dried apple products [3, 4]. Moreover, the polyphenol distribution in fruit may induce non-uniform browning on dried apple products, contributing to the different overall browning levels [11, 12]. Therefore, it is necessary to clarify the correlation of polyphenol’s spatial distribution in apple fruit with the overall browning level of the dried apple products. The aim of this research was to investigate the key polyphenols distributed in 20 cultivars of apple fruit and their correlation distance with the browning accumulation of dried apple slices in the drying process.
Material and methods
Apple
The information of twenty apple varieties is listed in Table 1, including the name, abbreviation, picking time, and place of origin. The selected apple varieties are the most representative and distinctive in both morphology and phytochemical compositions. Specifically, apples were randomly picked from three trees in the orchard of Xingcheng research base of Liaoning province (N40°33’, E+120°45’), Yangling experimental center in Shaanxi province (N34°16’, E+108° 4’), and Yantai in Shandong province (N36°16’, E+119°34’). Apples were packaged in plastic atmosphere bags and transported to Beijing in shockproof cartons within two days. The apples were then stored in the refrigerator under 4°C with 85% relative humidity until use.
Information of apple materials
| Name of varieties of apple | Abr | Picking time | Origin |
|---|---|---|---|
| Changhong | CH | Oct 15th, 2017 | Liaoning |
| Huajin | HJ | Oct 15th, 2017 | Liaoning |
| Huayue | HY | Oct 15th, 2017 | Liaoning |
| Qiaonajin | QNJ | Oct 15th, 2017 | Liaoning |
| Huafu | HUF | Oct 15th, 2017 | Liaoning |
| Xinhongxing | XHX | Oct 15th, 2017 | Liaoning |
| Huahong | HH | Oct 15th, 2017 | Liaoning |
| Jinguan | JG | Oct 15th, 2017 | Liaoning |
| Hanfu | HAF | Oct 15th, 2017 | Liaoning |
| Qiujin | QJ | Oct 15th, 2017 | Liaoning |
| Fuji | FS | Nov 7th, 2017 | Shaanxi |
| Qinguan | QG | Oct 11th, 2017 | Shaanxi |
| Huangyuanshuai | HYS | Oct 12th, 2017 | Shaanxi |
| Qingping | QP | Oct 23rd, 2017 | Shaanxi |
| Changmiou | CMO | Oct 12th, 2017 | Shaanxi |
| Ligala | LGL | Aug 14th, 2017 | Shandong |
| Hongjiangjun | HJJ | Oct 23rd,2017 | Shandong |
| Guoguang | GG | Oct 23rd, 2017 | Shandong |
| Honggailu | HGL | Aug 17th, 2017 | Shandong |
| Jinshuai | JS | Oct 14th, 2017 | Shandong |
Abr: abbreviations
Chemicals
High-performance liquid chromatography (HPLC)-grade methanol (product number: 106035) and acetic acid (product number: 159007) were purchased from Merck Co. (Darmstadt, Germany). Standard phenolic compounds listed in Table 2 were all HPLC grade and bought from Sigma-Aldrich Corporation (Missouri, USA). Ultra-pure water provided by ULUP-Ⅰ microanalysis system (Xi’an, China) was used throughout the experiment.
Polyphenols identified by photo diode array-HPLC (PDA-HPLC) in 20 apple varieties
| Number | tR (min) | Polyphenol compound | Formula |
|---|---|---|---|
| P1 | 11.17 | Neochlorogenic acid | C16H18O9 |
| P2 | 14.77 | Catechin | C15H14O6 |
| P3 | 17.25 | Chlorogenic acid | C16H18O9 |
| P4 | 20.25 | p-Coumaroylquinic acid | C16H18O8 |
| P5 | 20.94 | Caffeic acid | C9H8O4 |
| P6 | 22.79 | Epicatechin | C15H14O6 |
| P7 | 23.30 | Caffeoylquinic acid isomer | C16H18O9 |
| P8 | 24.32 | Kaempferol-hexoside | C27H30O15 |
| P9 | 29.95 | Coumaric acid | C9H6O3 |
| P10 | 30.89 | Ferulic acid | C10H10O4 |
| P11 | 37.50 | Protocatechuic acid | C7H6O4 |
| P12 | 38.29 | Quercetin 3,5-digalactoside | C27H30O17 |
| P13 | 38.77 | Rutin | C27H30O16 |
| P14 | 39.63 | Caffeoyl hexoside | C15H18O9 |
| P15 | 40.68 | Phlorizin | C21H24O10 |
| P16 | 41.42 | Syringic acid | C9H10O5 |
| P17 | 41.94 | Naringenin-7-hesperidoside | C27H32O14 |
| P18 | 42.82 | Procyanidin A-type trimer | C45H36O18 |
| P19 | 43.53 | Procyanidin B-type trimer | C45H36O18 |
| P20 | 45.08 | Quercetin rutinoside | C27H30O16 |
| P21 | 45.40 | Quercetin-3-O-deoxyhexosyl(1-2) deoxyhexoside | C27H30O15 |
| P22 | 45.58 | Quercetin-3-O-pentosyl-7-O-hexoside | C26H28O16 |
| P23 | 45.80 | Phloretin | C15H14O5 |
| P24 | 46.21 | 3-Hydroxyphloretin | C15H14O6 |
| P25 | 46.48 | Phloretin 2’-O-rhamnoside | C21H24O9 |
| P26 | 46.76 | Shikimic acid | C7H10O5 |
| P27 | 47.11 | Quercetin-3-O-pentosyl-pentoside | C25H26O15 |
| P28 | 47.81 | Procyanidin B-type dimer | C30H26O12 |
| P29 | 48.14 | Procyanidin A-type dimer | C30H24O12 |
| P30 | 48.43 | 3-Hydroxyphloretin-pentosyhexoside | C21H24O11 |
| P31 | 49.04 | Quercetin 3-O-(6’’-acetyl-glucoside) | C23H22O13 |
tR: retention time
Polyphenol preparation from apple peel, pulp, and core
The 20 varieties of apple fruit with similar sizes and quality were separately cleaned. The peel part of fruit was peeled in 0.8 cm thickness, the flesh of the fruit was cut at a semidiameter of 1.0 cm from the stem axis. The core section of the apple fruit was located at the stem axis with a semidiameter of 1.0 cm. Three parts of the apple were instantly frozen and ground into powders. The frozen powders were stored separately at –80°C for further use, and 10 g of frozen apple powder was made from core, flesh, and peel respectively, and 0.2 g NaCl was added (Sinopharm Chemical Reagent Co. LTD, China. Product number: 10019308) before 10 mL of 70% aqueous methanol (Beijing Tongguang Fine Chemical Co, China. Product number: 12395) and fully mixed for further extraction. The polyphenols were extracted 3 times with assisting of ultrasonic treatment (40 kHz, 30°C, 20 min). The extracts were then centrifuged (10,000 rpm, 4°C, 10 min), taken, and mixed with the supernatant, which was evaporated into dryness, and dissolved in 10 mL pure methanol for analysis.
Apple slices dried by hot pump processing
Apples used for drying treatment were cleaned to remove the impurities and dust, and the slices were prepared at 4°C by using the slicer with a 0.8 cm razor (DL50; Robot Coupe, Vincennes, France). The fresh cut apple slices were immediately wrapped in vacuum bags (Heyuan Huafeng Plastic Co., LTD, China. Product number: 1-12) to avoid browning. The apple slices were then subjected to hot pump drying (Zhengxu Co., Ltd, Dongguan, China. Product number: ZX-015YTHG) with air flow velocity of 2 m/s and temperature of 65°C. Apple slices were dried for 10 h until the moisture content of the apple slices was lower than 8 g/100 g.
Polyphenols profile analysis by PDA-HPLC
Apple polyphenol composition was analyzed by using the Waters 2,695 HPLC system equipped with ultraviolet (UV) vs. photo diode array (PDA) detectors (Waters, Milford, USA), and the detection wavelength was set in the range of 200–600 nm. A Promosil C18 column (4.6 mm × 250 mm, 100 Å, 5 µm) was equipped and kept in a 30°C column oven (Waters, Milford, USA. Product number: D145CH). Binary gradient elution was employed, in which eluent A was 2% (v/v) acetic acid aqueous solution and eluent B was 100% (v/v) methanol. The flow rate was set as 1.0 mL/min, and the injection volume was 10 µL. The gradient was optimized as follows: 5–25 mL eluent A/100 mL eluent B was kept for 20 min; 25–40 mL eluent A/100 mL eluent B was kept for 15 min; 40 mL eluent A/100 mL eluent B was kept for 5 min; 40–95 mL eluent A/100 mL eluent B was kept for 5min; 95 mL eluent A/100 mLeluent B was kept for 5 min; 95–5 mL eluent A/100 mL eluent B was kept for 3 min; and finally 5 mL eluent A/100 mL eluent B was kept for 2 min. Breeze software was applied for system control and data processing. Quantification was conducted by external standards using HPLC-grade standards polyphenol compounds. Each sample was prepared and tested in triplicate, and the results were expressed as the mean ± standard deviation (SD).
Chromatic value variation of apple slices during hot pump processing
The color of apple slices was evaluated by determining the variation of chromatic value of surface color by three parameters: the coherent infrared energy (CIE) L* value (brightness), CIE a* value (redness), and CIE b* value (yellowness). Color detection was conducted by V270-Digi Eye (Hunter Lab, Reston, VA, USA. Product number: 1103779). The picture was taken, and ten spots of dehydrated apple slices were chosen for measurement of their CIE L*, a*, and b* levels. An average of CIE L*, a*, and b* levels were calculated, the average of which was defined as the appearance color profile. Browning degree in the present study was defined as:
In which L1, a1, b1 is the value of dried processed apple slices, L0, a0, b0 is the initial value of fresh apple slices.
Statistical analysis
The data were statistically analyzed by using statistic package for social science (SPSS) 2.0 (SPSS Inc., Chicago, USA), and results were expressed as mean ± SD. Differences between groups were analyzed by one-way analysis of variance (ANOVA). Correlation analyses were performed using a two-tailed Pearson’s test. Statistically significant differences were defined as * P < 0.05 and ** P < 0.01. Multivariate modeling analysis by orthogonal projection to latent structures (OPLS) was performed in Ezinfo 3.0 (Umetrics, Umea, Sweden).
Results
Polyphenol distribution in core, flesh, and peel of 20 apple cultivars
Polyphenol distribution in apple core, flesh, and peel is intuitively illustrated in the heat map (Figure 1), and 34 phenolic compounds were detected and quantified, among which chlorogenic acid, catechin, phloretin, and quercetin were the most dominant constitutes. While their concentration and the distributions in core, flesh, and peel parts of different apple cultivars were markedly varied (* P < 0.05). Chlorogenic acid level was 15.22–196.85 ug g–1 wet weight in three parts of apple fruit from 20 Chinese apple varieties, the level of which was in a descending order in core, flesh, and peel of 20 apple varieties successively, which matched with several previous reports that the chlorogenic acid in flesh and peel were 2,731.73 ug g–1, 2,019.30 ug g–1 dry weight respectively [8]. Chlorogenic acid was one of the dominant phenolic compounds detected in all parts of the apple fruit, which was different from those reported in previous research that showed flavanols (catechin and proanthocyanidins) were the prominent phenolic components [7]. It might be caused by the polyphenol extraction method that accumulates more water-soluble phenolic components which were the predominant factors in browning reaction of the apple slices [13, 14]. Epicatechin was in a concentration of 0–94.4 ug g–1, which was more likely to accumulate in apple core and peel. Whereas other flavanols were found to predominate in peels of the fruit, quercetin derivatives were mainly found in apple peel with the level of 3.02–284.52 ug g–1 in wet weight, and their contents were more than 10 times higher than that in flesh. The results were comparable to the finding that indicate the quercetin derivatives in the peel of two Lithuania apple cultivars varied in the level of from 781 ug g–1 to 1,899 ug g–1 (dry weight), which predominantly existed in peel and more than 10 times that in flesh [8]. Phlorizin was regarded as a marker phenolic compound in the apple [12]. It was found to be peel-specific in several works of literature [8, 9]. Whereas in the present work, phlorizin was observed to be the most abundant in apple core, followed by peel and flesh, with the amount of 7.08–162.11 ug g–1 in core, 2.08–15.38 ug g–1 in flesh, and 0.33–77.75 ug g–1 in peel. These were comparable to the results in previous literatures [13, 15].

Heat map of polyphenol composition in 20 varieties of apple. (A) Apple core; (B) flesh; and (C) peel. The content of each phenolic compound was normalized to complete linkage hierarchical clustering. Each sample is visualized in a single row and each metabolite is represented by a single column. Red indicates high abundance, whereas blue indicates low content
Overall, apple core, flesh, and peel could be well grouped on their polyphenol composition by the partial least squares discriminant analysis (PLS-DA, Figure 2). The variance of apple flesh was the minimum followed by that of core, and the variance of peel polyphenol composition of different cultivars was the maximum. It indicates that peel polyphenol composition could be used to distinguish the apple varieties.
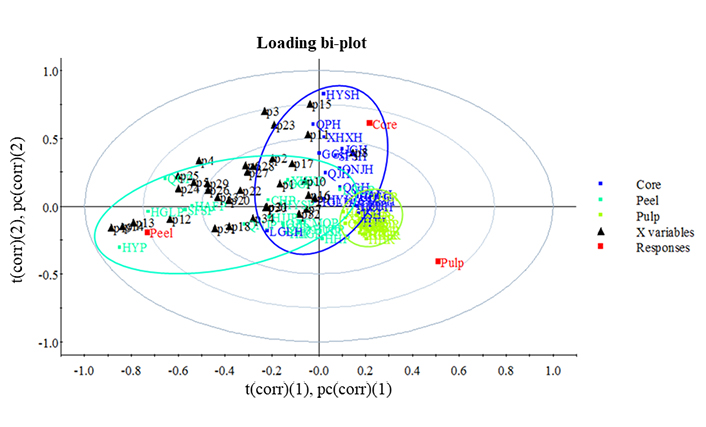
PLS-DA loading bi-plot of polyphenol composition distribution in core, pulp and peel of 20 apple variaties. (1) and (2) indicate principal component and were caculated by PLS-DA analysis. t(corr): the scores of the most important variables; pc(corr): how the variables correlated to each other
Dynamic variation of apple slices browning in the hot pump drying process
The surface color of apple slices was expressed by using CIE L*, a*, b*, and ∆E values (Figure 3). The overall browning expressed by ∆E value was observed in the first-hour of dehydration in almost all apples. The ∆E value at the first-hour of drying was as high as 13.35 in QG, and less than 5 in Huahong, Huajin, Qiaonajin, Jinguan, Hongjiangjun, Huangyuanshuai, and Ligala apple varieties. Specifically, L* value showed a marked decline, while a* and b* values significantly increased in the first-hour of drying in most varieties of apple slices, except for Huahong, Huajin, Qiaonajin, Jinguan, Hongjiangjun, Huangyuanshuai, and Ligala. It could be explained that at the initial stage of drying, polyphenol oxidase was triggered and accelerated browning induced by polyphenol oxidation.
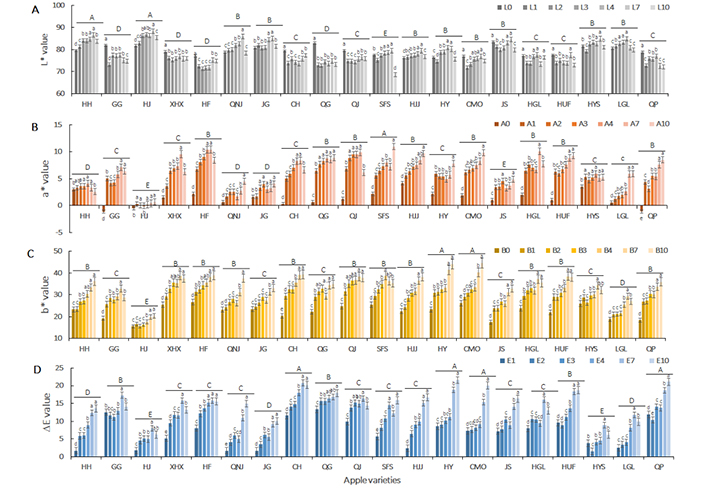
Variation of apple slices color (CIE L*a*b*, ∆E value) in hot air-drying process. Data are presented as mean ± SD of ten independent measurements. A–E showed significant differences (* P < 0.05) in different varieties of apple slices; a–d indicated the significance of the difference (* P < 0.05) at different drying stages
In metaphase of drying, with another six-hour of drying, the browning degree was gradually increased with some exceptions, mainly because the un-uniform browning distribution made overall browning deviation. L*, a*, and b* value was slowly increased during the process. The reason could be that water removal decreased the light refraction and absorbance, and therefore, increased the L* value. Meanwhile, polyphenol oxidation accumulated induced a further a* value increase, and triggered the Maillard reaction, thus induced the increment of b* value. Moreover, the browning rate on the surface of apple slices was distinct in different apple varieties, among which Huahong, Huajin, Qiaonajin, Jinguan, Hongjiangjun, etc. showed continuously high rates of browning as compared with other apple varieties.
At the end of drying, the ∆E value was sharply raised in Guoguang, Changhong, Hongjiangjun, Huayue, Changmiou, Jinshuai, Honggailu, Huafu, Huangyuanshuai, Ligala, and Qingping, with b* value rapidly increase, followed by a* value increment and L* decreasing. These were attributed to Maillard reaction products and intermediate product accumulation in low water activity.
Correlation distance of polyphenols distribution and apple slices browning in drying processing
Correlation between polyphenols distribution and surface browning on apple slices was evaluated by correlation distance (Figure 4). Chlorogenic acid, epicatechin, rutin, and phloretin were separately investigated to clarify their correlation distance with CIE L*, a*, b*, and ∆E value. The larger the absolute distance value, the higher the correlation is. The positive value indicates a positive correlation and the negative value indicates a negative correlation. The results showed that phenolic compounds located in the core, flesh, and peel affect the overall browning of apple slices differently at different stages of drying. Specifically, chlorogenic acid in apple core and peel was positively correlated to the ∆E value variation when drying started, while flesh chlorogenic acid negatively correlate to it. On the contrary, the core and peel chlorogenic acid were negatively correlated with a* and b* value variation when drying started. Moreover, flesh chlorogenic acid was highly correlated with a* value, whereas the correlation was more than 3 times lower with b* value. At the end of the drying process, the peel and core chlorogenic acid was positively correlated with a* value and negatively correlated with b* value. These results could be explained by chlorogenic acid performed differently in reacting with Maillard reaction in different apple fruit matrices [16].
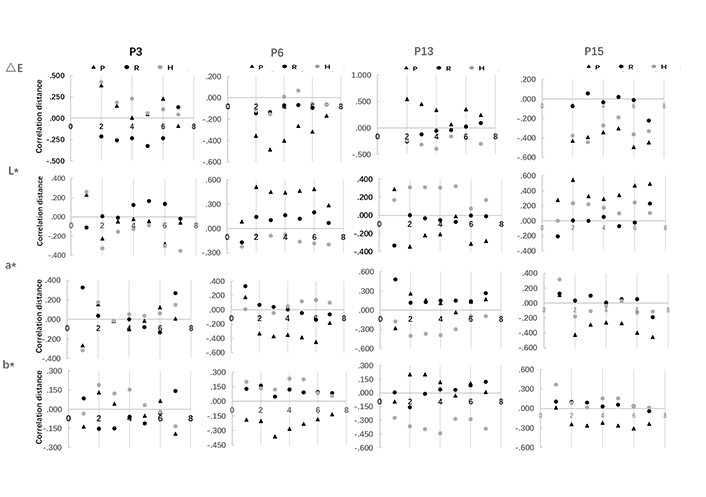
Correlation distance of polyphenol compounds in apple core, flesh, and peel with chromatic value (CIE L*a*b*, ∆E) on apple slices in hot air-drying process. P3 represents chlorogenic acid; P6 represents epicatechin; P13 represents rutin; P15 represents phlorizin; P indicates peel; R indicates flesh; H indicates core
Correlation distances of overall browning of apple slices with epicatechin in peel were positively correlated with CIE L*, but negatively correlated with CIE a* and b* in drying process. The correlation distance is relatively stable for CIE L*and a*, whereas increased in the first 3 h of drying and decreased afterward, which was in line with that of ∆E. In regard to rutin, its core content correlated with CIE L* positively and correlated with CIE a* and b* negatively, the correlation distance of which was found stable with CIE L* and increased in the first 4 h of drying and decreased afterwards with CIE a*and b*. It could be supported by that the rutin would participate in Maillard reaction to produce higher brown intensity [17]. As for phloretin, the peel distribution is negatively correlated with ∆E and CIE a* and b*, but positively correlated with CIE L*. The correlation distance was decreased in the first 4 h of drying and increased afterwards with CIE L* and a* respectively. The phenomena could be explained by different polyphenols as the substrate of enzymatic browning, which contribute differently to the surface browning of the apple slices. It involves the polyphenol distribution in the fruit, as well as the tissue density which could be supported by the polyphenol-enzyme regional distribution hypothesis of browning [18, 19]. Moreover, polyphenols act as both enzyme inhibitors and exacerbators in the browning control study, which could support that in the drying process, the correlation of polyphenols varied induced by the moisture continuously removed [20, 21]. Additionally, along with the drying, the Maillard reaction also activated when the water activity decreased to a certain extent, and polyphenol interaction with Maillard reaction also contributed to the browning variation of the apple slices [3, 4].
Discussion
Polyphenol composition in core, flesh, and peel of apple fruit from 20 varieties showed that chlorogenic acid, epicatechin, phlorizin, and quercetin were the most abundant component, and polyphenol composition in core, flesh, and peel were characterized by phlorizin, cholorgenic acid, and quercetin. Huahong, Huajin, Qiaonajin, Jinguan, Hongjiangjun, Huangyuanshuai, and Ligala out of 20 apple varieties showed significantly higher (* P < 0.05) browning (L*, a*, b*, and ∆E value) at the initial and metaphase of drying process, which was mainly induced by polyphenol oxidation. While in the later period, Maillard reactions were the dominant factor in browning formation. The correlation distance of CIE L*, a*, b*, and ∆E with chlorogenic acid, epicatechin, phlorizin, and rutin distributed in core, flesh, and peel with browning value (CIE L*, a*, b*, and ΔE) varied at different stages in the drying process.
Abbrevaitions
5-HMF: 5-hydroxymethyl furfural
CIE: coherent infrared energy
HPLC: high-performance liquid chromatography
PDA-HPLC: photo diode array-high-performance liquid chromatograph
PLS-DA: partial least squares discriminant analysis
SD: standard deviation
Declarations
Author contributions
WW and XL equally contributed to: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. JH: Conceptualization, Investigation, Writing—original draft. JB: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was funded by the earmarked fund for China Agriculture Research System [CARS-27]. The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Copyright
© The Author(s) 2023.