Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
†These authors contributed equally to this work.
Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
†These authors contributed equally to this work.
Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
3Liaoning Province Key Laboratory of Metabolomics, Dalian 116023, Liaoning, China
Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
3Liaoning Province Key Laboratory of Metabolomics, Dalian 116023, Liaoning, China
Affiliation:
2Key Laboratory of Phytochemical R&D of Hunan Province, Hunan Normal University, Changsha 410081, Hunan, China
Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
3Liaoning Province Key Laboratory of Metabolomics, Dalian 116023, Liaoning, China
Email: luxin722@dicp.ac.cn
ORCID: https://orcid.org/0000-0002-2164-2147
Affiliation:
1CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
3Liaoning Province Key Laboratory of Metabolomics, Dalian 116023, Liaoning, China
ORCID: https://orcid.org/0000-0003-4298-3554
Explor Foods Foodomics. 2023;1:72–82 DOI: https://doi.org/10.37349/eff.2023.00007
Received: February 22, 2023 Accepted: June 09, 2023 Published: June 30, 2023
Academic Editor: Jose Antonio Mendiola, Institute of Food Science Research (CIAL-CSIC), Spain
Aim: The aim of this study is to comprehensively investigate the distribution of amine and phenol compounds in different flavors of Baijiu.
Methods: 12C-/13C-dansyl chloride labeling was applied for untargeted and quantitative analyses of amine and phenol compounds in Baijiu.
Results: A total of 267 amine/phenol compounds were detected, and 30 of them were confirmed by the standards. 4 of 30 confirmed compounds were newly identified in Baijiu, and 16 ones were related with flavor or biological activity. After statistical analysis, 34 amine/phenol compounds were defined as potential markers for indicating sauce flavor, strong flavor, and light flavor Baijiu. 30 compounds in Baijiu were quantified with high precision, high accuracy, and high sensitivity. Results of the untargeted and quantitative analyses indicated that the number and contents of amine and phenol compounds were generally richest in sauce flavor Baijiu, while lowest in light flavor Baijiu.
Conclusions: The results obtained in the research are beneficial for comprehensively understanding the amine and phenol compounds in Baijiu and further provide the basis for the flavor blending of Baijiu.
Baijiu is one of the oldest distilled spirits in the world and has a history of more than 2,000 years in China [1]. Based on the differences in flavor, Baijiu is divided into four basic types [2]. Among them, sauce flavor Baijiu, strong flavor Baijiu, and light flavor Baijiu occupy most of the market. Amine and phenol compounds are widely present in Baijiu and play crucial roles in the flavor and quality of Baijiu [3]. For example, Arg-Asn-His in Baijiu possess antioxidant function [4], 4-methylphenol and 4-ethyl-2-methoxyphenol contribute to smoky aroma [5], phenolic acid such as ferulic acid possesses antioxidant properties [6], while excessive biogenic amines in Baijiu will lead to breathing disorders and headaches [7]. Therefore, the qualitative and quantitative analyses of amine and phenol compounds in Baijiu are not only beneficial to reveal the reason why Baijiu is seductive but also essential for quality control (QC) of Baijiu.
Some analytical methods for the determination of amine/phenol compounds have been reported, such as high-performance liquid chromatography (HPLC) coupled to ultraviolet detection [8], HPLC coupled to fluorescence detection [9], HPLC coupled to potentiometric detection [10], and gas chromatography-mass spectrometry (MS) with trifluoroacetamide derivatization [11]. However, the methods mentioned above were insufficient in sensitivity. Although the detection sensitivity of HPLC-MS/MS has been improved [12], capturing some trace compounds is still difficult. Meanwhile, the chemical and physical properties of amine and phenol compounds in Baijiu have great diversity. All of these issues hinder the global detection of amine and phenol compounds in Baijiu.
The global analysis of amine and phenol compounds in Baijiu are including two aspects: identification and quantitation. In general, the unambiguous identification of the amine and phenol compounds in Baijiu was based on authentic standards [13]. However, the authentic standards were expensive resulting in only a fraction of compounds can be positively identified. Putative identification mainly relied on MS/MS spectra of databases [14]. Similarly, MS/MS spectra in databases is shortage. Therefore, high coverage identification of amine and phenol compounds in Baijiu is hindered. Chemical isotope labeling-assisted ultrahigh-performance liquid chromatography coupled to high-resolution MS (CIL-UHPLC-HRMS) improved the MS response of the amine and phenol compounds by introducing easily protonated groups [15, 16]. In addition, due to the selective reaction with specific groups, the reaction products should contain amino or hydroxyl groups, so, the coverage of identification of amine and phenol compounds will increase by CIL-UHPLC-HRMS. As for quantitation analysis, stable-isotope-labeled (SIL) internal standards are usually applied for accurate quantification [17]. However, it is impossible to provide SIL internal standards for each compound because of the high cost and uneconomical availability. CIL-UHPLC-HRMS was reported to be able to improve quantification accuracy by providing one-to-one SIL internal standard [18]. Therefore, to comprehensively analyze amine and phenol compounds in Baijiu, a high sensitivity, high coverage, and high accurate CIL-UHPLC-HRMS analytical method is needed.
In the present study, 12C-/13C-dansyl chloride (12C-/13C-DnsCl) labeling combined with ultrahigh-performance liquid chromatography coupled to high-resolution MS (UHPLC-HRMS) was applied for untargeted and quantitative analyses of amine and phenol compounds in Baijiu for the first time (Figure 1). After being labeled by 12C-DnsCl or 13C-DnsCl, the samples were detected by UHPLC-HRMS. Subsequently, untargeted analysis was performed, and a total of 267 amine /phenol compounds were detected. Then, the features of amine or phenol compounds in different flavor of Baijiu were studied. Finally, 30 compounds confirmed with standards were quantitated in different flavor of Baijiu with one-to-one SIL internal standards.
Methanol, ethanol, and acetonitrile (ACN) were purchased from Merck (Darmstadt, Germany). Formic acid and 12C-DnsCl were purchased from J&K Scientific Ltd. (Beijing, China). Ultra-pure water was produced from a Milli-Q® system (Millipore, Massachusetts, USA). 13C-DnsCl was obtained from the University of Alberta. Sodium carbonate, sodium bicarbonate, and sodium hydroxide were purchased from Sinopharm (Beijing, China). All standards were purchased from Aladdin® (Shanghai, China).
All Baijiu samples including 5 sauce flavor, 4 strong flavor, and 6 light flavor Baijiu were obtained from the local market, and the detailed information is shown in Table S1. The blank sample was ethanol-water [53:47, volume/volume (v/v)]. QC sample was prepared by mixing equal volumes of Baijiu samples. The mixed standards labeled by 13C-DnsCl served as internal standards.
The amine and phenol standards were dissolved in ultra-pure water or ACN; 50 mL of Baijiu or blank samples were concentrated to 2 mL by evaporating with a vacuum distillation system (70 r/min, 45℃, and –0.1 MPa), and all prepared samples were stored at –20℃ before chemical labeling.
The chemical labeling was performed as that described in previous studies with slight modifications [19, 20]. Briefly, 30 μL of the concentrated sample was freeze-dried, and 30 μL of water was used to redissolve the residues. Then, 30 μL of sodium carbonate and sodium bicarbonate buffer solution (0.25 M, pH = 9.5), 30 μL 13C-DnsCl (10 mg/mL) or 12C-DnsCl (10 mg/mL), 30 μL of ACN were added for labeling 60 min at 40℃. After that, 5 μL of sodium hydroxide (250 mM) was added and reacted for another 10 min. Subsequently, 25 μL of formic acid (425 mM) was added and shocked for 5 min. The mixture was freeze-dried, and 80 μL of ACN was added for redissolving, after centrifugation for 10 min (4℃, 10,000 g), the 60 μL of supernatant was taken out for analysis.
Agilent 1290 Infinity UHPLC system coupled to 6546 Q-TOF (Agilent Technologies, California, USA) was used for data acquisition. Mobile phase A was composed of 0.1% (v/v) formic acid, 5% (v/v) ACN, and 94.9% (v/v) water. Mobile phase B was 0.1% (v/v) formic acid in ACN. The chromatographic column was ACQUITY BEH C18 column (100 mm × 2.1 mm, 1.7 μm, Waters™, Massachusetts, USA), column temperature was 50℃, and the column flow rate was 0.18 mL/min. The separation started with 20% B and increased to 35% B in 3.5 min, further increased to 65% B in another 14.5 min, to 99% B in another 6 min, and held for 4 min. Lastly, the gradient was back to 20% B in 0.1 min and maintained for 2.9 min [21]. The temperature of the sample manager was 4℃, injection volume was 5 μL.
Data were acquired in positive ionization mode. In full scan mode, the scan range was mass-to-charge ratios (m/z) 200–1,500 Da. In target MS/MS mode, the scan range was m/z 30–1,500 Da. Drying gas temperature and drying gas flow rate were set as 320℃ and 8 L/min, respectively. The fragmentation voltage was 175 V, and capillary voltage (Vcap) was 4,000 V. Sheath gas flow rate and Sheath gas temperature were 11 L/min and 350℃, respectively. The secondary collision energies were 10 eV, 20 eV, and 40 eV.
Analytical characteristics including linear range, the lower limit of detection (LLOD), the lower limit of quantification (LLOQ), intra-day precision, inter-day precision, and recovery were evaluated to ensure the robustness of the method. The mixed standards were diluted to 8–10 calibration solutions with the blank sample. Signal to noise ratio (S/N) ≥ 3 and S/N ≥ 10 were defined as the LLOD and LLOQ, respectively [22]. The intra-day precision and inter-day precision were evaluated based on the mixed standards of low, medium, and high concentrations. The mixed standards of the above three concentrations were diluted by the blank sample and prepared in 6 replicates in one day or 8 replicate samples within 3 consecutive days. Then, the relative standard deviation (RSD) of each compound was calculated. For the recovery experiment, mixed standards with three different concentrations were added to the QC samples. Afterward, the QC samples, spiked QC samples, and mixed standards were analyzed. The recovery of each compound was calculated by the peak area ratio of spiked QC samples subtracted from the peak area ratio of QC, and the difference was divided by the peak area ratio of standard, where peak area ratio means the ratio of peak area of analyte to internal standards. Each sample of the recovery experiment was prepared in 6 replicates.
The peak table was generated by MS-DIAL (version 4.24). In-house python programming was used to pick out the amine and phenol compounds from the peak table. In-house database, MassBank of North America (MoNA), and the Yeast Metabolome Database (YMDB) were used for annotation. The elements composition of amine and phenol compounds were set as C, H, O, N, and S. The formulas of amine and phenol compounds were obtained by removing the DnsCl residue (C12H11NO2S). The amine and phenol compounds were annotated at 5 different levels, Level 1: the amine and phenol compounds were positively identified based on matching the retention time (tR), precursor ions, and MS/MS with authentic standards; Level 2: the amine and phenol compounds were putatively identified by in-silico MS/MS; Level 3: the amine and phenol compounds were putatively identified by manually annotated fragments; Level 4: the amine and phenol compounds were putatively identified by searching the databases with precursor ions; and Level 5: the amine and phenol compounds were identified with the molecular formula. One-way analysis of variance (ANOVA) was performed for amine or phenol compounds to evaluate the differences between the three flavors of Baijiu. Partial least squares-discriminant analysis (PLS-DA) was carried out on SIMCA® P 13 software (Umetrics®, Umea, Sweden). The heat maps were plotted based on the online website of Omicstudio (https://www.omicstudio.cn).
To explore whether the retention and MS response of labeled amine/phenol compounds were improved, we analyzed 30 amine/phenol standards before and after labeling. The extracted ion chromatogram (EIC) profile before and after labeling was shown in Figure 2. It can be seen from Figure 2A, 30 compounds were eluted completely in 10 min, and most of the compounds were eluted in 5 min. However, after being labeled, the tR of 30 compounds was in the range of 26 min (Figure 2B). Therefore, since introducing hydrophobic DnsCl, the retention of labeled amine and phenol compounds on the reversed phase column was significantly enhanced. In addition, the MS response of most labeled amine/phenol compounds was greatly improved (Figure 2).
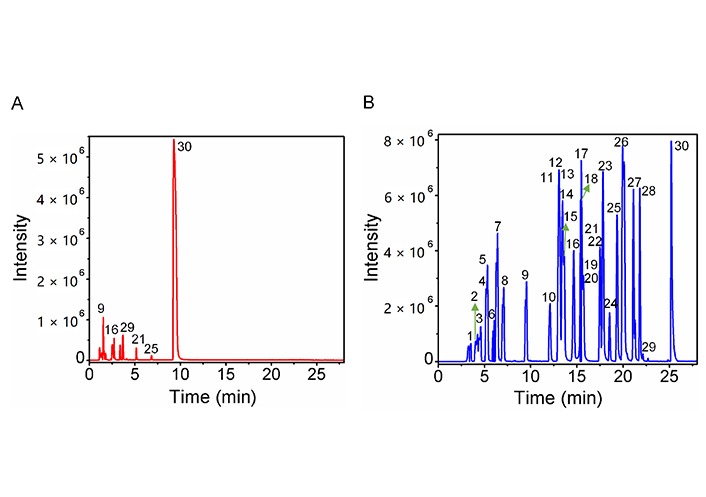
Extracted-ion chromatograms of 30 amine or phenol compounds. (A) before DnsCl labeling; (B) after DnsCl labeling. The compound information corresponding to the numbers is shown in Table 1
To show the DnsCl labeling derivatizing method was helpful for high coverage detection of amine/phenol compounds, 15 Baijiu were analyzed. It can be seen from Figure 3A, 261, 258, 261, 261, 249, 154, 237, 139, 232, 98, 76, 115, 83, 126, and 132 compounds were detected in S1–S15, respectively. After removing duplicates, a total of 267 amine/phenol compounds were obtained (Table S2). Among the 30 compounds unambiguously identified, pentan-1-amine, dodecan-1-amine, 4-allylsyringol, and 2-vanillin were identified in Baijiu for the first time, and the 3D structure of four newly identified were shown in Figure S1.
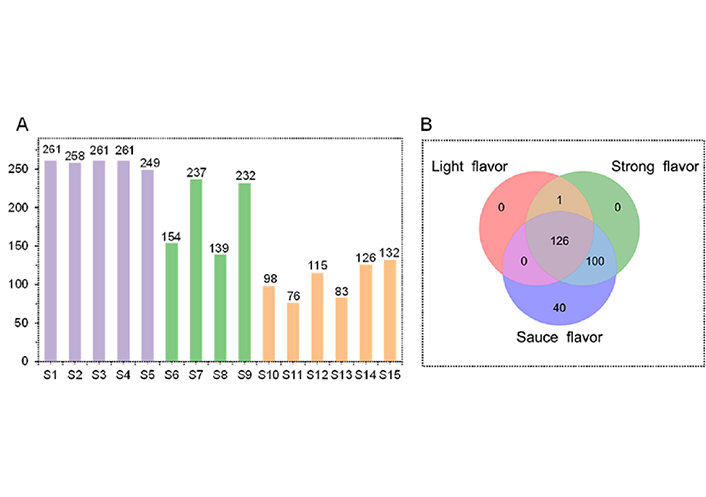
The number of amine or phenol compounds in Baijiu. (A) different samples; (B) different flavor
The numbers of amine/phenol compounds in sauce flavor Baijiu, strong flavor Baijiu, and light flavor Baijiu were 266, 227, and 127, respectively (Figure 3B). The PLS-DA was plotted, and the result is shown in Figure 4A, the distributions of amine/phenol compounds in strong flavor Baijiu and light flavor Baijiu were similar, while they were greatly different from in sauce flavor Baijiu. In Figure 4B, it shows the VIP distribution of all compounds, the number of compounds with VIP greater than 1 was about half of the total number (115), suggesting amine/phenol compounds in different types of Baijiu were greatly different. The cluster analysis was performed based on amine/phenol compounds of VIP > 1.2 and P < 0.05. As shown in Figure 4C, 34 amine/phenol compounds were divided into two categories, one of which showed the highest content in sauce flavor Baijiu (23), and the other showed the highest content in strong flavor Baijiu (11). In addition, 34 amine/phenol compounds had the lowest content in light flavor Baijiu. Therefore, it can be seen that significant differences in the number and content of amine/phenol compounds contribute to the discrimination of Baijiu with different flavor from each other.
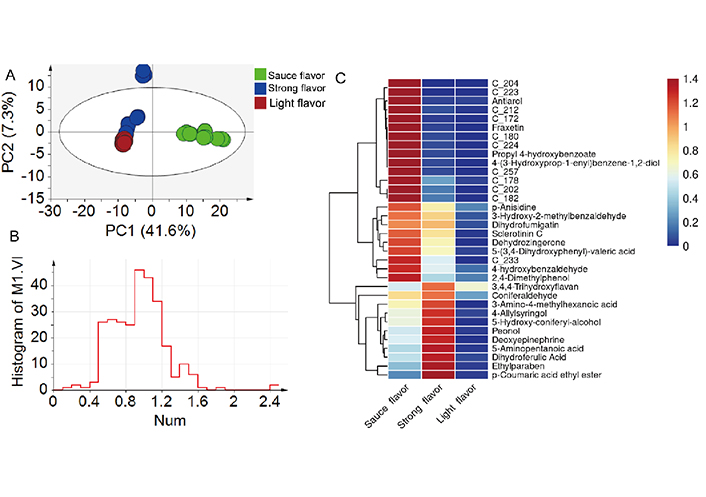
Statistical analysis. (A) PLS-DA R2 = (0.0, 0.0603), Q2 = (0.0, –0.238); (B) variable importance of projection (VIP) distribution of all of the compounds; (C) cluster analysis based on compounds of VIP > 1.2 and P < 0.05. The compound information corresponding to the numbers is shown in Table S2
In the untargeted analysis, we found that the relative content of some compounds in Baijiu with different flavor were different. To explore the differences in compound contents, 30 amine/phenol compounds confirmed with standard were quantitated by using the internal standard method.
The results of the analytical characteristic evaluation are shown in Table 1. First of all, the R2 of all compounds was larger than 0.99, indicating that the established method had good linearity between the concentration of the analyte and the MS response. Secondly, the LLODs and LLOQs were in the range of 1–14,564.63 ng/L and 0.01–61.90 μg/L, respectively, hence, high sensitivity was achieved. Thirdly, the RSDs of intra-day and inter-day were smaller than 15%, indicating good stability. Finally, the recovery rates were in the range of 80–120%, suggesting high accuracy. In summary, the established method can be used for the quantification of DnsCl labeled compounds.
Summary of validation results
| No. | Compound name | Liner range (ug/L) | LLOQ (ug/L) | LLOD (ng/L) | R2 | Intra-day precision (%) | Inter-day precision (%) | Recovery rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | Low | Medium | High | ||||||
| 1 | Serine | 25.21–43,768.10 | 0.26 | 66.18 | 0.996 | 8.40 | 10.49 | 11.77 | 7.14 | 7.01 | 1.85 | 104.50 | 108.29 | 108.92 |
| 2 | Threonine | 49.70–42,976.42 | 0.24 | 60.66 | 0.999 | 9.98 | 10.86 | 8.33 | 4.56 | 5.88 | 5.63 | 93.94 | 100.49 | 89.23 |
| 3 | 2-Aminoacetic acid | 9.00–10,421.20 | 0.19 | 47.27 | 0.997 | 9.36 | 12.35 | 8.98 | 4.17 | 8.61 | 5.59 | 89.43 | 80.28 | 115.28 |
| 4 | Alanine | 16.92–19,555.76 | 0.36 | 37.40 | 0.999 | 4.64 | 8.79 | 5.56 | 5.46 | 8.31 | 5.22 | 85.23 | 94.63 | 80.77 |
| 5 | 5-Aminopentanoic acid | 13.81–7,990.00 | 6.90 | 1,725.95 | 1.000 | 5.98 | 9.18 | 9.75 | 3.20 | 5.64 | 6.57 | 88.46 | 99.77 | 80.91 |
| 6 | 2-Aminobutanoic acid | 20.24–23,344.94 | 2.38 | 396.46 | 0.998 | 6.32 | 8.36 | 4.07 | 6.10 | 8.97 | 2.93 | 82.59 | 83.96 | 81.03 |
| 7 | 2-(Methylamino)acetic acid | 10.86–12,573.77 | 0.23 | 56.13 | 0.999 | 13.28 | 3.37 | 9.86 | 5.51 | 8.87 | 2.60 | 84.35 | 85.05 | 85.40 |
| 8 | Proline | 18.49–22,274.32 | 0.17 | 42.27 | 0.998 | 5.29 | 8.95 | 2.85 | 11.44 | 7.98 | 2.63 | 113.16 | 102.27 | 81.71 |
| 9 | Ile-Val | 61.90–35,683.35 | 61.90 | 14,564.63 | 0.993 | 7.69 | 7.13 | 1.94 | 5.36 | 8.28 | 5.45 | 90.53 | 80.24 | 88.67 |
| 10 | Octan-1-amine | 14.82–5,830.55 | 0.01 | 3.70 | 0.996 | 10.89 | 6.47 | 5.02 | 8.68 | 12.38 | 8.18 | 98.14 | 92.73 | 97.06 |
| 11 | Ornithine | 31.97–36,878.52 | 0.63 | 157.05 | 0.997 | 13.50 | 7.69 | 0.70 | 10.57 | 5.79 | 5.62 | 83.29 | 82.31 | 81.85 |
| 12 | Butan-1-amine | 7.31–8,218.44 | 0.01 | 1.00 | 0.999 | 2.72 | 6.29 | 6.74 | 3.57 | 8.72 | 9.47 | 93.91 | 93.91 | 115.95 |
| 13 | Peonol | 30.27–34,900.35 | 7.12 | 1,780.63 | 0.993 | 4.06 | 8.24 | 2.54 | 5.17 | 2.87 | 6.21 | 93.08 | 88.70 | 95.26 |
| 14 | 2-Phenylethylamine | 5.23–6,048.56 | 5.23 | 1,306.57 | 0.999 | 5.95 | 3.05 | 6.24 | 10.52 | 2.89 | 14.72 | 88.72 | 94.65 | 95.06 |
| 15 | 2-Vanillin | 39.86–69,191.81 | 0.42 | 104.62 | 0.997 | 12.09 | 14.09 | 12.92 | 5.37 | 5.02 | 7.37 | 80.24 | 107.46 | 108.28 |
| 16 | Syringic aldehyde | 18.37–21,261.14 | 2.30 | 382.70 | 0.998 | 13.23 | 9.06 | 5.23 | 14.68 | 9.16 | 5.88 | 97.16 | 95.25 | 96.17 |
| 17 | Pentan-1-amine | 6.23–7,111.35 | 0.77 | 128.23 | 0.998 | 4.48 | 6.88 | 5.53 | 10.99 | 8.08 | 9.95 | 95.43 | 90.46 | 101.00 |
| 18 | Tyramine | 14.65–16,954.74 | 14.65 | 3,662.19 | 0.999 | 6.67 | 4.24 | 8.42 | 11.78 | 8.25 | 5.02 | 100.00 | 99.22 | 99.00 |
| 19 | Coniferaldehyde | 11.65–13,477.91 | 11.65 | 1,552.75 | 0.999 | 5.99 | 5.82 | 7.91 | 11.04 | 8.29 | 3.54 | 100.73 | 97.70 | 97.78 |
| 20 | 4-Hydroxycinnamic acid | 17.19–19,819.61 | 4.04 | 1,011.20 | 0.999 | 4.79 | 8.21 | 4.00 | 10.02 | 9.27 | 12.24 | 92.73 | 87.48 | 105.26 |
| 21 | Phenol | 0.77–2,384.89 | 0.01 | 2.06 | 0.996 | 5.36 | 5.42 | 3.94 | 4.73 | 8.83 | 4.16 | 91.61 | 82.57 | 95.53 |
| 22 | Tyrosine | 20.56–35,480.10 | 1.37 | 228.93 | 0.997 | 10.72 | 8.32 | 10.14 | 8.56 | 9.23 | 8.51 | 84.23 | 101.85 | 83.86 |
| 23 | Hexan-1-amine | 7.02–7,968.40 | 0.86 | 144.01 | 0.997 | 7.17 | 5.06 | 6.12 | 8.34 | 12.69 | 12.28 | 91.91 | 90.38 | 99.12 |
| 24 | Lysine | 14.76–18,012.40 | 1.64 | 273.96 | 0.997 | 3.12 | 8.22 | 8.53 | 14.02 | 7.54 | 0.67 | 106.37 | 94.67 | 88.41 |
| 25 | 4-Allylsyringol | 3.66–12,966.41 | 4.88 | 366.11 | 0.999 | 13.65 | 14.88 | 14.36 | 14.71 | 14.93 | 12.95 | 111.97 | 103.58 | 84.74 |
| 26 | Heptan-1-amine | 1.43–4,582.55 | 0.25 | 62.14 | 0.996 | 6.12 | 4.77 | 6.47 | 6.00 | 4.00 | 8.17 | 91.63 | 94.16 | 97.30 |
| 27 | 4-Hydroxybenzaldehyde | 13.82–15,991.25 | 3.45 | 575.68 | 0.996 | 8.76 | 7.62 | 3.77 | 5.20 | 4.73 | 3.97 | 86.39 | 85.63 | 117.59 |
| 28 | Octopamine | 14.53–8,408.43 | 14.53 | 3,632.67 | 0.999 | 13.37 | 7.40 | 2.48 | 10.94 | 13.41 | 8.21 | 82.47 | 86.83 | 91.16 |
| 29 | 4-Ethylphenol | 2.10–20,511.88 | 0.09 | 18.08 | 0.996 | 8.79 | 13.26 | 13.09 | 6.14 | 4.53 | 5.81 | 100.16 | 95.48 | 100.90 |
| 30 | Dodecan-1-amine | 1.14–172.29 | 0.01 | 1.52 | 0.995 | 9.48 | 9.12 | 6.41 | 14.29 | 13.36 | 11.86 | 99.27 | 93.24 | 92.20 |
The quantification method was used for the quantitative analysis of 30 compounds (12 amino acids and derivatives, 6 aliphatic amines, 1 aromatic amine, 2 alkylphenols, and 9 other phenolic compounds) in different flavor of Baijiu, including 5 sauce flavor Baijiu, 4 strong flavor Baijiu, and 6 light flavor Baijiu. In Table 2 it shows that 12 compounds had significant differences among different Baijiu (P < 0.05). The concentration of tyrosine was in the range of 2,221.38–43,887.19 ug/L, and much higher than other compounds. The contents of phenol and 4-ethylphenol in sauce flavor Baijiu were higher than those in the other two types of Baijiu. Besides, the other 6 compounds including serine, 2-(methylamino) acetic acid, alanine, Ile-Val, proline, and 2-vanillin also showed the highest content in sauce flavor Baijiu, and the contents of the remaining 4 compounds were the highest in strong flavor Baijiu. However, most of the compounds in light flavor Baijiu had the lowest contents, especially 5-aminopentanoic acid, Ile-Val, peonol, 2-vanillin, and 4-ethylphenol.
Quantification result of amine/phenol compounds in different flavor of Baijiu samples (mean ± standard deviation)
| No. | Name | Sauce flavor (n = 5) | Strong flavor (n = 4) | Light flavor (n = 6) | P value |
|---|---|---|---|---|---|
| 1 | Serine | 35,550.79 ± 888.85 | 5,858.38 ± 492.57 | 1,931.11 ± 113.52 | 0.011* |
| 2 | Threonine | 7,891.51 ± 651.03 | 4,304.49 ± 160.47 | 2,451.41 ± 197.44 | 0.827 |
| 3 | 2-Aminoacetic acid | 7,017.45 ± 197.09 | 1,130.18 ± 105.83 | 946.76 ± 70.37 | 0.077 |
| 4 | Alanine | 29,084.91 ± 1,022.82 | 4,458.02 ± 329.44 | 2,017.54 ± 152.8 | 0.001* |
| 5 | 5-Aminopentanoic acid | 1,371.82 ± 94.44 | 2,950.72 ± 246.08 | 61.94 ± 3.67 | 0.002* |
| 6 | 2-Aminobutanoic acid | 312.92 ± 1.35 | 32.63 ± 0.62 | 37.49 ± 0.48 | 0.104 |
| 7 | 2-(Methylamino)acetic acid | 4,350.97 ± 518.89 | 1,208.32 ± 77.34 | 1,180.54 ± 90.66 | 0.001* |
| 8 | Proline | 28,889.48 ± 1045.96 | 8,376.64 ± 646.04 | 6,764.32 ± 556.85 | 0.001* |
| 9 | Ile-Val | 231.46 ± 19.20 | 61.41 ± 13.83 | NQ | 0.001* |
| 10 | Octan-1-amine | 2,122.98 ± 95 | 1,798.9 ± 117.67 | 1,848.48 ± 125.85 | 0.609 |
| 11 | Ornithine | 1,612.06 ± 7.96 | 252.33 ± 0.79 | 192.88 ± 2.67 | 0.949 |
| 12 | Butan-1-amine | 45.23 ± 2.87 | 23.31 ± 1.80 | 25.44 ± 2.09 | 0.755 |
| 13 | Peonol | 1,556.96 ± 63.3 | 5,307.81 ± 357.87 | 81.88 ± 8.65 | 0.007* |
| 14 | 2-Phenylethylamine | NQ | NQ | NQ | - |
| 15 | 2-Vanillin | 48,982.32 ± 1,194.81 | 16,045.59 ± 669.61 | 2,904.81 ± 125.16 | 0.001* |
| 16 | Syringic aldehyde | 334.95 ± 30.64 | 2,070.11 ± 2,393.69 | 311.10 ± 12.22 | 0.372 |
| 17 | Pentan-1-amine | 32.92 ± 12.50 | 84.34 ± 8.38 | 48.83 ± 3.02 | 0.246 |
| 18 | Tyramine | 419.91 ± 18.70 | 227.67 ± 23.53 | 195.22 ± 10.48 | 0.198 |
| 19 | Coniferaldehyde | 190.85 ± 27.57 | 226.43 ± 34.48 | 127.57 ± 18.63 | 0.811 |
| 20 | 4-Hydroxycinnamic Acid | 486.88 ± 35.35 | 3,247.54 ± 269.01 | 286.03 ± 15.15 | 0.325 |
| 21 | Phenol | 5.19 ± 0.20 | 3.57 ± 0.18 | 3.11 ± 0.18 | 0.002* |
| 22 | Tyrosine | 43,887.19 ± 1,619.57 | 4,246.4 ± 216.78 | 2,221.39 ± 109.23 | 0.024* |
| 23 | Hexan-1-amine | 8.6 ± 0.02 | 8.74 ± 0.13 | 9.37 ± 0.59 | 0.441 |
| 24 | Lysine | 18,271.58 ± 483.12 | 1,539.31 ± 90.61 | 1,468.75 ± 86.45 | 0.108 |
| 25 | 4-Allylsyringol | 3,722.24 ± 164.46 | 6,766.26 ± 316.15 | 413.78 ± 39.03 | 0.009* |
| 26 | Heptan-1-amine | 19.16 ± 1.04 | 1.45 ± 0.10 | 4.69 ± 0.32 | 0.362 |
| 27 | 4-Hydroxybenzaldehyde | 10,965.37 ± 16,311.02 | 4,989.17 ± 0.00 | 1,054.25 ± 0.00 | 0.110 |
| 28 | Octopamine | 62.64 ± 14.67 | NQ | NQ | 0.135 |
| 29 | 4-Ethylphenol | 189.72 ± 11.34 | 18.99 ± 1.49 | NQ | 0.001* |
| 30 | Dodecan-1-amine | NQ | NQ | NQ | - |
* P < 0.05. NQ: compounds that were not detected or the content was below the LLOQ; -: none. The unit of concentration was ug/L
After labeling, the improved MS response of amine and phenol compounds can be attributed to two aspects. On one hand, the labeled amine/phenol compounds have enhanced retention which weakened matrix interference, meanwhile, due to eluted in higher ACN content, the analytes were ionized with higher spraying efficiency and desolvation efficiency. On the other hand, the tertiary amino group in DnsCl attribute to the improved ionization efficiency of labeled products.
As far as we know, our study detected the most amine and phenol compounds in Baijiu [23]. In addition, according to records in PubChem, threonine, proline, butan-1-amine, pentan-1-amine, hexan-1-amine, and 4-hydroxybenzaldehyde are associated with flavor, while serine is a nutritional supplement. Moreover, as it was reported, syringaldehyde, glycine, alanine, tyrosine, tyramine, lysine, 4-ethylphenol, phenol, and 4-allylsyringol [24–32] were flavor compounds or related to flavor, meanwhile, coniferaldehyde [33] possessed antioxidant property.
Tyrosine is one of the most important precursors of aromatic compounds and greatly influences the flavor of Baijiu [34]. Tyrosine in Baijiu mainly comes from the hydrolysis of macromolecular substances during fermentation [35]. High temperature accelerated the hydrolysis of macromolecules, which may further lead to the highest content of tyrosine in the sauce flavor Baijiu than in the other two flavors Baijiu. Phenol and 4-ethylphenol are not only key aroma compounds in Baijiu [32] but also can improve the flavor and taste of Baijiu by interacting with proteins or peptides [36]. Therefore, the highest content of phenol and 4-ethylphenol in sauce flavor Baijiu may be related to the unique flavor of sauce flavor Baijiu. For the fermentation temperature was highest in sauce flavor Baijiu and lowest in light flavor Baijiu [37]. Accordingly, the fermentation temperature may play an important role in the distribution of amine/phenol compounds in different flavors of Baijiu.
In conclusion, 12C-/13C-DnsCl was used for the high coverage detection and accurate quantification of amine and phenol compounds in Baijiu. And, the number and content of most amine/phenol compounds were found rich in sauce flavor Baijiu and infertile in light flavor Baijiu. All in all, this work provides a novel understanding of amine/phenol compounds in Baijiu and is helpful for in-depth investigation of the flavor and quality of Baijiu.
ACN: acetonitrile
CIL-UHPLC-HRMS: chemical isotope labeling-assisted ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry
HPLC: high-performance liquid chromatography
LLOD: lower limit of detection
LLOQ: lower limit of quantification
MS: mass spectrometry
PLS-DA: partial least squares-discriminant analysis
QC: quality control
SIL: stable-isotope-labeled
UHPLC-HRMS: ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry
v/v: volume/volume
VIP: variable importance of projection
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/10107_sup_1.pdf.
XX: Conceptualization, Writing—original draft, Methodology, Writing—review & editing. FZ: Methodology, Investigation, Software. TC and X Liu: Investigation, Methodology. CH: Writing—review & editing. MM: Methodology. X Lu: Writing—review & editing, Validation. GX: Investigation, Writing—review & editing, Validation, Funding acquisition.
The authors declare that they have no conflict of interest.
Not applicable.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
This work was funded by the Key Foundation from the National Natural Science Foundation of China [
Not applicable.
© Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
