Affiliation:
1Department of Nutrition and Dietetics, Faculty of Health Sciences, Karamanoğlu Mehmetbey University, Karaman 70100, Türkiye
Email: busra.atbln@hotmail.com
ORCID: https://orcid.org/0000-0002-8485-1763
Affiliation:
2Department of Nutrition and Dietetics, Faculty of Health Sciences, Gazi University, Ankara 06500, Türkiye
ORCID: https://orcid.org/0000-0002-2213-4419
Explor Foods Foodomics. 2025;3:101073 DOI: https://doi.org/10.37349/eff.2025.101073
Received: December 19, 2024 Accepted: February 06, 2025 Published: February 13, 2025
Academic Editor: Luca Rastrellii, University of Salerno, Italy
The article belongs to the special issue Ketogenic Diet as Medical Nutrition Therapy
The ketogenic diet (KD) is a nutritional model that includes high fat, moderate protein, and low carbohydrate (less than 50 g). The “KD ratio” is used to determine the amount of macronutrients in the diet. In classical KD with the ratio of 3:1 or 4:1, 85–90% of the energy is provided from dietary fat. In addition to classical KD, the modified Atkins diet, low glycemic index therapy, and medium-chain triglyceride diet have also been used, and in some studies, ketosis has been achieved with exogenous ketone supplements. KD has long been recognized as a successful dietary approach in the treatment of refractory epilepsy. It is known that KD may also be effective in other neurological diseases such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and migraine through various mechanisms such as providing an alternative energy source for neurons, reducing inflammation and oxidative stress, stimulating neurotransmitter synthesis and regulation of microbiota, etc. However, existing evidence is insufficient to make definitive conclusions about the effect of the KD on neurological diseases other than epilepsy due to the short intervention time, the small sample size, and the heterogeneity in the study methods. Considering factors such as genetics, endocrine differences, timing, and diet composition, it is important to apply and follow precision nutrition programs to increase the benefits of KD and reduce its side effects. In this review, the mechanisms of the KD on neurological diseases, recent evidence on the use of the KD in neurological diseases other than epilepsy, the limitations and difficulties in the literature on the KD, and the contraindications of the KD were discussed in detail.
Since at least 500 BC, epilepsy has been treated with fasting and other dietary changes. In the 1920s, physicians developed the ketogenic diet (KD) to cure epilepsy by stimulating the metabolism of fasting [1]. The KD is a nutritional model that includes high fat, moderate protein and limits carbohydrate intake. In general, the goal of the KD is to reduce daily carbohydrate intake to less than 50 g. Protein intake is modestly restricted to less than 1 g/kg body weight [2]. The “KD ratio” is used to determine the amount of macronutrients in the diet. The KD ratio represents the ratio of dietary fat (g) to total protein and carbohydrate intake (g). The diet is usually started with a low KD ratio (1:1, 2:1, etc.) and the ratio is gradually increased over 3–4 days until the desired level of ketosis is achieved. The most used ratios are 3:1 and 4:1 (classical KD, 85–90% of energy is provided by dietary fats) [3, 4].
In addition to classical KD, the modified Atkins diet (1:1 ratio), low glycemic index therapy (1:1 ratio), and medium-chain triglyceride (MCT) diet (1:1 ratio) have also been used in the treatment of epilepsy. In the MCT diet, 60% of energy is provided by dietary fat. MCT intake should initially be 40–50% of energy, and the ratio should be increased according to tolerance. Consumption of medium-chain fatty acids, which do not require bile salts in digestion and absorption, causes the rapid synthesis of ketone bodies by fatty acids reaching the liver through the portal circulation. Therefore, this dietary model has a high potential for ketosis. However, this dietary pattern is less common due to the gastrointestinal side effects of MCTs [4–6]. The modified Atkins diet is a nutritional model that limits carbohydrate intake to 20 g/day, with no restriction on protein intake (however excessive protein intake is not desired) [7]. In low glycemic index treatment, carbohydrate intake is limited 40 g/day to 60 g/day from foods with a low glycemic index (GI < 50) (foods with high fiber contents, such as non-starchy vegetables, nuts, and seeds) to prevent fluctuations in blood glucose and insulin levels. Similar to the modified Atkins diet, there is no restriction on protein intake (20%), and fat makes up 60–70% of total calories [4, 5]. The degree of ketosis produced by the low glycemic index treatment varies individually, and it is lower than the classical KD. Nevertheless, the impact of low glycemic index treatment has been linked to reduced serum glucose levels but not to the degree of ketosis [5]. Figure 1 shows the macronutrient ratios of KD [4].
In some studies, ketosis has been achieved with exogenous ketone supplements. These supplements are available as salts or esters of beta hydroxy butyrate. Exogenous ketone supplements directly increase beta-hydroxy butyrate levels without requiring ketogenesis or increasing free fatty acid levels. Weak adverse effects on health have been reported. However, they can cause gastrointestinal symptoms, and their bitter taste and high cost limit their use. Research on their physiological effects is still in the early stages, so their long-term effects are unknown [8, 9].
KD has long been recognized as a successful dietary approach in the treatment of refractory epilepsy and is being investigated as a promising therapeutic option for a variety of diseases ranging from obesity to malignancies [10]. The effect of KD on neurological diseases is one of the frequently researched topics. According to the results of the bibliometric analysis study, a total of 2,808 publications and 88,119 citations were identified on this subject in the last twenty years [11]. It is known that ketone bodies may have a positive effect on neurological diseases through mechanisms such as providing energy sources to neurons, increasing gamma-aminobutyric acid (GABA) levels, improving blood flow to the cerebrum, positive regulation of the microbiota-gut-brain axis, reducing oxidative stress and neuroinflammation, and neuroprotection [12]. Dietary ketosis has been demonstrated to be beneficial for Alzheimer’s disease [13–17], Parkinson’s disease [18–20], multiple sclerosis (MS) [21, 22], and migraine [23–26].
This review aims to evaluate recent findings regarding the use of KD in neurological diseases other than epilepsy. In addition, limitations and difficulties regarding KD and contraindications to KD were discussed.
According to the consequences of ketone body metabolism, moderate ketosis may have therapeutic promise for neurological diseases. The liver produces large amounts of ketone bodies, including acetoacetate, 3-hydroxybutyrate, and acetone, as a result of the fatty acids oxidation. The first two can be utilized to produce adenosine triphosphate (ATP) by entering the citric acid or Krebs cycle. The obtaining of energy from ketone bodies has provided an alternative source of energy for neurons [27]. The KD has been shown to have a wide range of mechanisms of action in neurological diseases, including its effects on reducing inflammation and reactive oxygen species (ROS), restoring the myelin sheath of neurons, and forming and regenerating mitochondria and neuronal metabolism. It has also been shown to reduce glucose and insulin levels, reduce amyloid plaques, induce autophagy, reduce excessive neuronal activation, reduce microglia activation, gene expression, and dopamine production, and increase glutamine conversion into GABA [28]. Also, the ratio of nicotinamide adenine dinucleotide (NAD+) to NADH can be altered by a KD, increasing the amount of NAD+ available in the brain. This has a major effect on cellular pathways involved in DNA damage repair, inflammatory response, and circadian rhythm regulation [27].
The KD may also be effective in neurological diseases by regulating the gut microbiota. A systematic review study has shown that the KD can potentially improve memory, learning, and disease progression, and reduce the frequency of relapses and attacks. The KD can provide clinical improvement by regulating microbiota composition and bacterial metabolites. Following KD, there was a decrease in Firmicutes and Actinobacteria phyla, Eubacterium, Cronobacter, Saccharomyces, Claviceps, Akkermansia, and Dialister genera and an increase in Proteobacteria phylum, Escherichia, Bacteroides, Prevotella, Faecalibacterium, Lachnospira, Agaricus, and Mrakia genera [29]. According to the narrative review, KD has a major role in modifying the gut microbiota to alleviate disease symptoms, mostly by increasing the ratio of Bacteroidetes to Firmicutes and, in some situations, decreasing Proteobacteria [30]. However, while short-term studies have shown that KD may have positive effects on the gut microbiota, there remains uncertainty on the efficacy and potential for dysbiosis in long-term studies [31]. KD provides an energy source for colonocytes, has anti-inflammatory and antioxidant properties, and plays a role in protecting the immune system and intestinal barrier integrity. However, it has also been suggested that there are conflicting results regarding the effect of KD on microbiota. In a related systematic review, the findings offer compelling proof of a long-lasting decline in Bifidobacterium abundance after KD adherence. Although two studies with longer intervention periods suggest this may be time-limited, a decreased abundance of important Firmicutes butyrate-producing bacteria was determined to be a potential influence [32]. Intake of adequate amounts and quality of unsaturated fatty acids, reduction of animal protein, and addition of fermented foods to the diet are among the recommendations given to protect intestinal health in patients following KD. However, it is important to maintain ketonemia while making changes [33].
The mechanisms for the effect of KD in neurological diseases are summarized in Figure 2 [28, 34].
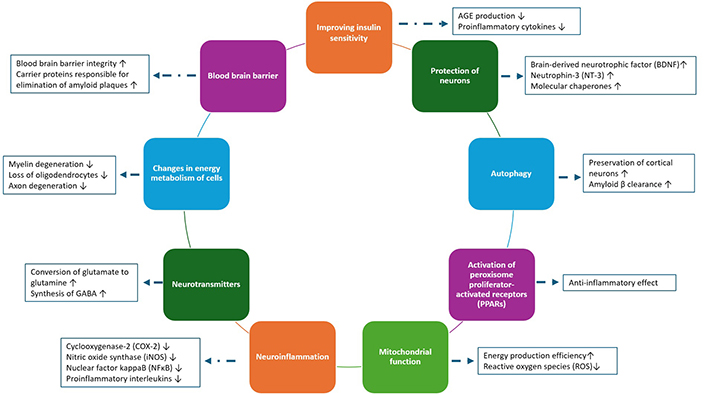
KD mechanisms in neurological diseases. AGE: advanced glycation end-product; GABA: gamma-aminobutyric acid
Alzheimer’s disease is clinically characterized by dementia that includes memory and cognitive impairment, personality, and behavioral changes. Genetic and environmental factors are effective in the formation of disease. Amyloid precursor protein mutations can lead to increased synthesis of amyloid-beta (Aβ) peptides, while apolipoprotein ε4 (APOε4) gene mutations can lead to impaired clearance of Aβ. In addition, tau protein accumulation and stimulation of neuroinflammation are other mechanisms thought to cause the formation of the disease [35]. KD may be protective against Alzheimer’s disease by reducing Aβ peptide accumulation, increasing Aβ clearance, reducing neuroinflammation and oxidative stress, increasing brain-derived neurotrophic factor levels, and stimulating neuronal autophagy [36].
Systematic reviews of the effects of KD on Alzheimer’s indicated that KD may have positive effects on memory [14, 16]. However, no changes were observed in psychological health, walking, and attention [14]. In another systematic review including 12 randomized controlled trials, it was reported that mild ketosis induced by the intake of medium-chain fatty acids could stimulate cognitive improvement in patients with Alzheimer’s disease [13]. In addition to KD, a normal diet with exogenous ketone supplementation may also be effective in the treatment of Alzheimer’s. It is not clear whether KD or exogenous ketone supplementation is more effective, but it has been stated that exogenous supplementation may provide longer-term adherence [15]. However, limitations were also stated regarding the studies, such as small sample sizes, lack of evaluation of adherence to the diet, heterogeneity of study protocols, use of MCT or exogenous ketone supplements, and the fact that the diet application period was generally not longer than 6 months (45–180 days) [14]. Moreover, it was emphasized that the results of studies on the effects of KD in diseases such as Alzheimer’s and Parkinson’s were contradictory, and the studies were insufficient [17].
When evaluating the effect of KD on Alzheimer’s, the APOε4 allele should also be evaluated. According to the results of a systematic review, KD was probably effective in improving cognition in individuals with mild to moderate Alzheimer’s who were negative for APOε4 allele. Insufficient and conflicting evidence was reported for cognitive stabilization in patients with mild to moderate Alzheimer’s disease who were positive for the APOε4 allele. These results demonstrate the importance of evaluating the APOε4 allele to define the effect of ketogenic intervention in Alzheimer’s patients [18]. Similarly, in another study, no effect of KD was found on APOε4 carriers. It was reported that diets rich in saturated fats may be particularly harmful for APOε4 carriers [37].
In conclusion, although positive study results have been shared regarding the effect of KD on Alzheimer’s, more studies are needed to reach a definitive conclusion. To slow down cognitive decline in older ages, recommendations are given to reduce the intake of saturated fatty acids, increase the intake of mono- or poly-unsaturated fatty acids, increase the consumption of fruits and vegetables, meet the need for vitamin D, and adherence to the Mediterranean diet. In addition, other promising dietary advice includes a KD, replacing whole dairy products with semi-skimmed products, or energy restriction, although definitive evidence on these issues is lacking [38].
Parkinson’s disease is caused by neuronal loss in the substantia nigra, which causes dopamine deficiency, or by pathological alpha-synuclein accumulation within the cells. The disease is the most common neurodegenerative movement disorder. The main motor symptoms are tremors, rigidity, bradykinesia/akinesia, and postural instability [39]. Pathophysiological mechanisms, motor and non-motor symptoms, drug interactions, disease course, and quality of life are the intersections of Parkinson’s disease and nutrition. An interdisciplinary team and evidence-based practices, including prevention of malnutrition and protein-redistribution diets, are necessary for the symptomatic management of nutrition-related problems in Parkinson’s disease [40]. The available literature supports a protective function of the Mediterranean diet against the onset and progression of Parkinson’s disease, even though the clinical trials have several possible limitations and more, well-designed, long-term researchers are required [41]. In addition to the Mediterranean diet, the effects of a protein-restricted diet, KD, and the Mediterranean-DASH intervention for neurodegenerative delay (MIND) diet on disease risk, progression, and severity have also been investigated [42].
Protective effects of KD on Parkinson’s disease include protecting neurons from toxicity by increasing mitochondrial complex II activity and ATP synthesis, relieving symptoms such as akinesia and rigidity by positively affecting the somatic nervous system, delaying or preventing neuron degeneration, and providing neuroprotection by increasing glutathione enzyme effect [12]. A systematic review evaluating the effect of the KD on Parkinson’s disease included twelve studies (4 randomized controlled trials and 8 animal studies). According to randomized controlled trials, KD did not improve motor coordination and functioning, cognitive impairments, blood lipids and glycemic control, exercise performance, or voice disorders in patients with Parkinson’s disease. Animal studies have shown more promising results with significant improvements in locomotor activity, dopaminergic activity, redox status, and inflammatory markers [43]. Another systematic reviews found that ketogenic intervention was probably effective in improving non-motor symptoms in Parkinson’s disease [18, 19], but the evidence for motor symptoms was insufficient and conflicting [18]. On the contrary, there is also a systematic review showing that the KD may also be effective on motor symptoms in Parkinson’s disease [20]. However, although the studies suggest that following a KD may alleviate a variety of disease symptoms, their generalizability is limited by their small sample size and short intervention durations [42]. It is recommended that before applying the KD as a therapeutic option, it is necessary to consider whether it will actually provide a symptomatic benefit or for how long it should be applied [19].
MS is a central nervous system autoimmune demyelinating disease that has both neurodegenerative and inflammatory aspects [44]. Although the cause of MS pathology is unknown, research shows that genetic, immunological, and environmental factors may all play a role in a complex etiology [45, 46]. However, inflammation that occurs when exposed to risk factors is one of the most important factors in the development of the disease [45]. Evidence from recent studies suggests that nutrition may also have an impact on the development, progression, and quality of life of MS patients. However, there are currently no specific guidelines for a particular diet for patients with MS. Nonetheless, there is indirect evidence from clinical and experimental research suggesting a healthy lifestyle and a balanced diet are associated with improvements in a number of clinical indicators and quality of life metrics for MS patients [47].
A KD could function by focusing on an immunological activity that is “out of control”. Adenosine, ketone bodies, mechanistic target of rapamycin pathways, peroxisome proliferator-activated receptor-gamma (PPAR-γ), the NLR family pyrin domain containing 3 (NLRP3) inflammasome, and gut microbiota are among the increasing number of possible inflammatory pathways of KD [48]. Studies on rodent models support the effectiveness of the KD in preventing and treating demyelination and inflammation in experimental MS models [49]. A phase II study evaluating the effects of the KD on patients with relapsing-remitting MS showed significant improvements in body fat mass, self-reported fatigue, and depression scores after six months of intervention. Significant improvements were noted in the quality-of-life scale score and the 6-minute walk test [21]. In a review evaluating 15 in vitro, preclinical, and clinical studies, it was reported that the KD may have a potential neuroprotective effect and a positive impact on disease outcomes [22]. However, in a systematic review evaluating the effects of different dietary models on MS, it was reported that in addition to studies showing a positive effect on the disease, there were also studies in which no clinical effect could be demonstrated or studies that were stated as KD but could not be evaluated as KD due to their high carbohydrate content (40% carbohydrate) [50]. Although it has been stated that the KD is safe and tolerable in individuals with MS, more studies with longer duration and larger samples are needed for definitive evidence.
If a KD is to be applied to patients with MS, some modifications to the nutrition plan are recommended compared to the classical KD: 20 g/day carbohydrate intake in the first 4-week adaptation phase, increasing carbohydrate intake by 5 g every week in the second period of the adaptation phase to reach a maximum of 40 g/day carbohydrate intake within a month, and keeping the ketone level between 0.5–3 mM in the maintenance phase [48]. However, question marks have been reported regarding the long-term sustainability of the KD interventions. Although adherence and interest in the KD are high within the scope of the studies, it is estimated that adherence to the KD outside the study will be around 58%. In addition, the cost of KD may be another factor affecting the sustainability of the diet [51].
Migraine is a highly disabling primary headache disorder with a 1-year prevalence of approximately 15% in the general population. It is clinically characterized by frequent headache attacks with a variety of concomitant symptoms. About one-third of migraineurs experience migraine aura, which is a temporary neurological disturbance that occasionally or always precedes or accompanies their headache. Additionally, a small percentage of afflicted patients get chronic migraine, which results in extremely frequent attacks. It is generally accepted that both central and peripheral stimulation of the trigeminovascular system has a role in the pathophysiology of migraine [52].
KD can cause an increase in brain-derived neurotrophic factor and a decrease in histone deacetylase via epigenetic regulation. These pathways are important in stimulating neuroplasticity and reducing oxidative stress. KD can also reduce cortical spreading depression via increasing mitochondrial function and GABA production. In addition, reduction in leptin and insulin levels, inhibition of nuclear factor kappa B (NF-κB) pathway, and regulation of microbiota may provide improvement in migraine through anti-inflammatory effects [53]. In a study, participants were followed for 12 weeks with a KD (3:1, 2:1, or 1:1) or migraine prevention diet (no energy restriction, based on the elimination of processed foods, caffeine, and foods containing monosodium glutamate). At the end of the study, no significant difference was found between the groups in terms of migraine frequency, severity, or duration. A clinically important trend towards a decrease in migraine duration was noted only in the KD group [25]. Similarly, in another study, a 3-month KD intervention was recorded as a significant reduction in symptom frequency, duration, pain level, and number of medications taken in patients with refractory chronic migraine [23]. In a systematic review, it was reported that the KD reduced the number and severity of migraine attacks in patients. However, the evidence for the effectiveness of the KD was stated as low [24]. A systematic review of 10 studies reported that there was inconsistency among studies regarding the evaluation of ketosis. Therefore, no relationship was established between the level of ketosis and the prevention or reduction of migraine attacks. Although high levels of heterogeneity were reported, all interventions were shown to have an overall significant effect [26].
In a position paper on the application of KD to patients with headaches, the necessity of multidisciplinary work, electrocardiography, and complex laboratory evaluation before starting the diet was emphasized. It was also stated that KD can be started gradually, if there is no response within 3 months, the ketogenic ratio can be increased, and similarly, if there is still no response, diet treatment should be stopped in the 6th month. There is no optimal duration of KD that can be generalized to all patients. However, the minimum duration of the diet should not be less than three months [53]. However, it has been emphasized that KD should not be recommended in elderly patients without clear answers to questions such as what should be the ratio of animal and plant sources in KD? Should KD be adjusted specifically for gender? When should KD be started? Should characteristic features of migraines (presence/absence of aura, nausea, vomiting, etc.) be considered in KD planning [54]? The increase in circulating ketone bodies for older ages increases all-cause mortality, raising questions about the KD recommendation for elderly patients [55, 56].
In the KD, the shift in energy metabolism from carbohydrates to fatty acids and ketone bodies may cause symptoms such as headache, fatigue, nausea, dizziness, gastrointestinal discomfort, decreased energy, and heartbeat alterations within the first few weeks. The condition presented with these findings is defined as “keto flu” [57]. The timing and intensity of keto flu symptoms can vary depending on metabolism, eating patterns before KD, hydration level, electrolyte balance, and general health. it is critical to maintain electrolyte balance, be well hydrated, and ensure that sufficient nutrients are available throughout the early stages of the KD [58]. Side effects have also been noted in studies evaluating the effects of the KD on neurological diseases. In Alzheimer’s and Parkinson’s, gastrointestinal discomfort is the most reported side effect [18]. Also, worsening rigidity and tremor [59] and increased cases of cardiac arrhythmias [43] in Parkinson’s disease and weight loss and malnutrition in Alzheimer’s have been noted [16]. Side effects such as airway infection and increased expanded disability status scale (EDSS) scores have occurred in MS patients [22]. Fatigue, gastrointestinal symptoms, bloating, and difficulty sleeping have been reported in patients with migraine [25, 26]. In fact, although the KD has been studied for therapeutic purposes in neurological diseases, in some cases it can cause an increase in disease-related symptoms and worsen the existing condition. Therefore, patients undergoing KD should be regularly monitored for their outcomes and side effects.
When evaluated in terms of potential long-term side effects, it is known that KD can cause nutritional deficiencies. Limiting fruits, vegetables, whole grains, and legumes to reduce carbohydrate intake in the KD causes a decrease in diet quality. KD is generally deficient in nutrients such as fiber, thiamine, folic acid, vitamins A and E, vitamin B6, calcium, magnesium, iron, potassium, and phytochemicals [60]. In preventing constipation due to decreased fiber intake, it is important to prefer low-carbohydrate vegetables with high fiber content (lettuce, spinach, cabbage, etc.) and adequate fluid intake [3]. Hypoglycemia, dyslipidemia, gastrointestinal problems, carnitine deficiency, bone disorders such as osteopenia and osteoporosis, and nephrolithiasis are examples of other common side effects in the long term [31]. Patients with or without underlying hereditary hyperlipidemia may develop or worsen hypercholesterolemia as a result of following a KD [61]. To prevent and control dyslipidemia, practices such as reducing the KD ratio, increasing mono and polyunsaturated fatty acids in the diet (using olive oil instead of saturated fats such as butter, cream, etc.), replacing some of the long-chain fats with MCT, and supplementing with carnitine and omega-3 fatty acids can be recommended [3]. A recent study also showed that KD can induce p53 signaling via AMP-activated protein kinase (AMPK) activation and increase cellular senescence in multiple organs (heart and kidney). Accumulation of senescent cells may contribute to systemic inflammation and toxicity [62].
Although KD is a nutritional model whose effectiveness has been evaluated in neurological diseases, the use of KD is contraindicated in some cases. KD is not recommended for patients using sodium-glucose transport protein-2 (SGLT-2) inhibitors, pregnant and breastfeeding women, in case of active/serious infection, and elective surgery or invasive procedures. It can increase damage in liver failure and chronic kidney disease and can stimulate hypoglycemia and diabetic ketoacidosis in type 1 diabetes. Therefore, an evaluation by a specialist should be made and the feasibility of the diet should be evaluated. KD is also contraindicated in cases of cardiac arrhythmia, recent stroke or myocardial infarction, heart failure, respiratory failure, malignancy, elevated serum uric acid levels, abnormal lipid profile, and in frail elderly patients with a history of mental illness [63].
In conclusion, KD can play a protective and therapeutic role in neurological diseases through many mechanisms such as reducing inflammation and oxidative stress, improving insulin sensitivity, increasing mitochondrial function, neurotransmitter synthesis, protecting neurons, and modulation of microbiota. There are studies showing that KD may have positive effects on the progression of neurological diseases such as Alzheimer’s [14, 16], Parkinson’s disease [18, 19], MS [21, 22], and migraine [23, 24]. However, a definitive conclusion cannot be reached due to the short intervention time, the small sample size, and the heterogeneity in the study methods, such as the use of different types of KD or exogenous ketone supplements to achieve ketosis. Although small study groups or short-term studies may produce rapid results, the reliability and predictive power of the results may be low. Therefore, such studies should be used in methodologically stronger, longer-term studies with larger sample sizes. However, one of the difficulties in planning long-term studies may be low adherence to a KD. Since it is a limited nutritional model, adherence to the diet may decrease in the long term, side effects may make adherence difficult, or nutrient deficiencies may develop in the long term, so monitoring and supplementation are necessary. In addition, the aim should be to find out which KD model is more effective, reliable, and tolerable for which neurological disease. For example, the effect of saturated fatty acids on the development and course of MS is a known fact. In addition, the risk of cardiovascular morbidity is high in this patient group. Therefore, disease-specific modifications can be made in the KD, such as replacing saturated fatty acid food sources with monounsaturated fatty acid and reducing the KD ratio. Regarding these recommendations, the modified Mediterranean-KD, which prefers olive oil as a fat source, includes high biological value protein sources and low glycemic index fruits and vegetables, which has become widespread in recent years [64].
It is evident that a KD has complicated effects, and that using it will probably have different health impacts depending on multiple factors (timing, dietary composition, genetics, endocrine considerations, and the health status of patients) [62]. Due to individual genetic differences, the risks of KD can be greater than the advantages. This situation increases the need for precision nutrition and nutrigenomics research. In clinical practice, precision nutrition should also be considered as small changes in diet composition may affect/reduce potential side effects of the KD in certain populations [65].
APOε4: apolipoprotein ε4
ATP: adenosine triphosphate
Aβ: amyloid-beta
GABA: gamma-aminobutyric acid
KD: ketogenic diet
MCT: medium-chain triglyceride
MS: multiple sclerosis
NAD+: nicotinamide adenine dinucleotide
BA: Conceptualization, Writing—original draft, Writing—review & editing. YA: Conceptualization, Writing—review & editing, Supervision.
The authors declare they have no conflicts of interest to report.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2213
Download: 224
Times Cited: 0
Giulia Izzo ... Mario Vitale
Leo Karl Hanke ... Paola Molettieri
Paola Pellegrini ... Maria D’Elia
Xin Qi, Richard Tester
Alejandro Borrego-Ruiz, Juan J. Borrego
