Abstract
Aim:
The study investigates the probiotic potential of exopolysaccharide (EPS)-producing lactic acid bacteria (LAB) isolated from yoghurt samples. It assesses their antimicrobial efficacy against foodborne pathogens, particularly Escherichia coli and Staphylococcus aureus. The objective is to identify LAB strains that can be used as natural preservatives and health-promoting probiotics in functional foods.
Methods:
Yoghurt samples were collected from household local markets in Rawalpindi, Pakistan. LAB was isolated and identified using selective media, Gram staining, and biochemical tests. EPS production was quantified using the phenol-sulfuric acid method. Probiotic properties, including antimicrobial activity against E. coli and S. aureus, were evaluated using the disc diffusion method. Strains producing the highest EPS were biochemically characterised using the API Strep system.
Results:
Of 29 LAB isolates, 12 were identified as significant EPS producers, with Streptococcus thermophilus, Lactococcus lactis, and Limosilactobacillus fermentum demonstrating the highest EPS production (up to 62 µg/mL). These strains exhibited strong antimicrobial activity against E. coli and S. aureus, with inhibition zones ranging from 2 mm to 32.1 mm. The results confirmed the dual functionality of these strains as both texture enhancers and natural preservatives in food products.
Conclusions:
The EPS-producing LAB strains, particularly S. thermophilus, L. lactis, and L. fermentum, showed significant potential as probiotics and natural preservatives. Their antimicrobial activity and ability to enhance food texture suggest their applicability in the food industry to promote health and improve food safety. Further research should explore their stability in different food matrices for commercial use.
Keywords
Probiotics, exopolysaccharides, lactic acid bacteria, anti-microbial activity, foodborne pathogensIntroduction
The focus on food applications has recently expanded beyond taste and nutritional requirements to encompass their potential health benefits. One promising area in this domain is the use of probiotics, which are live bacteria that confer health benefits [1, 2]. Among these, lactic acid bacteria (LAB) stand out due to their long history of safe use in food fermentation and their presence in the gastrointestinal tract. LAB is Gram-positive bacteria known for its ability to ferment sugars into lactic acid, including several genera, with Lactobacillus being particularly significant due to its extensive use in dairy fermentation [3–6].
Probiotics, derived from the Greek word for life, are recognised for their health-promoting properties. According to the World Health Organization (WHO), probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [7, 8]. This concept was popularised by Elie Metchnikoff, who observed the longevity of Bulgarian peasants consuming fermented milk, attributing it to the probiotics in these foods. Probiotics have since been shown to offer various health benefits, including improving gut health, boosting the immune system, and protecting against harmful pathogens [9].
LABs are vital for producing fermented dairy products like yoghurt and cheese and play a crucial role in maintaining gut health. These bacteria can survive the acidic environment of the stomach and colonise the gastrointestinal tract, where they contribute to the inhibition of pathogenic bacteria through the production of organic acids and bacteriocins [10]. Furthermore, exopolysaccharides (EPSs) produced by LAB is gaining attention for potential applications in the food industry. These polysaccharides serve as natural thickening agents, enhancing the texture and stability of food products. EPS can improve the viscosity and uniformity of fermented milk products, such as yoghurt, and help prevent syneresis.
Additionally, some EPS exhibit health benefits, such as anti-ulcer and cholesterol-lowering activities, making them valuable for their functional properties in food and their direct health effects [11–13]. EPS-producing LAB can enhance the structural properties of fermented dairy products while also contributing to their probiotic benefits. Similarly, the potential of EPS in pharmaceutical applications is attributed to its bioactive properties [11, 14].
Recent advancements have focused on optimizing EPS production by LAB and understanding the genetic and environmental factors that regulate its synthesis [15]. Key studies have explored using different carbon sources and fermentation conditions to maximize EPS yield and quality. Furthermore, the dual role of EPS as a texturizing agent and a bioactive compound underscores its potential as a sustainable alternative to synthetic additives in the food industry. For instance, Korcz et al. [16] reviewed the applications of EPS in extending food shelf life, highlighting its antimicrobial properties when produced by LAB strains.
The incorporation of LAB and their EPS into food products not only improves their sensory attributes but also enhances their health benefits. As the demand for functional foods continues to grow, the role of probiotics and LAB in promoting health and preventing disease becomes increasingly important [15]. Their dual functionality in food production and health promotion underscores the significance of ongoing research and development. LAB and their metabolic products, including EPS, have significant applications in the food industry. The ability of LAB to produce EPS in situ during fermentation offers a natural means to enhance food products’ sensory and functional properties. This is particularly beneficial in making yoghurt, where EPS contributes to creaminess and firmness, addressing common issues like low viscosity and gel fracture [17–19].
This research investigates the probiotic potential of LAB that produces EPS and their effectiveness against foodborne pathogens. We will isolate and characterise various LAB strains from yoghurt samples to explore their antimicrobial properties, EPS production capabilities, and overall health benefits. Our approach involves isolating and identifying LAB strains, screening them for EPS production, and evaluating their ability to combat common pathogens such as Escherichia coli and Staphylococcus aureus. We expect to identify potent probiotic strains with significant antimicrobial activities and high EPS production, which could be used to develop functional foods and natural preservatives. This research will contribute to the growing knowledge of probiotics and their applications in enhancing food safety and human health.
Materials and methods
Reagents
The reagents and chemicals used in this study include M17 broth (115029), MRS broth (110661), and Brain-Heart Infusion (BHI) agar (110493), all purchased from Merck. Sigma-Aldrich provided the sugars for fermentation tests (sucrose, fructose, glucose, maltose, galactose, lactose, mannitol, and sorbitol), MRS broth, and glucose for EPS quantification. Nutrient agar was obtained from HiMedia Laboratories. Hydrogen peroxide (3%), phenol red, trichloroacetic acid [80% weight/volume (w/v)], ethanol (90%), and glycerol (20%) were all sourced from VWR International. The API 20 Strep kit was supplied by bioMérieux. All reagents were prepared and used according to the manufacturer’s instructions.
Sample collection and isolation of LAB
Twenty-six yoghurt samples were collected randomly from households and local markets in Rawalpindi, Pakistan (Table S1). Samples were stored in sterile glass bottles at 4°C and processed within two hours under hygienic conditions. LAB was isolated using M17 and MRS media. M17 broth (1.96 g) was mixed with 1 g lactose and 0.92 g agar in 50 mL distilled water, and MRS broth (2.6 g) was similarly prepared. Both media were sterilized at 121°C using traditional autoclaving for 15 minutes. After cooling down, they were poured into Petri plates in a laminar flow hood. After ensuring no contamination, samples were streaked on MRS media and incubated at 37°C for 48 hours. Aerobic conditions were maintained by placing the plates in a standard incubator. Anaerobic conditions were maintained using an Oxoid Anaerobic Jar HP0011 with an atmosphere generation system AN0035 (Thermo Fisher Scientific), which created an anaerobic environment suitable for the growth of anaerobic bacteria. The BHI agar medium was used to grow pathogenic bacteria E. coli and S. aureus, and nutrient agar media for the antimicrobial evaluation.
Identification of microflora
Morphological identification of colonies included observing colour, shape, size, and structure. Gram staining was done following the basic protocol [20]. Catalase activity was tested by placing isolates on a slide and treating them with 3% hydrogen peroxide, observing for bubble formation [21]. Sugar fermentation tests used eight sugars (sucrose, fructose, glucose, maltose, galactose, lactose, mannitol, and sorbitol) to identify microorganisms at the species level. Each sugar (1 g) was dissolved in 10 mL distilled water, sterilised by syringe filtration, and mixed with nutrient broth containing phenol red as an indicator. Tubes were inoculated with isolates and incubated at 37°C for 48 hours.
Further molecular analysis, such as 16S ribosomal RNA (rRNA) sequencing, is recommended to confirm species-level identification. While this study primarily focused on screening isolates for EPS production and their probiotic properties, future work will include 16S rRNA sequencing and alignment for precise taxonomic classification of the isolated strains.
Preservation of isolates and screening for EPS production
Isolates were preserved in MRS and M17 broth with 20% glycerol at 4°C. Cultures were incubated for 24 hours at 37°C, centrifuged, and stored. Isolated strains were screened for EPS production by streaking on MRS and M17 agar plates containing 5% lactose and incubating anaerobically at 37°C for 48 hours. EPS-producing strains exhibited mucoid or ropy colonies and were preserved in MRS broth with 10% glycerol at –20°C.
The production of EPS from the isolated strains was achieved by using sucrose and glucose (5% w/v) as carbon sources. Samples were incubated at 37°C for 24–48 hours, and cells were removed by centrifugation at 13,000 rpm for 10 minutes. The supernatant was treated with 2.5% (v/v) of 80% (w/v) trichloroacetic acid to remove proteins, followed by centrifugation at 12,000 rpm for 15 minutes. The resulting supernatant was mixed with three volumes of 90% cold ethanol and incubated overnight at 4°C to precipitate the EPS. The EPS was recovered by centrifugation, dried at 42°C, and resuspended in distilled water.
Quantification and biochemical characterization
EPS was quantified using the phenol-sulfuric acid method [22]. Absorbance was measured at 490 nm with a spectrophotometer (Thermo Scientific Evolution 201). A standard curve for glucose was made for quantification. Strains with a high amount of EPS were compared with the help of a graph of standard sugar solutions. Total EPS was estimated in each sample by the phenol-sulphuric acid method using glucose as a standard. Higher-producing EPS strains were identified using the API Strep system. Strains were inoculated into API Strep kits and incubated at 36°C for 24 hours, and results were recorded based on colour changes.
Evaluation of probiotic properties
Antagonistic effects of probiotics against foodborne pathogens were tested. Preserved cultures were activated in MRS and M17 broth with 1% tryptone, streaked on MRS and M17 agar plates, and incubated at 37°C for 24 hours. Bacteriocin production was evaluated by inoculating isolated LAB in MRS and M17 broth. Foodborne pathogens were grown on nutrient agar, and bacteriocin production was tested using the disc diffusion method. The antimicrobial activity was evaluated by placing sterile discs impregnated with bacteriocin solution onto the agar plates inoculated with the test pathogens. After incubation at 37°C for 24 hours, the clear inhibition zones around the discs were measured using a digital calliper. The diameter of each inhibition zone was recorded in millimeters. Each experiment was performed in triplicate to ensure reproducibility.
Results
Isolation of LAB
Out of 29 bacterial isolates, 12 species belonging to the cocci family and 17 species belonging to the rods family were identified from 26 yoghurt samples (Table S2). Twelve isolates were divided into three main groups of cocci according to their morphological and biochemical behaviour. Five Enterococcus faecium isolates, four were Lactococcus lactis, and three were Streptococcus thermophilus. On the other hand, according to their biochemical evaluation, seventeen Lactobacillus species showed their belongingness to 5 different groups. Five Lactobacillus groups include Lactobacillus delbrueckii (specie delbrueckii), Lactobacillus plantarum, Limosilactobacillus fermentum, Lacticaseibacillus helveticus, and Lacticaseibacillus paracasei subsp. paracasei.
LAB was isolated by directly streaking raw samples on autoclaved, solidified, and selective M17 and MRS media. After incubation for 24 hours at 37°C, visible colonies were selected visually, as shown in Figure 1a and 1b, and picked for further purification. The pH of the media was adjusted to 6.5–6.9 for the optimum growth of LAB.
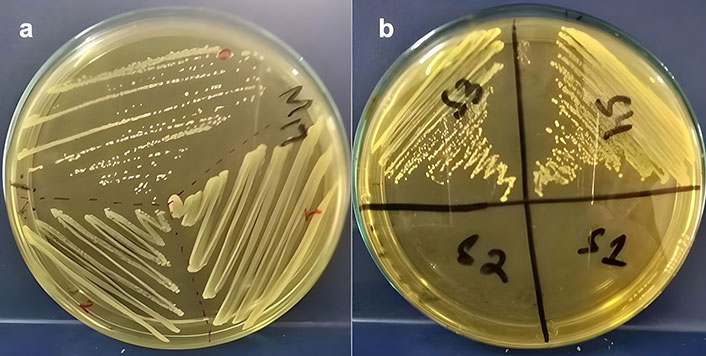
Isolation of lactic acid bacteria (LAB) on selective media. (a) Lactococcus colonies on M17 media; (b) Lactobacillus colonies on MRS media
Morphological characteristics
The isolated colonies on Petri plates were mostly circular and creamy white. Most colonies have a smooth, margined, and shiny appearance. All the isolates were Gram-positive, making Gram-positive rods and cocci-making chains and clusters, as shown in Figure 2a and 2b, when observed under a 100× microscope lens. The rods were in the form of long threads, and cocci were in chains and pairs. Furthermore, isolated species showed negative behaviour during the catalase test when treated with 3% H2O2 because they lacked catalase enzyme, as shown in Figure 2c. Table S3 shows the details of the colony shape, colour, margins, cell shape, and Gram staining results for various LAB species.
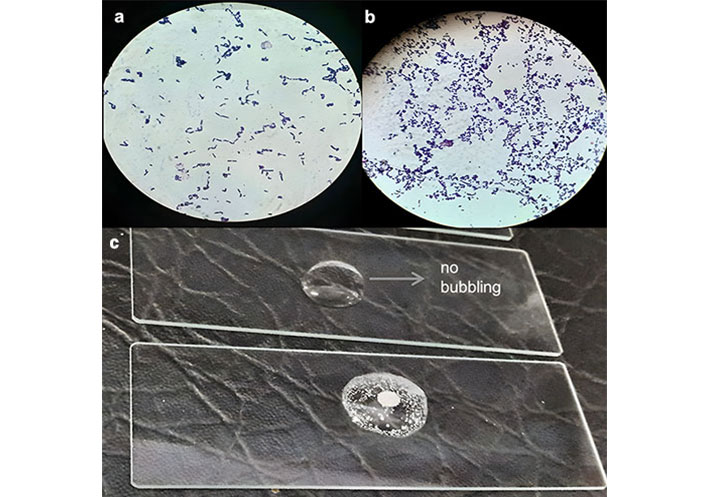
Morphological and biochemical characteristics of lactic acid bacteria (LAB) isolates. (a) Gram strain of Lactobacillus plantarum (P1) showing Gram-positive rods at 100×; (b) Gram strain of Streptococcus thermophilus (S3) showing Gram-positive cocci at 100×; (c) isolate of Streptococcus thermophilus (S1) showing catalase negative result
Screening for EPS-producing bacterial strains and their biochemical characteristics
Out of twenty-nine identified strains of LAB, only twelve strains that showed mucoidity (Table S4) were subjected to screening for EPS production, as shown in Table 1. This includes three strains of S. thermophilus, two strains each of L. lactis, E. faecium, and L. fermentum, and one strain each of L. plantarum, L. paracasei subsp. paracasei, and L. helveticus. These strains were then subjected to a sugar fermentation test for their identification according to their fermentation pattern of 8 sugars: fructose, glucose, galactose, maltose, sucrose, lactose, sorbitol, and mannitol. E. faecium showed 100% fermentation with some variability for fructose, while S. thermophilus showed fermentation of sucrose sorbitol, glucose, and lactose.
Fermentation pattern of lactic acid bacteria isolates from yoghurt
| Isolates | Glucose | Fructose | Sorbitol | Manitol | Maltose | Lactose | Sucrose | Galactose |
|---|---|---|---|---|---|---|---|---|
| Lactobacillus plantarum | + | + | + − | + | + | + | + | + |
| Limosilactobacillus fermentum | + | + | − | + | + | + | + | + |
| Lacticaseibacillus paracasei subsp. paracasei | − | − | + | − | − | − | + | − |
| Lacticaseibacillus helveticus | + | − | − | − | + | + | − | + |
| Lactococcus lactis | + | + − | − | + − | + | + − | + − | + |
| Enterococcus faecium | + | + − | + | + | + | + | + | + |
| Streptococcus thermophilus | + | − | − | − | + | + | + | − |
Symbols in the table represent the carbohydrate fermentation patterns of lactic acid bacteria (LAB) isolates: ‘+’ indicates positive fermentation, meaning the carbohydrate is utilized by the isolate. ‘−’ indicates negative fermentation, meaning the carbohydrate is not utilized by the isolate. ‘+ −’ indicates variability in fermentation patterns, which may reflect strain-specific differences, weak or inconsistent fermentation, or experimental conditions that partially support carbohydrate utilization
EPS isolation and quantification
Table 2 gives the EPS for each strain. The highest values of EPS were observed in two strains of S. thermophilus and L. lactis, 62 and 52 µg/mL, respectively. In contrast, the lowest value of EPS was observed in the strain of E. faecium. The difference in EPS amount might be the time coupled with a suitable carbon source and incubation time and temperature at which the culture was tested for their analysis.
Exopolysaccharide (EPS) produced by different strains of lactic acid bacteria
| Isolates and species | µg of polysaccharides/mL |
|---|---|
| Lactobacillus plantarum (P1) | 34 |
| Lacticaseibacillus helveticus (H1) | 32 |
| Lacticaseibacillus paracasei subsp. paracasei (CP1) | 40 |
| Limosilactobacillus fermentum | |
| F1 | 43 |
| F2 | 40 |
| Enterococcus faecium | |
| E1 | 37 |
| E2 | 28 |
| Lactococcus lactis | |
| L1 | 52 |
| L2 | 49 |
| Streptococcus thermophilus | |
| S1 | 48 |
| S2 | 40 |
| S3 | 62 |
Identification and biochemical characterization of high EPS-producing strains
Standardized identification of higher-producing EPS strain was performed using API Strep. Results were obtained based on colour change after 24 hours of incubation at 34°C. API Strep was used to identify streptococci and test the fermentation ability of 10 carbohydrates. After 24 hours of incubation, all the tubes showed positive results (Table 3). API Strep tests were positive for the fermentation of ribose. Arabinose, mannitol, sorbitol, lactose, trehalose, inulin, raffinose, starch, and glycogen. Moreover, positive results were also recorded for acetoin production, β-glucuronidase, α- and β-galactosidase, alkaline phosphatase, leucine arylamidase, and arginine dihydrolase production. Test results concluded that there was 99.9% homology with the S. thermophilus strain, an essential strain for yoghurt starter culture.
Results of API Strep
| Serial number | Reaction tested | Substrate | Results |
|---|---|---|---|
| 1 | Voges-proskauer test | Acetoin production | Red |
| 2 | Hippurate hydrolysis | Hippurate | Violet |
| 3 | Aesculin hydrolysis | Aesculin | Black |
| 4 | Pyrrolidonyl arylamidase test | Pyrrolidonylaryl-amidase | Orange |
| 5 | α-Galactosidase test | α-Galactosidase | Violet |
| 6 | β-Glucuronidase test | β-Glucuronidase | Blue |
| 7 | β-Galactosidase test | β-Galactosidase | Violet |
| 8 | Phosphatase test | Alkaline phosphatase | Violet |
| 9 | Leucine arylamidase test | Leucine arylamidase | Orange |
| 10 | Arginine dihydrolase test | Arginine dihydrolase | Red |
| 11 | Ribose fermentation | Ribose | Positive |
| 12 | Arabinose fermentation | Arabinose | Positive |
| 13 | Mannitol fermentation | Mannitol | Positive |
| 14 | Sorbitol fermentation | Sorbitol | Positive |
| 15 | Lactose fermentation | Lactose | Positive |
| 16 | Trehalose fermentation | Trehalose | Positive |
| 17 | Inulin fermentation | Inulin | Positive |
| 18 | Raffinose fermentation | Raffinose | Positive |
| 19 | Starch fermentation | Starch | Positive |
| 20 | Glycogen fermentation | Glycogen | Positive |
Antimicrobial characteristic
LAB isolated from yoghurt were subjected to test their anti-microbial activity against food-borne pathogens, mainly E. coli and S. aureus, as shown in Table 4. All investigated isolates showed mostly antagonistic activity against E. coli and S. aureus. Our findings suggest that wild strains of EPS-producing LAB isolated from local yoghurt samples with antimicrobial properties are essential. When they are used for starter culture in fermented dairy products, they show effective control against E. coli and S. aureus grown in products.
Quantitative measurement of antimicrobial activity of exopolysaccharide (EPS)-producing isolates
| Isolates and species | Inhibitory action against pathogenic bacteria (± SD) | |
|---|---|---|
| Escherichia coli | Staphylococcus aureus | |
| Lactobacillus plantarum (P1) | 22.3 ± 1.2 mm | 19.4 ± 0.4 mm |
| Lacticaseibacillus helveticus (H1) | 18.3 ± 0.9 mm | 4.0 ± 0.1 mm |
| Pseudo plantarum (CP1) | 23.6 ± 0.4 mm | 18 ± 0.4 mm |
| Limosilactobacillus fermentum | ||
| F1 | 21.5 ± 0.4 mm | 32.1 ± 0.2 mm |
| F2 | 11.6 ± 0.3 mm | -- |
| Enterococcus faecium | ||
| E1 | -- | 24.6 ± 0.5 mm |
| E2 | -- | 23 ± 0.8 mm |
| Lactococcus lactis | ||
| L1 | 18.8 ± 0.8 mm | 2 ± 0.1 mm |
| L2 | 17 ± 0.4 mm | -- |
| Streptococcus thermophilus | ||
| S1 | 24.4 ± 0.7 mm | 24.8 ± 0.4 mm |
| S2 | 24.1 ± 0.6 mm | 23.3 ± 1.2 mm |
| S3 | 21 ± 0.8 mm | 17.6 ± 1.1 mm |
--: no inhibitory action. E1 and E2 isolates showed no inhibitory action against Escherichia coli. Similarly, F2 and L2 isolates exhibited no inhibition against Staphylococcus aureus; therefore, triplicate measurements were not performed for these isolates in the respective cases
Discussion
This study effectively investigated the probiotic potential of EPS-producing LAB isolated from yoghurt samples, demonstrating their significant antimicrobial activity against foodborne pathogens, particularly E. coli and S. aureus. The findings emphasize the potential of these LAB strains, especially S. thermophilus, L. lactis, and L. fermentum, as effective natural preservatives and probiotics, underscoring their dual role in food safety and human health promotion. However, this study has certain limitations, including the reliance on biochemical and phenotypic methods for microbial identification, which may not provide definitive species-level resolution. Future research should include molecular techniques such as 16S rRNA sequencing for precise identification.
Our findings align with those of a previous study, which identified LAB as effective biocontrol agents against foodborne pathogens owing to their broad-spectrum antimicrobial properties [23]. A notable finding in this study is the high EPS production by S. thermophilus (62 µg/mL), L. lactis (52 µg/mL), and L. fermentum (43 µg/mL). Their antimicrobial activity against E. coli and S. aureus suggests a potential link between EPS production and pathogen inhibition. For instance, L. fermentum F1 exhibited inhibition zones of 21.5 ± 0.4 mm and 32.1 ± 0.2 mm against E. coli and S. aureus, respectively, the largest among the studied strains. This could indicate that higher EPS levels enhance antimicrobial efficacy, possibly due to biofilm formation or through the secretion of metabolites like bacteriocins [24, 25]. Further research should quantify these interactions under controlled conditions to establish causality.
The ability of these LAB strains to produce EPS, alongside their antimicrobial properties, suggests their potential to enhance the functional and sensory qualities of fermented food products, contributing to their texture, stability, and nutritional value. A study by Han et al. [14] supported these observations, indicating that EPS produced by LAB enhances the viscosity and creaminess of dairy products like yoghurt, which improves consumer acceptability. Furthermore, EPS has been linked with health-promoting benefits such as anti-ulcer, cholesterol-lowering, and immunomodulatory effects, making these strains valuable components in functional foods [11].
A crucial aspect of the study was evaluating antimicrobial activity, where LAB strains exhibited substantial inhibition against E. coli and S. aureus. The strongest inhibitory effects were observed with S. thermophilus, L. lactis, and L. fermentum, consistent with previous research indicating LAB’s production of organic acids, bacteriocins, and other bioactive compounds [4]. This antimicrobial activity enhances food safety and suggests that LAB strains could effectively reduce the reliance on synthetic preservatives.
Additionally, EPS production appears to contribute to the antimicrobial efficacy of LAB, likely due to its role in forming protective biofilms that inhibit pathogen growth [19]. This is supported by research from Prabhurajeshwar and Chandrakanth [26], which reported similar antimicrobial efficacy of LAB against foodborne pathogens, suggesting that LAB can produce a range of bioactive compounds, including organic acids, hydrogen peroxide, and bacteriocins, that inhibit pathogenic growth. In particular, L. lactis is known for producing nisin, a potent bacteriocin with proven efficacy against various foodborne pathogens, reinforcing its application as a natural preservative [27].
The growing demand for natural and health-promoting ingredients in the food industry aligns with these findings, suggesting that incorporating EPS-producing LAB could offer a sustainable and safer alternative to synthetic preservatives. The review by Korcz et al. [16] highlighted the commercial potential of using LAB as a natural preservative, noting that their application can significantly extend the shelf life of dairy products while improving their nutritional profile.
Conclusion
The study demonstrated the probiotic potential of EPS-producing LAB from yoghurt, specifically S. thermophilus, L. lactis, and L. fermentum, showing significant antimicrobial activity against E. coli and S. aureus. Their EPS production suggests these LAB strains can act as natural preservatives, enhancing food safety and improving the texture, stability, and health benefits of fermented products. Additionally, EPS offers health benefits like anti-ulcer, cholesterol-lowering, and immunomodulatory effects, making them ideal for functional foods. The study highlights EPS-producing LAB as a natural preservative alternative to synthetic chemicals, meeting the demand for safer, health-promoting ingredients. Future research should optimize EPS production and test these strains in various food matrices to evaluate long-term stability, aiding the development of novel functional foods and preservatives that promote healthier choices.
Abbreviations
| BHI: | Brain-Heart Infusion |
| EPSs: | exopolysaccharides |
| LAB: | lactic acid bacteria |
| rRNA: | ribosomal RNA |
| w/v: | weight/volume |
Supplementary materials
The supplementary tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/101075_sup_1.pdf.
Declarations
Author contributions
NI: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing. SA: Validation, Software, Visualization, Writing—original draft, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The data is available in the supplementary materials. The data for each sample that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.