Affiliation:
Department of Nutrition and Dietetics, Fethiye Faculty of Health Sciences, Muğla Sıtkı Koçman University, Muğla 48300, Türkiye
ORCID: https://orcid.org/0009-0007-4720-6187
Affiliation:
Department of Nutrition and Dietetics, Fethiye Faculty of Health Sciences, Muğla Sıtkı Koçman University, Muğla 48300, Türkiye
Email: deryaalkan@mu.edu.tr
ORCID: https://orcid.org/0000-0003-0608-296X
Explor Foods Foodomics. 2025;3:101077 DOI: https://doi.org/10.37349/eff.2025.101077
Received: December 12, 2024 Accepted: February 23, 2025 Published: March 10, 2025
Academic Editor: Miguel Herrero, Institute of Food Science Research (CIAL-CSIC), Spain
Aim: Dried fruits are consumed by many people around the world as a convenient alternative to fresh fruits with a long shelf life. As well as dried fruits, the manufacturing of baked chips based on fruits having good nutrition and sensory properties represents an alternative to healthier food. The aim of this study is to determine the different chemical properties of dried fruits and fruit chips when they are being fried in ovens. Another aim was to evaluate the changes in total phenolic content (TPC), antioxidant activity, ascorbic acid and hydroxy methyl furfural (HMF) content of chips and dried forms.
Methods: In this study, apple, pear, orange, and kiwi were dried in a convection oven at 100°–120°C. Moreover, apple-orange (A-O) and kiwi-pear (K-P) chips were produced in order to develop an alternative product. Dry matter, ash, TPC, ascorbic acid and HMF contents, pH, total acidity and antioxidant activity were determined in fresh, dried and chips samples. Sensory analysis was also carried out in the prepared fruit chips samples using the hedonic scale test.
Results: The results revealed that dry matter and ash content increased in dried fruit and fruit chip samples. Drying caused a slight increase in pH and total acidity of all fruit samples. The ascorbic acid contents of kiwi and apple significantly decreased during the drying process. The drying process significantly impacted the total phenol content and antioxidant activity in dried slices. The dramatic increase of HMF was observed during oven-drying and chip production.
Conclusions: Based on results, it can be concluded that drying and baking processes had variable effects on the chemical, sensory and bioactive properties of fruit samples. Sensory analysis revealed that A-O chips were more acceptable in terms of sensorial properties compared to K-P chips.
With the influence of developing science and technology, consumers prefer foods that are beneficial for human health and easier to consume. As a result of technological developments in the food industry, alternative ready-made foods are produced to meet this trend of consumers. Snack foods constitute an important part of the eating habits of both children and adults [1]. Snack foods have become an integral part of modern diets, often consumed between meals or as a quick source of energy. The most important features of these snack foods are their lightness, long shelf life, and suitability for storage [2]. Snacks can be sweet or savoury, light or substantial, and they may possess qualities such as being healthy or purely for enjoyment. Sensory properties such as appearance, texture, smell, and taste are also gaining importance in snack foods that have their own quality criteria. The nutritional value of snack foods varies widely depending on their ingredients and preparation methods. While some snacks can provide essential nutrients, others may be high in sugars, unhealthy fats, and sodium. Snack foods include potato and corn chips, alkali-cooked corn tortilla chips, pretzels, popcorn, extruder-puffed and dried/fried products, half-products, animal product snacks, and cheese snacks [3, 4].
Dried fruits are considered as an alternative to unhealthy snacks [5, 6]. Epidemiological evidence has shown a relationship between dried fruit consumption and diet quality. Dried fruits contain a wide variety of phytochemicals such as phenolic acids, flavonoids, phytoestrogens and carotenoids. In particular, flavonoids, which are commonly found in fruits and vegetables, exhibit strong antioxidant activity [7]. Consuming foods such as fruits and vegetables that contain high amounts of antioxidants in the diet reduces the damage that can be caused by oxidation [8]. Various studies have reported that the bioactive components and antioxidant activities of dried fruits are higher than their fresh counterparts. This is because the antioxidants are concentrated after the dehydration process [9–11]. Fruit chips are one group of the dry food products that can extend the shelf life of fruit and increase its sales value. Dried fruits and vegetables have been sold in the market in the form of sliced fruit chips, and consumers use these products throughout the day, mainly as snacks. The manufacturing of fried chips based on fruits having good nutritional and sensory properties represents such an alternative. There are studies in the literature on many products such as cassava, papaya, banana, mango, and tortilla chips fortified with broccoli flour, and enriched gluten chips. The common aim of all these studies is to develop healthier products with desirable features that meet consumer demand [12–14]. Considering the growth in the chips market, it is seen that this is a necessity [15, 16]. Although drying of fruits is a common preservation method, it is known that drying has important effects on both chemical and physical properties of fruits. Changes that occur with drying can be expressed as the decrease in vitamin and mineral contents in dried fruits due to high temperature and long drying time. In addition, the nutritional value of dried fruits may decrease due to the degradation of nutritional components such as phenolic and flavonoid compounds and the decrease in antioxidant activity. Drying also affects the physical properties of fruits such as color, texture, and appearance [17, 18]. This study aims to investigate the effect of drying process on the different chemical, sensory and bioactive properties, as well as antioxidant activity of dried fruits and fruit chips during oven frying. The information obtained from this study will be useful for consumers and manufacturers who try to obtain attractive and healthy snacks.
In this study, apple, orange, kiwi, and pear were selected to obtain dried fruit and fruit chips because they are valuable sources of vitamins (Vit A, Vit C, folate, etc.) and phenolic compounds (chlorogenic acid, naringenin, quercetin, protocatechuic acid, p-hydroxybenzoic acid, etc.). Besides their nutritional values, their availability in all seasons has been effective in choosing these fruits. Apple, orange, kiwi, and pear were obtained from the market in Fethiye, Türkiye. Two different types of fruit chips were produced in different formulations using apple, orange, kiwi, and pear. In the production of fruit chips, apple pectin and starch were used as thickeners, citric acid and ascorbic acid were used as acidity regulators and preservatives. Maltodextrin and water were used to give crispness to the chip form. No sweeteners used in formulations. The formulations of apple-orange (A-O) and kiwi-pear (K-P) fruit chips are given in Table 1. Fruit mixtures prepared for the production of chips were placed in heat-resistant molds with an inner diameter of 5 cm and a depth of 3 cm, dried by controlled drying at 120°C for 2 h (ILD-EKH-55, Ildam, Türkiye) and then frying at 180°C for 2 min. Except for fruit chips, all fruits were sliced into 0.5 cm thick slices using a stainless-steel slicing tool and dried after being immersed in a solution containing 0.3% citric acid and 0.05% ascorbic acid for 15 min. The sliced fruit samples were dried at 100°C for the first 2 h and then at 120°C for approximately 6 h [19]. All analyses were performed immediately following sample preparation.
Ingredients used in the production of fruit chips
| Sample codes | Ingredients | Amount (g/100 g) |
|---|---|---|
| A-O* | AppleOrangePectinStarchCitric acidAscorbic acidMaltodextrinWater | 3939170.30.051.6512 |
| K-P* | KiwiPearPectinStarchCitric acidAscorbic acidMaltodextrinWater | 3939170.30.051.6512 |
K-P*: kiwi-pear chips; A-O*: apple-orange chips
Dry matter (DM) contents of dried fruits and fruit chips were determined gravimetrically by drying them to the constant weight at 105°C in the drying oven (ILD-EKH-55, Ildam, Türkiye). After dehydration, the dry weight was measured and percentage of DM content was calculated from Equation 1. DM determination was repeated three times for each sample.
For ash determination, approximately 5 g of dried samples were carbonized in the muffle furnace (Protherm furnaces, PLF 110/6) at 550°C for 6 h until white ash was obtained. The percentage of ash content was calculated using Equation 2. Ash determination was repeated three times for each sample (AOAC, 1997).
For pH measurements, 10 g of the samples were weighed and a 10% mixture was prepared with pure water in a volumetric flask. pH measurements of the samples were made by immersing the glass electrode of pH meter (Mettler Toledo, Five easy plus FP20) directly into the sample at 25°C [20].
Total acidity of the samples was determined by titration method described in the study of Bayrakdar [19]. After diluting 10 g of the samples with 100 mL of distilled water, it was filtered on filter paper to remove the solid particles. Total acidity was measured by titrating an aliquot (10 mL) of the samples with 0.01 N NaOH until a pink color was observed. The results were expressed as % citric. Measurements were performed in triplicate.
Extraction of phenols from dried fruits and fruit chips samples was carried out according to the method described by Zhu et al. [21] with some modification. Two grams of small pieces of sample were mixed with 10 mL of methanol solution (80% v/v) for 1 h, and then centrifuged at 7,000 rpm for 10 min, and the supernatant was obtained. The total phenolic content (TPC) of the samples was determined by using the Folin-Ciocalteu (1.09001.0100, Supelco, Germany) reagent as described in the study of Singleton and Rossi [22]. The absorbance was measured at 765 nm by using a spectrophotometer (Agilent Cary 60 UV-VIS). Phenolic content was expressed as mg of gallic acid equivalents (GAE) per gram of the extract (mg GAE g–1).
The antioxidant activity was determined by the standard 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method modified by Ankelokar et al. [23]. For the determination of antioxidant activity, the samples were extracted with methanol (80% v/v) by using a shaker at room temperature for 1 h. Then the extracts were centrifuged at 7,000 rpm for 10 min. A 250 μL aliquot of the sample extract was mixed with 1,250 μL of DPPH (60 μM in ethanol). The reaction mixture was shaken and incubated in the dark for 30 min. The absorbance was measured at 517 nm using the spectrophotometer. The readings were compared with the controls, containing 95% ethanol instead of sample extract. The percentage inhibition was calculated by:
First, 10 g of fruit and chips samples were weighed; 70 mL of 4% oxalic acid solution (060121-ALF-578, Kimbiotek, Alfasol, Türkiye) was added, then mixed and filtered. Then, as a reference solution, 1 mL of oxalic acid solution and 9 mL of dye solution (2.6 dichlorophenolinedephenol; 102442190, Sigma Aldrich, Germany) were mixed, and permeability values (L1) were determined by reading at 520 nm in the spectrophotometer. The same process was applied to the mixture of 1 mL filtrate and 9 mL dye solution, the second permeability value was determined (L2) and the amount of ascorbic acid (mg/100 g) was determined by taking the difference of the two values obtained [19].
For the determination of hydroxymethylfurfural (HMF) in fruits and chips, 20 g of sample was transferred to a 100 mL measuring flask and completed to 100 mL with pure water. After the samples were mixed well, they were filtered. Two millilitres of the filtrate were taken into three test tubes, and 5 mL of the p-toluidine reagent (N2OZI-RK, TCI, Japan) and 1 mL of barbutyric acid solution (AKJ8M-JH, TCI, Japan) were added to the first two tubes. The blank was prepared by adding 5 mL of p-toluidine reagent and 1 mL of pure water to the 3rd tube. After the tubes were mixed with vortex, the absorbance of the samples was determined within 3 min by reading against the blank at 550 nm [20].
Produced fruit chips were used for the sensory test [24]. Ten adult and healthy panelists from Fethiye Faculty of Health Sciences participated in the sensory panel. The panelists were informed about the details and implications of participating in the experiment. A written consent was obtained from all participants. For two fruit chips, the participants took the preference test of appearance, aroma, taste, color, texture, and overall acceptability using a 9-point hedonic scale (1: extremely dislike, 9: extremely like). This procedure was applied with the permission of Muğla Sıtkı Koçman University Ethics Committee (protocol no: 210063).
Data from parallel measurements obtained from the physicochemical and sensory properties of the studied samples were expressed as mean ± standard deviation (SD). All data were analyzed by one-way analyses of variance using the IBM SPSS Statistical Software (SPSS 22 Inc. Chicago, IL, USA). The significant means were compared by Tukey’s multiple range tests (P < 0.05).
DM and ash content, pH and total acidity of fresh fruit, dried fruit and fruit chip samples are shown in Table 2. It is clearly seen that the dried and chip forms of all fruit samples have higher DM content than their fresh forms. Significant differences were noted between the analyzed samples (P < 0.05). The DM content of samples ranged from 15.60% to 96.34%, with the highest DM content being found in K-P chip (96.34%), followed by A-O chip (94.55%) and the least in fresh orange (15.60%). Table 2 shows that the ash content in all fruits increased significantly (P < 0.05) during the drying process. Dry apple sample had significantly higher ash content of 3.51%. The ash contents of fruit chips were found to be lower than the ash contents of dried fruit samples. pH values of fresh fruits ranged from 2.84 to 4.25, the highest pH being in apple and the lowest in orange (Table 2). The pH value of fresh apple was in average 4.25, being a fruit with low acidity compared to other studied fresh fruits. Total acidity of fresh kiwi (1.13%) was high compared to fresh orange (0.88%), apple (0.26%) and pear (0.22%). However, the dried and chip samples had higher total acidity than the fresh samples.
Dry matter (%), ash (%), pH and total acidity (%) values of the samples
| Samples | Dry matter | Ash content | pH | Total acidity (%) |
|---|---|---|---|---|
| K | 15.84f ± 0.01 | 0.71cd ± 0.16 | 2.97g ± 0.03 | 1.13cd ± 0.08 |
| P | 18.15e ± 0.10 | 0.16d ± 0.06 | 3.93c ± 0.02 | 0.22e ± 0.08 |
| O | 15.60f ± 1.00 | 0.45cd ± 0.03 | 2.84h ± 0.09 | 0.88d ± 0.04 |
| A | 18.25e ± 0.04 | 0.19d ± 0.00 | 4.25a ± 0.06 | 0.26e ± 0.07 |
| DK | 93.90bc ± 0.29 | 2.80ab ± 0.85 | 3.12f ± 0.00 | 1.99b ± 0.07 |
| DP | 87.34d ± 0.29 | 2.30abc ± 0.92 | 4.06b ± 0.02 | 0.94d ± 0.03 |
| DO | 92.80c ± 0.42 | 1.93abcd ± 0.82 | 3.45d ± 0.01 | 2.05b ± 0.06 |
| DA | 86.75d ± 0.46 | 3.51a ± 0.93 | 4.12b ± 0.04 | 0.88d ± 0.04 |
| K-P | 96.34a ± 0.08 | 1.52bcd ± 0.03 | 2.97g ± 0.04 | 1.41c ± 0.13 |
| A-O | 94.55bc ± 0.05 | 1.05bcd ± 0.13 | 3.27e ± 0.02 | 2.52a ± 0.27 |
Results are presented as mean ± standard deviation (SD), and different letters within columns indicate statistically significant differences (P < 0.05) by Tukey’s multiple range test. K: fresh kiwi; P: fresh pear; O: fresh orange; A: fresh apple; DK: dried kiwi; DP: dried pear; DO: dried orange; DA: dried apple; K-P: kiwi-pear chips; A-O: apple-orange chips
Results of experimental data for the ascorbic acid, total phenol content, HMF and antioxidant activity of studied samples are shown in Table 3 and Figure 1. Ascorbic acid is an important nutritional parameter for dried food products [18]. Fresh fruits used in this study had almost the same ascorbic acid content. There were no significant differences (P > 0.05) for the ascorbic acid contents of fresh fruits (Table 3). It is observed that ascorbic acid changes in dried and chip samples differ. It was found that there was a significant decrease in the ascorbic acid content of kiwi and apple with the drying process, while there was no change in the ascorbic acid content of pear (Figure 1C). The drying process has significant effects on the TPC of fruits and vegetables. Figure 1B shows the total phenol content changes of fruits during the drying and chips production process. An ANOVA showed that the total phenol content was significantly different (P < 0.05) among the samples. Higher TPC values of in average 73.62 and 50.02 mg GAE g–1 were observed in fresh orange and A-O chips (Figure 1B). DPPH is a free radical compound that has been widely used to determine the free radical scavenging capacity of various samples because of its stability, simplicity and fast assay [25]. In terms of antioxidant activity, a clear difference was observed between fresh fruits and dried fruit & fruit chips samples (P < 0.05). The highest decrease in antioxidant activity with drying was observed in kiwi (from 98.91% to 19.99%) and orange (from 98.94% to 26.12%) samples (Figure 1A). HMF content in fruit products provides information about the process and the heat treatment applied [26]. The contents of HMF in analyzed samples presented significant differences (P < 0.05). The amounts of HMF in fresh fruits were found to be lower than other samples, similar to each other (Figure 1D).
Ascorbic acid, total phenolic, antioxidant activity and HMF values of the samples
| Samples | Ascorbic acid (mg/100 mL) | Total phenol content (mg GAE g–1 extract) | Antioxidant activity (%) | HMF content (mg/L) |
|---|---|---|---|---|
| K | 19.59cd ± 1.67 | 44.47abc ± 14.11 | 98.91a ± 0.02 | 7.23d ± 0.21 |
| P | 19.89cd ± 1.01 | 24.99bc ± 8.67 | 98.94a ± 0.00 | 6.39d ± 0.38 |
| O | 24.12cd ± 0.51 | 73.62a ± 20.93 | 98.94a ± 0.00 | 10.63d ± 0.02 |
| A | 23.77cd ± 0.42 | 44.69abc ± 13.28 | 98.99a ± 0.01 | 23.05d ± 1.10 |
| DK | 7.79e ± 1.42 | 19.56bc ± 0.92 | 19.99de ± 13.83 | 440.62b ± 55.01 |
| DP | 20.09cd ± 2.05 | 6.58c ± 0.48 | 49.64c ± 6.22 | 596.14a ± 64.21 |
| DO | 68.24a ± 9.78 | 29.94bc ± 1.41 | 26.12d ± 9.53 | 355.55b ± 47.16 |
| DA | 17.28de ± 0.78 | 34.91abc ± 3.66 | 75.71b ± 0.49 | 321.04bc ± 59.10 |
| K-P | 38.89b ± 6.18 | 24.34bc ± 1.23 | 46.07c ± 5.17 | 175.05c ± 32.27 |
| A-O | 30.67bc ± 3.55 | 50.02ab ± 0.23 | 5.25e ± 5.21 | 296.22bc ± 2.66 |
Results are presented as mean ± standard deviation (SD), and different letters within columns indicate statistically significant differences (P < 0.05) by Tukey’s multiple range test. K: fresh kiwi; P: fresh pear; O: fresh orange; A: fresh apple; DK: dried kiwi; DP: dried pear; DO: dried orange; DA: dried apple; K-P: kiwi-pear chips; A-O: apple-orange chips
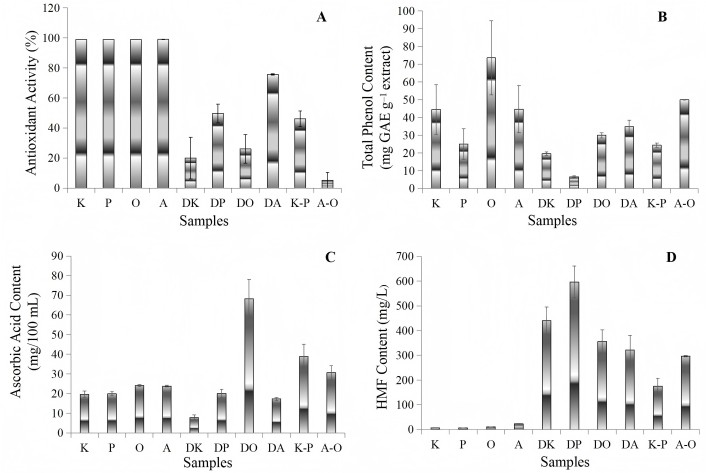
Chemical analysis results of products. Antioxidant activity (A), total phenolic content (B), ascorbic acid (C), and HMF (D) values of the samples. K: fresh kiwi; P: fresh pear; O: fresh orange; A: fresh apple; DK: dried kiwi; DP: dried pear; DO: dried orange; DA: dried apple; K-P: kiwi-pear chips; A-O: apple-orange chips
The sensory scores of two fruit chips are given with a radar graph in Figure 2. The radar graph summarized the mean scores of hedonic sensory evaluation for appearance, aroma, taste, texture, color, and general acceptability of the fruit chip samples. The sensory evaluation based on hedonic scale test revealed that A-O chip sample was more acceptable than K-P chip sample. Highest rating scores were recorded for A-O chip in all sensory characteristics including general acceptability. It is observed that fruit chip samples were evaluated with higher scores by the panelists, especially in terms of color and appearance characteristics.
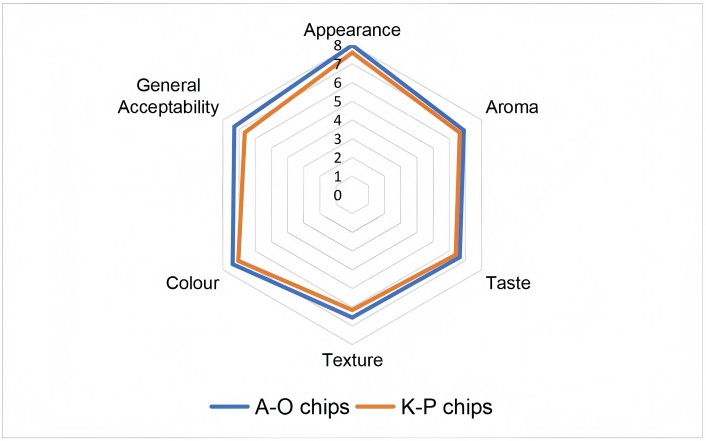
Radar graph of hedonic sensory evaluation of fruit chips. K-P: kiwi-pear chips; A-O: apple-orange chips
When the DM results of fresh fruit samples were compared, apple was found to have the highest DM content, followed by pear, kiwi and orange. However, it was observed that the drying process increases the DM content of orange (494.87%) and kiwi (492.80%) more than pear (381.21%) and apple (375.34%). This result shows that the moisture of pear and apple was more difficult to remove during drying than for orange and kiwi. However, it is observed that the process of obtaining chips from fruits increases their DM content even more. This difference could be explained by differences in structure, soluble solids, and texture. The drying process lowers the high moisture content of fresh fruits and concentrates the DM content such as organic acids and sugars, extending their shelf life [27]. Dag et al. [28] described that the DM content of apricot increased to 90% by sun drying. This value is lower than our findings for dried kiwi (93.90%), dried orange (92.80%), K-P fruit chips (96.34%), and A-O fruit chips (94.55%). Krajewska et al. [29] investigated the effects of contact-drying and freeze-drying at various hotplate temperatures on the drying kinetics of pear pomace. They found higher DM content for pear pomace (92.10–93.69%) compared to the present study. The microwave-assisted contact drying process at 40°C was found to be the fastest among the selected drying methods.
As a result of the drying process, the ash content of apple and pear increased by 1,747.37% and 1,337.5%, respectively. It was also observed that the ash contents of the chip samples were higher than those of fresh fruits. In the study by Eze and Akubor [30], the effects of blanching and drying methods on the physicochemical properties of okra (lady’s fingers) were studied. The researchers found the decreases in the crude fibre, crude fat and crude carbohydrate contents of the dried samples on dry weight basis. However, there were increases in the ash contents of the dried samples. The increase in the ash content of the dried samples could be linked to the decreases in the other components such as crude fibre, crude fat and crude carbohydrate.
It is observed that the pH values of dried fruits, except for apple, are statistically higher than their fresh forms (P < 0.05). This result shows that the acidity rates of the fruits decrease with drying. However, the increase in pH value is less observed in prepared fruit chips. In particular, the pH value of the chip sample prepared with kiwi and pear (2.97) was found to be the same as the pH value of fresh kiwi (2.97). Among all dried fruits, fruit chips and fresh fruits, the highest total acidity was found in A-O chips (2.52%). Removing moisture during the drying process concentrates the DM content of dried fruits, such as organic acids and sugars, effecting their pH and total acidity [24].
Ascorbic acid content of dried orange (in average 68.24 mg/100 mL) was found to be higher than its fresh product (in average 24.12 mg/100 mL). Also, fruit chip samples contained significantly higher amounts of ascorbic acid (in average 38.89 mg/100 mL for K-P chips and 30.67 mg/100 mL for A-O chips) compared to other samples, except for dried orange. This increase in the content of the ascorbic acid was due to ascorbic acid oxidase inactivation during heating leading to ascorbic acid protection towards enzymatic oxidation as previously observed in cherries, nectarines, apricots, peaches, plums, carrots [31] and tropical fruits [32].
Total phenol contents of all fruits except for apple decreased significantly with the drying process. There were no significant variations in TPC of fresh apple and dried apple samples (P > 0.05). Loss of phenolic compounds during drying processes may be caused by the activation of oxidative enzymes such as polyphenoloxidase and peroxidase, as well as the binding of phenolic compounds to proteins, changes in their chemical structures or low extraction efficiencies [33]. Akturk Gumusay et al. [34] reported that tomatoes dried using the thermal method had lower phenolic content than that of fresh tomatoes. The impact of different drying processes (air dried, oven dried, vacuum dried) on the concentration of phenolic compounds of “Redfield” apple tissue has been investigated [35]. Although the concentration of individual phenolic compounds was influenced by drying, the TPC measured by the Folin-Ciocalteu assay in the apple slices was not affected by drying. This result is in good agreement with our findings for dried apple and A-O fruit chips.
As a result of our study, it was found that the antioxidant activity of fresh apple (from 98.99% to 75.71%) decreased slightly with drying. Fruits and vegetables contain many different antioxidant components. The sample with the lowest antioxidant activity was A-O chips with a value of in average 5.25%. The antioxidant activity is most significantly correlated with the contents of total phenolics. Shofian et al. [32] evaluated the antioxidant activity of the methanolic extract of different tropical fruits by free radical scavenging assay. They found that there were no significant (P > 0.05) differences observed for free radical scavenging activity between fresh and freeze-dried fruits, except for fresh starfruit, which showed significantly (P < 0.05) higher scavenging activity compared to the freeze-dried sample. The effect of drying (air-, oven- and vacuum-drying) on antioxidant capacity of slices of “Redfield” apple has been measured using oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assay [35]. The antioxidant capacity measured using ORAC assay remained constant for vacuum and air-dried slices but lowered significantly in oven-dried apple slices. However, this difference was not observed when the antioxidant capacity was measured using the FRAP assay.
It was observed that drying and chip production processes significantly increase the HMF content of the samples. In particular, dried fruits were found to have higher HMF amounts than chip samples. The highest content of HMF was observed in dried pear with a value of 596.14 mg/L. It is known that the formation of HMF in foods is affected by the pH value, the duration and intensity of the applied heat treatment, and the contents of sugar, protein and other substrates in the product. In our study, the highest HMF formation observed in pear fruit may be associated with its high fructose, sucrose, and glucose sugars content [36]. At the same time, chlorogenic acid was identified as the major phenolic compound in pear fruit. There is information in the literature that the presence of chlorogenic acid in plant materials will contribute to the formation of HMF during thermal processing. The dramatic increase in the HMF content of pear may be related to the chlorogenic acid it contains [36]. Similar to our findings, exposure of Asian pear samples to prolonged drying under hot air drying and infrared drying caused a dramatic increase in HMF [34]. Wojdyło et al. [26] demonstrated significantly higher content of HMF in red-fleshed apple fruit snack samples exposed to vacuum-microwave drying versus the other analyzed methods (freeze drying, convective drying and hybrid drying). HMF concentration rose together with increasing microwave power during vacuum-microwave drying and increasing temperature during convective drying. They also reported low correlation coefficients for the content of sugars and HMF, for fructose (r2 = 0.255), glucose (r2 = 0.227), sorbitol (r2 = 0.382) and sucrose (r2 = 0.416).
In conclusion, dried fruits and fruit chips are recommended by nutritionists as healthy snacks. In the study, dried apple, pear, orange, kiwi, and two fruit chips were analyzed in terms of some chemical, sensory, and bioactive properties. Oven-drying resulted in significant loss of total phenol and ascorbic acid content and antioxidant activity. Fruit chips production resulted in better retention of ascorbic acid and total phenolics than the oven-drying process. In addition, the increase in the amount of HMF was found to be lower in fruit chip samples than in oven-dried fruit samples. Based on sensory evaluation of fruit chips, it can be concluded that A-O chips sample was more acceptable in terms of all sensory parameters. Drying is one of the most used methods for preserving fruits, but it is very important to find the most appropriate drying method to preserve the quality characteristics of dried fruits. From the consideration of the scope of the present study, it turns out that it included chemical and sensory properties of oven-dried fruits and fruit chips, however, it did not include different drying methods. For this reason, other potential alternative processes could be examined for manufacturing fruit chips and drying fruits that can provide the benefit of retaining the heat-sensitive Vitamin C and bioactive compounds in fruits.
A-O: apple-orange
DM: dry matter
DPPH: 2,2-diphenyl-1-picrylhydrazyl
FRAP: ferric reducing antioxidant power
GAE: gallic acid equivalent
HMF: hydroxymethylfurfural
K-P: kiwi-pear
ORAC: oxygen radical absorbance capacity
TPC: total phenolic content
We appreciate the Fethiye Faculty of Health Sciences in Muğla Sıtkı Koçman University for enabling us to use their laboratory facilities.
DA: Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing. BK: Funding acquisition, Data curation, Formal analysis, Conceptualization.
The authors declare that they have no conflicts of interest.
Sensory analysis in this study was carried out with the permission of Muğla Sıtkı Koçman University Ethics Committee (approval no: 210063).
A written consent was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This project was supported by TUBITAK (the Scientific and Technical Research Council of Turkey) [project ID: TUBITAK-2209A-1919B012102629]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
