Abstract
Aim:
Livestock production plays a significant role in meeting global protein demands but is a major contributor to climate change. With the world population projected to reach 9 billion by 2050, identifying sustainable alternative protein sources has become more critical than ever. Edible insects offer an affordable protein option compared to beef, chicken, and fish, especially in many African and Asian cultures, where these conventional protein sources are considered relatively expensive. This study aimed to investigate the potential of mulberry silkworm pupae and African palm weevil larvae as alternative proteins to conventional protein sources for use in gluten-free wraps.
Methods:
Five gluten-free breakfast wraps were developed using oat flour and fillings made from beef, chicken, mackerel fish, palm weevil larvae, and silkworm pupae. The nutritional composition (amino acid and fatty acid profiles, micronutrient contents) and chemical, microbial, and sensory properties were determined using standard methods.
Results:
The wraps had protein contents ranging from 23.78% to 35.60%. Breakfast wrap with palm weevil larvae had slightly more fiber (4.01%) and carbohydrate (36.11%) contents and lower fat (10.22%) compared to the other wraps. It also had an impressive vitamin A content (528.96 μg RAE/100 g) and an exceptional amino acid profile. The insect wraps had more vitamin B12 (0.02 mg/g) contents than the conventional wraps. The fish-based version was the most preferred of all the wraps, with an overall acceptability score of 7.80. All developed products were within permissible limits for microbial quality.
Conclusions:
Edible insects, such as palm weevil larvae and silkworm pupae, could serve as an alternative source of protein in the production of gluten-free foods.
Keywords
Oat breakfast wrap, conventional proteins, silkworm pupae, palm weevil larvaeIntroduction
Oat, an underutilized cereal grain, ranks seventh among the most produced grain crops in the world, behind corn, wheat, rice, barley, sorghum, and millet [1–4]. Oats predominantly comprise carbohydrates, with 14% protein, 6% fat, and 4% beta-glucan [5]. Oats are rich sources of bioactive compounds, including avenanthramides, avenacosides, avenacins, flavonoids, lignans, phenolic acids, saponins, sterols, and tocols, which have high antioxidant capacity [3, 6]. According to Tosh and Bordenave [7], the dietary fibers and phytonutrients present in oats play a particular role in the prevention of cardiovascular diseases by reducing serum low-density lipoprotein and type 2 diabetes, regulating postprandial blood glucose levels and helping to maintain the gut health. Oats have tremendous potential for development into functional foods and food additives as the only cereal classified by the World Health Organization (WHO) as healthy [4]. Hence, developing food products from oat grain would improve nutrition and serve as a therapy against various diseases [8].
Wraps are unleavened, flat, and circular bread 1–2 mm thick, usually made of white bread flour [9]. Flatbreads include pancakes, pizzas, crepes, chapattis, and tortillas. Flatbreads are becoming more popular due to increasing population size and urbanization, where people desire ready-to-eat and convenient meals on the go [10]. In response to this demand, some fast-food companies have developed innovations such as flatbread wraps and sandwiches. Flat breads can be a great carrier of other nutrient-dense dietary items when used for toast, sandwiches, and rolled up as wraps [11]. Various ingredients that can be incorporated into wraps include vegetables, spices, condiments, and particularly protein sources such as beef, fish, chicken, etc.
Conventional sources of dietary proteins are often thought to be of plant or animal origin. Animal sources such as fish, chicken, and beef provide a complete source of protein [i.e., containing all essential amino acids (AA)] but significantly contribute to greenhouse emissions, deforestation, and extensive water usage. On the other hand, vegetable sources generally lack one or more of the essential AA. Edible insects are often overlooked, and many are unaware of the nutritional importance of insects. The search for alternative food sources, especially protein, to meet the nutritional demand of the projected 9 billion world population by 2050 is now critical. Edible insects can serve as an alternative source of protein in many African and Asian cuisines where beef, fish, and chicken are perceived to be relatively expensive.
Edible insects are a quality and quantity protein source that can enrich human diets across all climes [12]. Edible insects have been proposed as a preserver of the ecosystem through limited water requirement, less greenhouse gas emissions, and minimal or no deforestation to set up insect rearing centers, etc. [13]. African palm weevil (Rhynchophorus phoenicis) larvae are the most consumed in Africa and have a high protein content. The protein content of silkworm pupae is about 21.5%, higher than that of usual animal products. On a dry-weight basis, the protein content of silkworm pupae has been reported to be as high as 49–54% [14]. Insects’ proteins are considered complete proteins because of the high content of essential AA and high digestibility. In fact, silkworms contain all the AA required by the human body and in the appropriate proportions based on the recommendations of the FAO/WHO [15].
Although different products have been developed from palm weevil larvae (PWL) and mulberry silkworm pupae (SWP), such as high-energy biscuits [16], pie and samosa [17], and tomato paste [18], the development and characterization of more edible insect-enriched products will encourage the use of insects as an alternative source of protein and enlighten consumers on its nutritional benefits. This study was intended to develop gluten-free (GF) oat-based breakfast wraps using different protein sources (namely beef, chicken, fish, PWL, and silkworm pupae) and determine the AA profile, fatty acid profile, some vitamins and minerals, microbial quality, sensory properties and oxidative stability of the five wraps.
Materials and methods
Source of materials
Canned GF (old-fashioned) oat was purchased from Shoprite supermarket, Akure. Raw PWL (Rhynchophorus phoenicis) were purchased from Ajagba market, Irele local government, Ondo State. Silkworm pupae (Bombyx mori) was procured from the Wealth Creation Agency (WECA) sericulture section of Akure, Ondo state, Nigeria. All other ingredients, such as beef, chicken, fish, cabbage, carrots, pepper, tomatoes, seasonings, salt, and vegetable oil, were sourced from local markets in Akure locality, Ondo State, Nigeria. All chemical reagents used were of analytical grade.
Preparation of raw materials
Protein sources
The PWL were sorted and degutted by cutting off the edible insects’ black end (fecal part). It was then washed adequately in potable water and drained. Two grams of seasonings and a pinch of salt were added to 400 g of edible insects and stir-fried on low heat for 15 min. The stir-fried insects were set aside for further preparation.
The silkworm pupae were thoroughly sorted. The discolored (black) insects were removed, and the others that retained their natural brown color were further processed. About 400 g of the pupae were thoroughly washed, minimally salted, seasoned (2 g), and stir-fried on low heat for 15 min. After cooling, the insects were set aside for later use.
The conventional proteins (beef, chicken, and mackerel fish) were obtained in small cut sizes from the market. The beef, chicken, and fish were prepared separately. The fish was degutted, and the fins were removed. After thoroughly washing with potable water, each protein source (400 g) was prepped with 4 g seasonings, 4 g salt, 30 g of diced onions, and 30 mL of water. The meat, chicken, and fish were boiled for 10 min under medium heat and later stir-fried for 5 min under low heat.
Other ingredients: The outer layer of the carrot was scraped and then washed before grating. The grated carrots were then stir-fried in 20 mL of soya bean oil for 5 min and divided into five portions after cooling. The outer layer of the cabbage was removed and washed in a mild salt solution before shredding with a sharp knife into thin layers. The pepper sauce was prepared by blending tomatoes, habanero pepper, and onion bulbs (60 g). The resultant mixture was fried in 35 mL of soya bean oil with a pinch of salt and 4 g of seasoning cube for 15 min.
Preparation of the fillings
Each filling (Figure S1) was prepared by mixing 150 g shredded cabbage, 80 g stir-fried carrot, 70 g protein source (beef, fish, chicken, PWL or silkworm pupae), and 35 g pepper sauce. A total of five different fillings were then obtained.
Oat-based break wrap
The old-fashioned oat (285 g) was weighed in three batches into the cup of the grinder (1,500 W Euro Premium Domestic mixer/grinder, India) and 1,275 mL of potable water was used in blending in total. The grinding period took a total of 30 min until a smooth texture was obtained. The slurry was carefully transferred into a bowl, and 0.5 g of table salt was added with a little stir. The mixture was allowed to rest for 20 min. After resting, a full scoop of the batter was evenly spread into the pre-heated non-stick frying pan and heated on both sides for 4 min each. The resultant flatbread was 40 g in weight after cooling. The prepared fillings (20 g) (Figure S1) were then incorporated into different flatbreads and wrapped up to obtain the GF oat-based break wraps (Figure S2). Five wraps were obtained based on the protein sources (Table 1).
Proportion of breakfast wrap ingredients and fillings
| Ingredients | Samples (g) | ||||
|---|---|---|---|---|---|
| Beef | Chicken | Fish | PWL | SWP | |
| Beef | 70 | ||||
| Chicken | - | 70 | - | - | - |
| Fish | - | - | 70 | - | - |
| PWL | - | - | - | 70 | - |
| SWP | - | - | - | - | 70 |
| Carrot | 80 | 80 | 80 | 80 | 80 |
| Cabbage | 150 | 150 | 150 | 150 | 150 |
| Pepper sauce | 35 | 35 | 35 | 35 | 35 |
| GF oat wrap (after cooking) | 40 | 40 | 40 | 40 | 40 |
PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae; GF: gluten-free
Analyses
Proximate composition
The AOAC [19] standard methods were employed to evaluate the proximate composition of the five breakfast wraps. Hexane was used for fat extraction using the Soxhlet method. The Kjeldahl method was employed to determine the nitrogen content of the samples. The nitrogen was converted to protein using 6.25 as the conversion factor. Crude fiber content was determined by the acid and alkali digestion of the samples. To obtain percentage ash, the samples were incinerated at 550°C in a muffle furnace (Carbolite, S33-6RB, Hope, England). The GF wraps were oven-dried until constant weights were obtained to obtain the moisture content. All analyses were replicated.
Mineral elements
The nutritive elements of the breakfast wraps were determined using the AOAC methods [20]. One gram of the sample was digested with nitric/perchloric/sulphuric acid (9:2:1, v/v/v) and filtered. The obtained filtrate was made up to mark (5 mL) in a volumetric flask. Iron, calcium, zinc, magnesium, and selenium were determined using an Atomic Absorption Spectrophotometer. Phosphorus was determined using the Vanado-molybdate method. For sodium and potassium, a flame photometer (Sherwood Flame Photometer 410; Sherwood Scientific Ltd., Cambridge, UK) was used, and sodium chloride and potassium chloride were used as standards.
Vitamins
Determination of vitamin A
One gram of the milled breakfast wraps was mixed with 30 mL of absolute alcohol and 3 mL of 5% potassium hydroxide. The resultant mixture was allowed to boil gently under reflux for 30 min and cooled rapidly. Thirty milliliters of water were added to the mixture and transferred into a separator to wash it with petroleum ether. It was washed in 3 × 50 mL ether, and the mixture was extracted for 1 min with careful shaking. When clear separation was achieved, the bottom layer was gradually run off, and the extract was washed with 4 × 50 mL water with cautious mixing during the first two washings. The resultant extract was evaporated to about 5 mL, and residual ether was removed at room temperature in a nitrogen stream. The residue was dissolved in sufficient isopropyl alcohol to obtain a solution containing nine to fifteen units per milliliter. Extractions were measured at wavelengths of 300, 310, 325, and 334 nm [21].
Determination of vitamin B1 (thiamine)
Five grams of samples (with beef, chicken, fish, PWL, and silkworm pupae) were homogenized with 50 mL of 0.2 M ethanoic sodium hydroxide. It was filtered into a 100 mL conical flask, 10 mL of the filtrate was pipetted, the color was developed by adding 10 mL of 1% potassium dichromate, and the absorbance read at 360 nm. A blank solution was also prepared [22].
Determination of vitamin B12
Milled breakfast wraps (0.2 g) were extracted for 30 min in a boiling water bath in 10 mL of extraction buffer (8.3 mmol/L sodium hydroxide/20.7 mmol/L acetic acid, pH 4.5) and l00 μL of Na-cyanide (1% w/v in water). After cooling, the extract was centrifuged twice, and the supernatants were combined. The extract was filtered, the pH was adjusted to 6.2, and the volume was set to 25 mL [23]. Standard solutions were prepared at 15–35 µg/mL concentrations, and their absorbances were measured at 354 nm and calibration curves were plotted using absorptivity coefficient values. The samples’ absorbance and absorptivity (A 1%, 1 cm) were measured at the selected wavelength. The wavelengths were then determined as the mean of three independent determinations. Sample concentrations were then obtained.
Determination of vitamin C
The vitamin C content of the breakfast wraps was determined according to the method of Benderitter et al. [24]. One gram of the samples was dislodged in 10 mL distilled water, allowed to stand for 5 h, and decanted. After this period, about 200 µL of the extract was pipetted and mixed with 300 µL of 13.3% trichloroacetic acid (TCA) and 75 mL of 2,4-dintrophenylhydrazine (DNPH). The mixture was incubated at 37°C for 3 h, then 0.5 mL of 65% H2SO4 was added and the absorbance was read at 520 nm. Ascorbic acid was used as the reference compound.
Determination of vitamin E
One gram of the samples was placed in a 100 mL flask fitted with a reflux condenser. Ten milliliters of absolute alcohol and 20 mL of 1 M alcoholic sulphuric acid was added to it. The mixture was refluxed for 45 min and cooled. Water (50 mL) was added to the mixture and transferred into a separating funnel of low actinic glass with 50 mL of water. The unsaponifiable matter of the mixture was extracted using 5 × 30 mL diethyl ether, washed, and combined with ether extract free from acid, and dried over anhydrous sodium sulfate. The extract was then evaporated at a low temperature. With protection from sunlight, the residue was dissolved in 10 mL absolute alcohol. The standard and the sample were transferred into a 20 mL volumetric flask, 5 mL of absolute alcohol was added to it, followed by 1 mL of concentrated nitric acid. Then, the flask was placed in a water bath for three minutes at 90°C. Cooling was carried out under running water, and the volume was made up to 20 mL with absolute alcohol. The absorbance was measured at 470 nm against a blank containing absolute alcohol [19].
Amino-acid profile
The AA profiles of the five wraps were determined using Applied Bio-systems PTH Amino Acid Analyzer following the procedure of AOAC [20]. A known sample was dried to constant weight, defatted using chloroform/methanol mixture of ratio 2:1 in a Soxhlet extraction apparatus for 15 h, hydrolyzed with 7 mL of 6 N HCl, evaporated in a rotary evaporator and loaded into the Amino Acid Analyzer. One hundred and 15 mg of the ground samples were then analyzed for nitrogen. Tryptophan in the wraps was determined as described by Yust et al. [25]. Samples were hydrolyzed with sodium hydroxide (4 M), dried, and defatted appropriately before hydrolysis. The hydrolyzed samples were evaporated in a rotary evaporator before the AA analysis using the analyzer. Protein indices such as total AA, total essential AA, total sulphur AA were calculated from the amino acid profile.
Fatty acid profile
Samples were extracted using the Soxhlet extraction method [19]. Fifty milliliters of the extracted oil were esterified using 3.4 mL of 0.5 M KOH in dry methanol at 95°C for 5 min. Neutralization of the resultant mixture was done using 0.7 M HCl. Approximately 3 mL of boron trifluoride (14%) in methanol was added. To achieve a total methylation process, the concentration of the mixture was carried out at 90°C for 5 min. Re-distilled normal hexane was then used to extract the fatty acid methyl esters. For the gas chromatography analysis, the content was concentrated to 1 mL and 1 g/L was injected into the injection port of the GC coupled with a flame ionization detector (FID) (Buck Scientific 91). The fatty acid analysis was carried out under the following conditions: column type: RESTEK 10 m MXT-2887, carrier gas: nitrogen, nitrogen gas: 10 psi, compressed air: 35 psi, hydrogen gas pressure: 15 psi, detector: FID, initial temperature: 50°C, final temperature: 250°C. The peaks were identified by comparison with standard fatty acid methyl esters.
Estimation of glycemic index (GI)
The glycemic index (GI) of individual wrap was estimated by determining how many carbohydrates (i.e., ingredients that contributed to CHO of the meal) were in each portion of the food. Then, the proportion of each carbohydrate added to the breakfast wrap was determined. The number of grams contributed by each component was divided by the total grams of carbohydrates in the food. The proportions of each wrap component were multiplied by the predetermined GI (obtained from the online GI database) of that component. The total GI of the breakfast wrap was then obtained by summation of the results (proportion of each component multiplied by its GI) of the individual components [26].
Glycemic load
The method described by Salmerón et al. [27] was employed to determine the glycemic load (GL) for each breakfast wrap. Each food’s unique GL was determined by multiplying its GI rating by the proportion of carbohydrates it contains in a typical serving using the equation below: GL = (Net carbohydrate g × GI)/100, where, net carbohydrate = total carbohydrates in the food served.
Microbial safety assessment
The milled wraps were assessed microbiologically using the method of Harrigan and McCance [28]. The diluents and different agars were diluted and sterilized appropriately by autoclaving at 121℃ and 0.15 MPa for 15 min. One gram of the sample was aseptically weighed, added into 9 mL sterile saline solution, and thoroughly homogenized. The samples were serially diluted up to 10–3 using sterile syringes. The process was repeated for the other four samples. Ultimately, 1 mL of an appropriate dilution was aseptically taken with the aid of a sterile syringe and transferred into the sterile petri dishes; the appropriate sterilized agar (cooled to 45°C) was poured into the petri dish, allowed to set, and incubated at 35°C for 24 h and 25°C for 72 h for bacteria and fungi, respectively. Enumeration of observable growths was carried out at the end of the incubation period. The colony-forming unit was obtained by multiplying the number of colonies by the dilution factor. Total mesophilic viable bacteria were examined using nutrient agar (NA), while fungi were determined using potato dextrose agar (PDA). Other microorganisms like Salmonella spp., Escherichia coli, and total coliforms were determined using deoxycholate citrate agar (DCA), eosin-methylene blue (EMB), Mac Conkey agar (MCA), respectively.
Chemical stability
Free fatty acid content
The free fatty acids (FFA) in the sample were estimated by titrating its fat against potassium hydroxide using phenolphthalein as the indicator. Extracted oil (1 mL) of the wrap was dissolved in 50 mL of the neutral solvent in a 250 mL conical flask, and about 3 to 4 drops of phenolphthalein indicator were added; it was titrated against 0.1 M KOH and was shaken constantly until a pink color which persisted for about 15 s was obtained [29]. The equation below was used to calculate the acid value:
Acid value (mg/KOH/g) = (Titre value × 0.1 M × 56.10)/Weight of sample
Free fatty acid = (Acid value)/2
Peroxide value
The peroxide value of the samples was determined by titration against thiosulphate in the presence of potassium iodide using starch as the indicator. This analysis was carried out in the dark. One gram of samples’ oil was weighed into a clean, dry boiling tube, and a gram of powdered KI and 20 mL of solvent mix was added. The tube was transferred into boiling water so the liquid boiled within 30 s and allowed to boil vigorously for not more than 30 s. The contents were quickly transferred into a conical flask containing 20 mL of 5% KI solution. The tube was washed twice with 25 mL of water each time and collected into the conical flask. Titration was carried out using 0.002 M Na2S2O3 solution until the disappearance of the yellow color. Then, 0.5 mL of starch was added to the obtained mixture, shaken vigorously, and titrated carefully until the blue color disappeared. A blank was set at the same time [30]. The peroxide value was calculated using the equation below:
Peroxide value (mmol peroxide/kg/sample) = (T × M × 1,000)/Sample weight (g)
where, T = titre value of sodium thiosulphate = sample titre – blank titre; M = molarity of sodium thiosulphate.
Lipid peroxidation inhibitory assay
An aliquot of homogenized tissue (100 mL) was incubated at 37°C for 1 h in the presence of extracts, with and without the prooxidant, sodium nitroprusside (SNP) (final concentration 3 mM). This was then used for lipid peroxidation determination. The production of thiobarbituric acid reactive species (TBARS) was determined as described by Liu et al. [31], except that the buffer of the color reaction had a pH of 3.4. The color reaction was developed by adding 300 mL 8.1% sodium dodecyl sulfate (SDS) to tissue, followed by the addition of 500 mL acetic acid/HCL (pH 3.4) and 500 mL 0.8% thiobarbituric acid (TBA). The mixture was incubated at 95ºC for 1 h. TBARS produced were measured at 532 nm using a 721G Visible spectrophotometer search tech instrument, and the absorbance was compared to that of the controls [31].
TBARS (μg/g) = (Abssample – Abscontrol)/S × 3 × 100
Where, Abssample = the absorbance of the sample; Abscontrol = the absorbance of the control; and S is the weight of the sample (g).
Sensory evaluation
The sensory attributes of the five breakfast wraps were evaluated by twenty untrained panelists who were healthy and familiar with the consumption of edible insects and other conventional protein sources. The purpose of the assessment was communicated to the testers, and they were required to state allergies, if any. None of them were allergic to any of the sources of protein. The samples were presented to the panelists in a randomized-coded manner. Panelists had to rinse their mouths before and after tasting each sample and rate the wraps on a nine-point hedonic scale. The GF oat breakfast wraps were evaluated for taste, appearance, texture, aroma, and overall acceptability.
Statistical analyses
The data obtained after the experiment were prepared using Microsoft Excel and subjected to analysis of variance (ANOVA) to obtain the descriptive analysis. The significant differences between the GF wraps were assessed using the New Duncan Multiple Range Test (DMRT) of Statistical Package for Social Science (SPSS) software (version 22.0). Statistical significance was accepted at P < 0.05.
Results
Nutrient composition of GF oats breakfast wraps
Proximate composition of GF oat-based breakfast wraps
Table 2 shows the nutritional composition of five breakfast wraps in terms of moisture, ash, crude fat, crude fiber, protein, and carbohydrates. Moisture content was within the range of 15.01–22.18%. The protein content of GF-oat wraps followed this significant order: chicken > fish > beef > PWL > silkworm pupae. Breakfast wraps with PWL had the highest significant crude fiber content compared to the other wraps. The ash contents ranged from 2.62% to 5.54% for the wraps. GF-wrap with beef had a higher significant fat content of 18.96 % than the other wraps. The wraps with PWL and SWP had significantly higher carbohydrate content.
Proximate composition of the GF oat-based breakfast wraps filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| Proximate constituents (%) | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP |
|---|---|---|---|---|---|
| Moisture | 17.90 ± 0.81c | 16.78 ± 0.13d | 15.01 ± 0.78e | 22.18 ± 0.35a | 20.62 ± 0.25b |
| Crude protein | 25.37 ± 2.98c | 35.60 ± 3.25a | 28.06 ± 2.44b | 23.82 ± 0.91d | 23.78 ± 0.30e |
| Crude fiber | 2.46 ± 0.54c | 2.43 ± 0.12d | 3.18 ± 0.37b | 4.01 ± 0.44a | 2.27 ± 0.24e |
| Crude fat | 18.96 ± 0.57a | 16.31 ± 0.23c | 17.78 ± 0.22b | 10.22 ± 0.64e | 15.79 ± 0.27d |
| Ash | 5.54 ± 0.13a | 4.26 ± 0.12b | 2.66 ± 0.68d | 3.66 ± 0.34c | 2.62 ± 0.25e |
| Carbohydrate | 29.77 ± 2.13d | 24.62 ± 2.87e | 33.31 ± 4.49c | 36.11 ± 1.13a | 34.92 ± 0.96b |
| Energy (KJ) | 408.8 | 387.67 | 405.5 | 331.7 | 376.91 |
Values are means ± standard deviation (SD) of three replicates. Mean values in the same row with different superscripts translate significant differences (P < 0.05) between samples. GF: gluten-free; PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae
Vitamin composition of GF oat-based wraps filled with different proteins
The vitamin composition of GF oat wrap filled with beef, chicken, fish, African PWL, and SWP is displayed in Figure 1. Comparatively, the vitamin A content of GF oat wrap with PWL competed well with that of beef, with an average value of 528.96 μg RAE/100 g. The vitamin B1 content of silkworm pupae wrap almost doubled that of the other wraps. The methylcobalamin (vitamin B12) in the insect wraps was significantly higher, followed by the wrap with fish, then chicken and beef. Contrary to the vitamins mentioned above, vitamin C was prominent in chicken and beef wraps, although they were not significantly different from GF-wraps with edible insects. In terms of vitamin E, all the GF-wraps were not significantly different except the chicken wrap. The values ranged from 4.29 mg/g to 4.97 mg/g. Sabolová et al. [32] reported that edible insect larvae can be a good dietary source of tocopherols and phytosterols. In 2010, Kinyuru et al. [33] reported α-tocopherol levels in Ruspolia differens from 161 mg/100 g to 170 mg/100 g (dry weight basis).
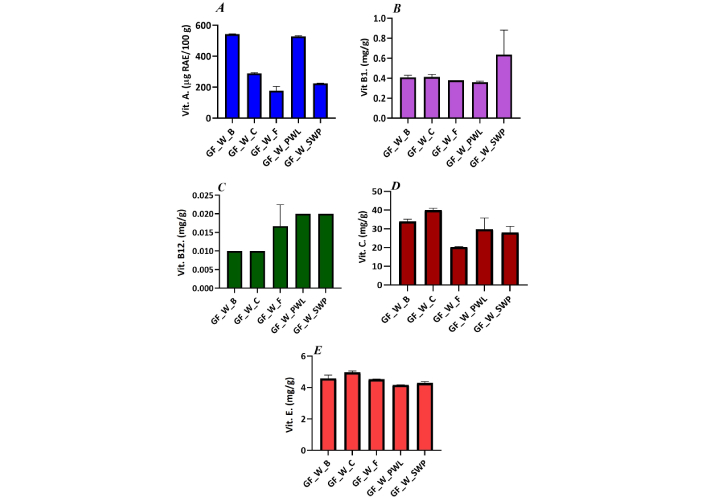
Contents of (A) vit A, (B) B1, (C) B12, (D) C, and (E) E of the breakfast wraps filled with different protein sources. Vit: vitamin; GF: gluten-free
Mineral composition of GF oat-based wraps filled with different proteins
The content of mineral elements, expressed in mg per 100 g, is presented in Figure 2 and Table 3. In the present study, eight nutritive elements were evaluated in the individual wraps, five breakfast wraps in total, and variations in the levels of minerals resulting from the protein filling were observed. From the results, wrap with PWL could be regarded as a good source of calcium, while silkworm pupae wrap could be a good source of calcium and magnesium. Banjo et al. [34] reported a high magnesium content (7.54 to 8.21 g/100 g) found in grasshoppers and weevils. GF-wrap with beef had the predominant amount of potassium, phosphorus, and sodium, with 46.99 mg/100 g, 6.22 mg/100 g, and 4.67 mg/100 g, respectively. The zinc content for insect fillings and the conventional proteins differs significantly. Selenium and iron were abundant in wrap with mackerel fish fillings.
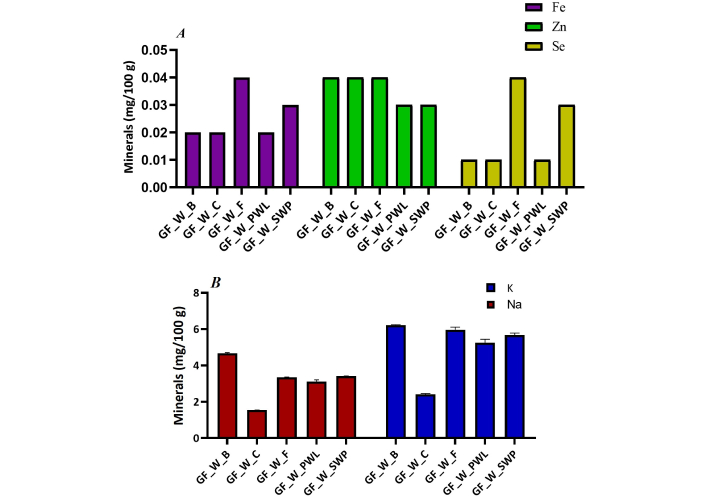
Contents of (A) iron (Fe), zinc (Zn), and selenium (Se) and (B) sodium and potassium of the breakfast wraps filled with different protein sources. GF: gluten-free
Mineral composition of GF oat-based wraps filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| Minerals | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP |
|---|---|---|---|---|---|
| Calcium (mg/100 g) | 2.45 ± 0.09b | 3.26 ± 0.16a | 2.53 ± 0.04b | 3.48 ± 0.06a | 3.33 ± 0.19a |
| Phosphorus (mg/100 g) | 46.99 ± 0.33a | 39.73 ± 0.07b | 20.20 ± 0.04e | 36.65 ± 0.10d | 38.12 ± 0.33c |
| Magnesium (mg/100 g) | 1.27 ± 0.07c | 0.99 ± 0.01d | 1.47 ± 0.01b | 1.49 ± 0.03b | 1.77 ± 0.01a |
Values are means ± standard deviation (SD) of three replicates. Mean values in the same row with different superscript letters translate significant differences (P < 0.05) between samples. GF: gluten-free; PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae
AA composition of GF oat-based wraps filled with different proteins
The AA composition of the GF oat breakfast wraps filled with different conventional and unconventional (insect) protein sources is shown in Table 4. Nutritionally, all the essential AA in all the wraps exceeded the FAO/WHO [35] recommended daily allowances except for lysine, tryptophan, and methionine. However, they can still meet above 50% of the recommended dietary allowances (RDA) for adults. Likewise, the quantity of the protein in the wraps can be adjusted to meet the daily protein needs. Leucine was discovered to be one of the most prevalent essential AA in the wraps. Overall, GF wrap filled with PWL had significantly higher essential and non-essential AA than the other wraps. Histidine from dietary sources is pivotal for children because of their body’s inability to effectively produce histidine, making it nutritionally essential for infants. The histidine contents of the GF wraps were about 18% higher than the daily requirement. The oat wraps contain appreciable amounts of glutamate and aspartate with values ranging from 9.49–12.67 mg AA/100 g protein and 6.37–8.51 mg AA/100 g protein. The total essential AA of the wraps with edible insects exceeded that of those with chicken and mackerel fish and were comparable to wraps with beef (Table 5).
AA profile of GFs filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| AA | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP | *RDA |
|---|---|---|---|---|---|---|
| Essential AA (mg AA/100 g protein) | ||||||
| Leucine | 8.39 ± 0.02b | 7.44 ± 0.08d | 6.73 ± 0.04e | 8.61 ± 0.08a | 8.13 ± 0.04c | 6.60 |
| Lysine | 3.55 ± 0.05b | 3.32 ± 0.04c | 3.17 ± 0.02d | 3.81 ± 0.02a | 3.46 ± 0.04b | 5.80 |
| Isoleucine | 4.35 ± 0.05ab | 3.79 ± 0.07d | 4.09 ± 0.08c | 4.53 ± 0.02a | 4.16 ± 0.05bc | 2.80 |
| Phenylalanine | 4.73 ± 0.07b | 4.16 ± 0.03c | 3.51 ± 0.10d | 5.09 ± 0.08a | 4.55 ± 0.05b | 2.80 |
| Tryptophan | 0.87 ± 0.02b | 0.77 ± 0.01c | 0.70 ± 0.00d | 0.93 ± 0.02a | 0.82 ± 0.01bc | 1.10 |
| Valine | 5.06 ± 0.04a | 4.12 ± 0.06c | 4.09 ± 0.09c | 4.89 ± 0.02a | 4.55 ± 0.04b | 3.50 |
| Histidine | 2.25 ± 0.02ab | 2.26 ± 0.02ab | 2.04 ± 0.03c | 2.34 ± 0.03a | 2.14 ± 0.06bc | 1.90 |
| Methionine | 1.25 ± 0.01bc | 1.16 ± 0.02d | 1.28 ± 0.01ab | 1.33 ± 0.03a | 1.20 ± 0.00cd | 2.20 |
| Threonine | 3.58 ± 0.09b | 3.18 ± 0.04c | 3.04 ± 0.03c | 4.10 ± 0.06a | 3.46 ± 0.06b | 3.40 |
| Non-essential AA (mg AA/100 g protein) | ||||||
| Proline | 3.52 ± 0.04bc | 3.44 ± 0.03c | 3.25 ± 0.04d | 3.85 ± 0.04a | 3.62 ± 0.04b | - |
| Arginine | 5.28 ± 0.03b | 3.93 ± 0.03d | 3.81 ± 0.05d | 5.50 ± 0.10a | 4.71 ± 0.06c | 2.00 |
| Tyrosine | 3.45 ± 0.02b | 3.52 ± 0.03b | 3.04 ± 0.03d | 3.91 ± 0.03a | 3.25 ± 0.04c | - |
| Cysteine | 1.35 ± 0.04a | 1.09 ± 0.03b | 0.88 ± 0.03c | 1.37 ± 0.03a | 1.27 ± 0.02a | - |
| Alanine | 4.68 ± 0.02a | 3.89 ± 0.00c | 3.49 ± 0.08d | 4.82 ± 0.03a | 4.14 ± 0.03b | - |
| Glutamate | 12.14 ± 0.13b | 10.64 ± 0.12c | 9.49 ± 0.17d | 12.67 ± 0.06a | 11.03 ± 0.03c | - |
| Glycine | 4.30 ± 0.07a | 3.39 ± 0.02c | 3.37 ± 0.05c | 3.87 ± 0.03b | 3.72 ± 0.04b | - |
| Serine | 4.09 ± 0.07ab | 3.90 ± 0.05ab | 3.51 ± 0.05b | 4.42 ± 0.10a | 3.73 ± 0.03ab | - |
| Aspartate | 8.05 ± 0.05b | 6.90 ± 0.07d | 6.37 ± 0.08e | 8.51 ± 0.11a | 7.56 ± 0.05c | - |
Values are means ± standard deviation (SD) of three replicates. Mean values in the same row with different superscript letters translate significant differences (P < 0.05) between protein filling samples. AA: amino acids; RDA: recommended dietary allowances; GF: gluten-free; PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae
Predicted nutritional indices for GF oat-based wraps filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| Predicted indices | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP |
|---|---|---|---|---|---|
| TEAA | 34.01 ± 0.18b | 30.18 ± 0.24d | 28.63 ± 0.00e | 35.61 ± 0.14a | 32.44 ± 0.12c |
| TNEAA | 46.84 ± 0.01b | 40.17 ± 0.20d | 37.28 ± 0.05e | 49.35 ± 0.33a | 43.01 ± 0.10c |
| TAA | 80.85 ± 0.17b | 70.37 ± 0.42d | 65.91 ± 0.05e | 84.50 ± 0.01a | 75.45 ± 0.22c |
| TSAA | 2.60 ± 0.05a | 2.24 ± 0.01c | 2.16 ± 0.03c | 2.70 ± 0.06a | 2.47 ± 0.02b |
| TEAA/TAA% | 42.06 ± 0.13c | 42.89 ± 0.09b | 43.44 ± 0.03a | 42.13 ± 0.15c | 42.10 ± 0.04b |
| TAEAA | 5.60 ± 0.09b | 4.93 ± 0.04c | 4.21 ±0.10 d | 6.02 ± 0.10a | 5.37 ± 0.04b |
Values are means ± standard deviation (SD) of three replicates. Mean values in the same row with different superscript letters translate significant differences (P < 0.05) between protein filling samples. GF: gluten-free; PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae; TEAA: total essential amino acids; TNEAA: total non-essential amino acids; TAA: total amino acids; TSAA: total sulfur-containing amino acids (cysteine + methionine); TAEAA: total aromatic essential amino acids (phenylalanine + tryptophan) [41]
Fatty acid composition of GF oat-based wraps filled with different proteins
The wrap with PWL displayed the highest composition of linoleic acid and overall polyunsaturated fatty acid (PUFA) (Table 6). There was a considerable variation in monounsaturated fatty acid (MUFA) levels among the samples, with the highest value of 31.78% in beef breakfast wrap, while the GF-wrap with chicken had the lowest value of 26.08%. Although beef is high in saturated fat, it also has some monounsaturated fat [36]. The SFA ranged from 50.69 % to 53.93 %, with wrap with silkworm pupae having the highest value. Arachidic acid (C20:0) was the most predominant SFA, followed by palmitic acid and then lauric acid.
Fatty acid composition of GF oat-based wraps filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| Fatty acids (%) | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP |
|---|---|---|---|---|---|
| Saturated fatty acids | |||||
| Butyric acid (C4:0) | 1.1 | 1.53 | 1.29 | 1.67 | 1.75 |
| Caproic acid (C6:0) | 1.26 | 1.45 | 1.41 | 1.76 | 1.96 |
| Caprylic acid (C8:0) | 0.73 | 1.32 | 1.28 | 1.67 | 1.66 |
| Capric acid (10.0) | 3.59 | 4.25 | 3.3 | 4.02 | 3.55 |
| Lauric acid (12:0) | 5.18 | 6.32 | 4.76 | 6.49 | 5.95 |
| Tridecanoic acid (13:0) | 3.75 | 4.59 | 4.42 | 5.25 | 5.12 |
| Myristic acid (14:0) | 1.94 | 2.41 | 1.33 | 1.71 | 1.88 |
| Palmitic acid (14:0) | 9.79 | 7.2 | 8.59 | 7.62 | 7.07 |
| Heptadecanoic acid (17:0) | 6.03 | 4.64 | 5.11 | 4.27 | 5.95 |
| Stearic acid (C18:0) | 3.51 | 5.37 | 4.78 | 4.31 | 4.62 |
| Arachidic acid (20:0) | 13.48 | 10.05 | 11.92 | 10.22 | 11.32 |
| Heneicosanoic acid (C21:0) | 1.91 | 1.8 | 1.09 | 2.3 | 0.92 |
| Behenic acid (C22:0) | 0.92 | 1.59 | 1.41 | 1.6 | 2.18 |
| £SFA | 53.19 | 52.52 | 50.69 | 52.89 | 53.93 |
| MUFAs | |||||
| cis-10-Pentadecanoic acid (C15:1) | 5.31 | 4.34 | 4.62 | 4.8 | 4.28 |
| Palmitoleic acid (16:1) | 3.25 | 3.51 | 3.55 | 3.92 | 4.25 |
| Oleic acid (C18:1) | 20.18 | 14.86 | 18.98 | 14.62 | 16.66 |
| cis-11-Eicosenoic acid (20:1) | 3.04 | 3.37 | 2.29 | 3.85 | 3.18 |
| £ MUFA | 31.78 | 26.08 | 29.44 | 27.19 | 28.37 |
| PUFAs | |||||
| Linoleic acid (C18:2) | 6.66 | 9.75 | 6.92 | 10.05 | 8.92 |
| Gamma-linolenic acid | 1.26 | 1.71 | 2.15 | 2.15 | 1.62 |
| Alpha-linolenic acid (C18:3) | 6.89 | 7.2 | 7.04 | 6.68 | 7.76 |
| cis-11-14-Eicosadienoic acid (C20:2) | 6.39 | 4.38 | 5.62 | 4.6 | 4.9 |
| £ PUFA | 21.2 | 23.04 | 21.73 | 23.48 | 23.2 |
GF: gluten-free; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae
The GI of oat-based wraps filled with different proteins
The estimated GI of the GF-oat wraps is shown in Figure 3. The GI of the wraps was in this ascending order: GF-oat with PWL < GF-oat with SWP < GF-oat with fish < GF-oat with beef < GF-oat with chicken. A similar observation of low GI was reported by Zielińska et al. [37] for muffins enriched with cricket and mealworm powders. This shows that an insect diet may be preferable to fish, beef, and chicken for a low GI diet.
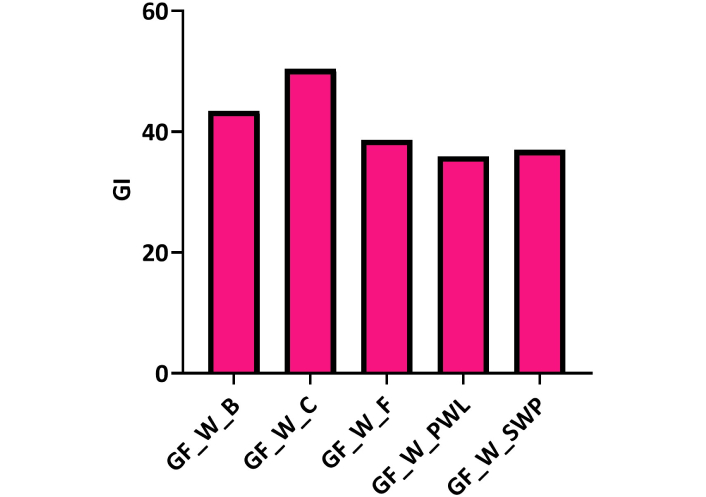
Glycemic index (GI) of the GF oat breakfast wraps filled with different protein sources. GF: gluten-free
Microbial quality of oat-based wraps filled with different proteins
The characterization of the oat-based breakfast wraps revealed the absence of E. coli, total coliforms, and Salmonella spp., indicating no fecal or other contamination forms (Table 7). However, regarding the aerobic mesophilic bacteria culture, wraps with chicken and fish recorded low levels of these organisms. The counts of yeasts and molds were lower than that reported by Roncolini et al. [38] for bread.
The microbial load of GF oat-based breakfast wraps filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| Microorganisms | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP |
|---|---|---|---|---|---|
| AMB | ND | 6 × 10–3 | 3 × 10–3 | ND | ND |
| Escherichia coli | ND | ND | ND | ND | ND |
| Total coliforms | ND | ND | ND | ND | ND |
| Salmonella spp. | ND | ND | ND | ND | ND |
| Yeasts and molds | 1 × 10–3 | 2 × 10–3 | ND | 2 × 10–3 | 2 × 10–3 |
ND: not detected; GF: gluten-free; PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae
Free fatty acid, peroxide values, and lipid peroxidation content of the wraps
Wraps with conventional proteins tend to be significantly higher than that of edible insects in terms of the free fatty acid value, with beef recording the highest value of 0.47 mg/KOH/g and PWL with the lowest value of 0.29 mg/KOH/g (Figure 4). The reverse was the case for peroxide value and lipid peroxidation content. It was observed that the peroxide values (PVs) of all the wraps were below the FAO/WHO [39] standard of 10 meq. O2/kg for fatty foods.
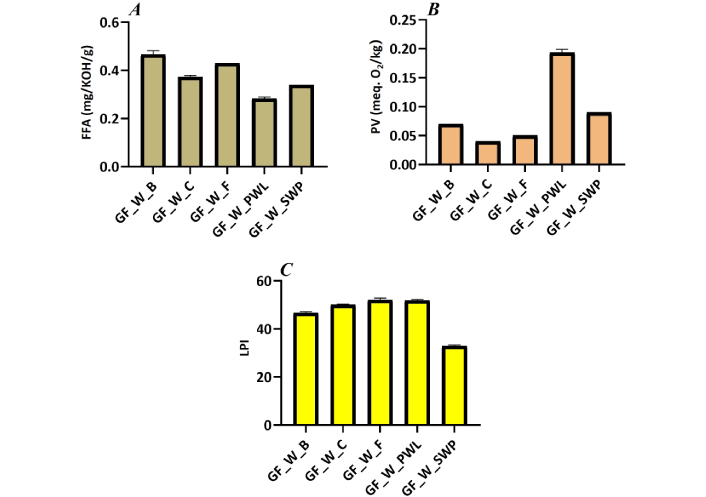
(A) FFA, (B) PV, and (C) LPI of the breakfast wraps filled with different protein sources. GF: gluten-free; FFA: free fatty acids; PV: peroxide value; LPI: lipid peroxidation inhibition
Sensory quality of oat-based wraps with different protein fillings
As shown in Table 8, the breakfast wrap with fish was ranked significantly higher than other wraps in appearance, taste, aroma, and overall acceptability. The panelists preferred the mouthfeel of GF wrap with beef fillings. Silkworm pupae wrap compares well with beef and chicken regarding taste, aroma, and overall acceptability. On average, the insect wraps were moderately liked.
Sensory quality of GF oat-based breakfast wraps filled with beef (GF_W_B), chicken (GF_W_C), fish (GF_W_F), PWL (GF_W_PWL), and SWP (GF_W_SWP)
| Sensory parameters | GF_W_B | GF_W_C | GF_W_F | GF_W_PWL | GF_W_SWP |
|---|---|---|---|---|---|
| Appearance | 7.90 ± 0.18b | 8.00 ± 0.26a | 8.00 ± 0.21a | 7.10 ± 0.23d | 7.40 ± 0.22c |
| Texture | 7.70 ± 0.21a | 6.90 ± 0.18c | 7.40 ± 0.27b | 6.30 ± 0.40e | 6.60 ± 0.48d |
| Taste | 6.90 ± 0.55b | 7.00 ± 0.26ab | 7.60 ± 0.27a | 5.20 ± 0.71c | 7.10 ± 0.28ab |
| Aroma | 6.30 ± 0.56b | 6.70 ± 0.26ab | 7.49 ± 0.22a | 6.10 ± 0.31c | 6.30 ± 0.50b |
| Overall acceptability | 7.50 ± 0.17b | 7.20 ± 0.13b | 7.80 ± 0.25a | 5.20 ± 0.49c | 7.40 ± 0.27b |
Values are means ± standard deviation (SD) of three replicates. Means with different superscript letters in the same row translate significant differences (P < 0.05) between samples. PWL: palm weevil larvae; SWP: Mulberry Silkworm pupae; GF: gluten-free
Discussion
Nutrient composition of GF oats breakfast wraps
Proximate composition of GF oat-based breakfast wraps
Moisture is one of the components that constitutes the proximal composition of foods. The high moisture contents of the breakfast wraps may not be a concern because wraps are products expected to be consumed almost immediately after production. The quantity of protein in the various wraps can be attributed to the protein quantity in the different protein sources. Nonetheless, the SWP and PWL were good protein sources and compared favorably well with beef, chicken, and mackerel fish. According to Parker et al. [40], complementary foods incorporated with PWL had elevated protein contents comparable with the study results. High fiber in PWL wrap may be attributed to the fibrous exoskeleton associated with PWL. Beef is a known source of high fat [41] which could have contributed to the high fat content of the beef wrap. According to Akande et al. [16], the presence of chitinous polymer of the insects’ cuticles may be responsible for the higher carbohydrate content of wraps with PWL and SWP. Since, the energy contents of the wraps followed the same trend as the fat content, the energy levels mainly depend on the fat content, which favors the fillings with beef because of their higher fat content.
Vitamin composition of GF oat-based wraps filled with different proteins
Vitamins and minerals, often called micronutrients, are vital elements required by humans and other forms of life in diverse quantities throughout life to coordinate various physiological processes for the maintenance of health [42]. The high vitamin B1 content of the SWP wrap confirms the assertion of Rumpold and Schlüter [12] that edible insects are good sources of B vitamins. The significant quantity of cobalamin in the insect wraps revealed the products may help combat degeneration of the spinal cord, megaloblastic, and pernicious anemia, amongst other illnesses associated with the deficiency of the vitamin. Similarly, Banjo et al. [34] reported the inclusion of edible insects (termites) to improve the β-carotene, niacin, vitamin B6, and B12 content of maize. The presence of vitamin C, also known as ascorbic acid, in the wraps can make the products a suitable source of antioxidant in the human body that helps prevent cell oxidation caused by free radicals. Variations in the vitamin E contents of the wraps may be attributed to the composition of the protein sources. Vitamin E comprises four tocopherols and four tocotrienols and is important for human metabolic processes.
Mineral composition of GF oat-based wraps filled with different proteins
Many studies have demonstrated that edible insects are good sources of nutrients, including minerals [43–47]. The variations in the levels of mineral contents of the wraps can be attributed to the differences in the mineral composition of the protein fillings. The higher calcium content of the insect wraps when compared with the other wraps supports the view of Ghosh et al. [48]. According to the authors, the calcium content in all studied insects was much higher than that in conventional foods of animal origin except chicken eggs. The supply of Fe and Zn is very important in human diets because of the worldwide deficiencies in these minerals among humans [48]. A diet rich in SWP can help prevent diseases associated with iron deficiency such as anemia and stunting. Based on the findings of the study, conventional proteins are preferable when a high zinc diet is desired. The lower zinc content of the insect wraps may be attributed largely on the food consumed by the insects during growth [48]. Chicken, fish, PWL and silkworm pupae may be considered for utilization for low-sodium diets. According to the study, chicken wrap is not a good source of potassium.
AA composition of GF oat-based wraps filled with different proteins
Edible insects are good sources of essential AA and have been reported to increase or improve the AA of foods such as pastries, complementary foods, and baked foods, to mention a few [17, 40, 43]. Comparatively, PWL wrap was outstanding in terms of all the AA. Elemo et al. [49] reported that PWL can act as a dietary AA supplement, particularly in developing nations where they are readily available because of their AA profile. As demonstrated by Williams et al. [50], tryptophan is a limiting AA in ants, bee (Apis mellifera), and silkworm (Bombyx mori), and lysine in termites. The study support the findings of limited lysine, tryptophan, and methionine in the wraps.
Fatty acid composition of GF oat-based wraps filled with different proteins
Linoleic (C18:2n6) and alpha-linolenic (C18:3n3) acids are essential fatty acids in the human diet because the human body cannot produce them [51]. Meanwhile, these acids have been linked with inflammatory regulation and overall body health [52, 53] because they belong to the omega-6 and omega-3 fatty acid groups, respectively. It is also known that consuming a diet rich in linoleic acid can help prevent hypertension and cardiac conditions such as coronary heart disease and atherosclerosis [54, 55]. This shows that the wrap with edible insects offers more potential health benefits than the conventional proteins. This agrees with the study of Cheseto et al. [56], who incorporated oil extracted from Schistocerca gregaria and Ruspolia differens into cookies. It was discovered that the insects’ oils had higher concentrations of omega-3 fatty acids, flavonoids, and vitamin E compared to oils from olive and sesame. Eventually, the cookies mirrored the fatty acid profile of the parent oils. Regardless, the distinctive nutritional concentration of PWL and, to some extent, silkworm pupae, in terms of PUFA, may be linked to the environment and the diet they feed or were reared on [56, 57].
The GI of oat-based wraps filled with different proteins
According to Brouns et al. [58], the glycemic index, or GI, is a method of ranking foods from 0 to 100 based on the influence of such foods on blood sugar levels. Higher GIs indicate foods that will cause the blood sugar to rise higher and faster than foods with lower GIs. Patients with type 1 and type 2 diabetes can benefit from consuming low-GI diets because of their ability to control blood sugar and insulin levels and insulin resistance [59].
Microbial quality of oat-based wraps filled with different proteins
Microbial assessment of insect products is expedient in establishing their safety for consumption, particularly when wildly sourced edible insects are incorporated in food products. In the present study, microbial characterization was carried out to evaluate the total viable aerobic mesophilic bacteria, Escherichia coli, total coliforms, Salmonella spp., and yeasts and molds of the five break wraps enriched with edible insects and conventional protein sources.
Free fatty acid, peroxide values, and lipid peroxidation content of the wraps
Beef, fish, and chicken are good sources of saturated fats, while edible insects such as PWL and SWP contain predominantly unsaturated fats. Although unsaturated fats are healthy for the body, they are subject to oxidation reactions because of the level of unsaturation. According to Tenyang et al. [60], It has been discovered that one of the leading causes of off-flavors and lower quality in fatty foods is lipid oxidation. The food becomes more prone to oxidation when the quantity of double bonds increases. Rapid assessment of fatty acid oxidation can be achieved by quantifying the hydroperoxide content. Under certain treatment conditions, the interaction of free unsaturated fatty acids with molecular oxygen can accumulate hydroperoxides. The samples’ high heat and moisture content have been implicated to speed up these reactions. A food is termed rancid when it has a peroxide value between 20 and 40 meq. O2/kg [60]. It was observed that the PVs of all the wraps were below the FAO/WHO [39] standard of 10 meq. O2/kg for fatty foods.
Sensory quality of oat-based wraps with different protein fillings
Sensory quality is a key parameter in determining the acceptability of new and existing products. Although the panelists were familiar with the consumption of edible insects, their preference for beef, fish, and chicken wraps was evident. This can be attributed to the fact that despite the nutritional quality of insects, beef, chicken and fish still forms part of the everyday diet of a typical Nigerian.
Conclusions
This study demonstrated the fact that edible SWP and African PWL could serve as an alternative to beef, fish, and chicken in GF oat-based wrap production, as this is evident in the high protein, lipid, and micronutrient contents. The AA and fatty acid profiles also showed high-quality protein and fat. The chemical and microbial quality of the insect-enriched wraps were within permissible limits and may pose no threat to consumption. In addition, insect wraps, particularly those of silkworm pupae, received acceptable scores comparable to those of conventional proteins. The low GI of the insect wraps could potentially position them as a healthier substitute for meat, fish, and chicken. Incorporation of edible insects into GF oat wrap may be one of the ways to encourage entomophagy, an environmentally friendly alternative to beef, fish, and chicken consumption. This may be a very useful low-cost alternative food source, especially in most developing nations with severe malnutrition.
Abbreviations
| AA: | amino acids |
| GF: | gluten-free |
| GI: | glycemic index |
| GL: | glycemic load |
| MUFA: | monounsaturated fatty acid |
| PUFA: | polyunsaturated fatty acid |
| PVs: | peroxide values |
| PWL: | palm weevil larvae |
| SWP: | Mulberry Silkworm pupae |
| TBARS: | thiobarbituric acid reactive species |
| WHO: | World Health Organization |
Supplementary materials
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/101078_sup_1.pdf.
Declarations
Author contributions
OA: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing—review & editing, Validation, Supervision. AF: Resources, Writing—review & editing, Validation, Supervision. TO: Investigation, Data curation, Writing—original draft. ET: Investigation, Data curation, Writing—original draft. TA: Investigation, Data curation, Writing—original draft. DA: Formal analysis, Writing—review & editing, Visualization. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Informed consent was obtained from participants to participate in the sensory evaluation of the wraps.
Consent to publication
Not applicable.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.