Abstract
Among the most common medical problems experienced by older adults (over 60 years) are diabetes, Parkinson’s disease (PD), and erectile dysfunction (ED). The potential use of Mucuna pruriens in treating type 2 diabetes (T2D), PD, and ED is being investigated. Literature searches were conducted using the PubMed, MEDLINE, and Mendeley databases (1990–2023). Key words related to Mucuna pruriens, PD, diabetes, and EDs were used. An analysis of 26 preclinical and clinical trials suggested that Mucuna pruriens may be used to treat PD, diabetes, and ED. This study revealed a correlation between diabetes, Parkinson’s, and ED, with metabolic disorders being the common cause. Mucuna pruriens-based therapeutics could be a positive source of leva-dopa (LD) medications. It is well tolerated and beneficial for brain function and overall health. Evidence suggests that it has positive effects on libido, testosterone levels, and PD. It is important to note that PD and ED are linked by multiple mechanisms. In many clinical trials (in humans and animals), Mucuna pruriens were found to be effective at treating ED, PD, and diabetes. It is further necessary to conduct additional scientific studies to confirm the molecular mechanisms and biomarkers that link Mucuna pruriens phytochemicals with PD, ED, and diabetes.
Keywords
Erectile dysfunction, Mucuna pruriens, metabolic disorders, mitochondria, Parkinson’s diseaseIntroduction
Approximately one in three older adults suffer from diabetes, however, obesity, physical inactivity, and poor eating habits have led to an increase in the incidence of diabetes in children, adolescents, and young adults [1]. There are complications associated with diabetes that affect the brain, such as cerebrovascular disease (CVA), and Parkinson’s disease (PD). The disability and death rates in patients with PD are greater than those in patients with any other neurological disorder. An estimated 5.8 million disability-adjusted life years were lost in 2019 due to PD; an increase of 81% since 2000, and 329,000 deaths occurred due to the disease [2]. Numerous epidemiological studies have linked type 2 diabetes (T2D) with PD progression risk [3, 4]. There is promising evidence that antidiabetic medications may be used as treatments for PD, suggesting that T2D could be modified in terms of its pathogenesis. PD patients with T2D may benefit from personalized antidiabetic treatments that also provide neuroprotection due to their high prevalence. Therefore, understanding how T2D affects specific molecular pathways in the brain and how these pathways interact mechanistically is important. The pathogenesis of PD and diabetes involves insulin signaling, mitochondrial function, autophagy, and inflammation [5, 6]. A strong correlation exists between diseases of the body and diseases of the mind [7]. PD impairs physiological responses to sexual stimulation. It is common among patients with erectile dysfunction (ED). PD also impairs a patient’s ability to have an erection when sexually stimulated [8]. Many medicinal plants contain antioxidants that help control blood sugar levels in human bodies, and scientists are keen to learn more about the process. It has been demonstrated that Mucuna pruriens, also known as velvet bean, may be effective in treating diabetes, PD, and ED [9]. Leva-dopa (LD) is present in Mucuna pruriens at a mean concentration of 5.3%. Due to the absence of alkaloids, it is a sustainable therapeutic option for PD patients in the long term [10]. Studies also suggest that Mucuna pruriens is effective in the treatment of ED [11]. The antioxidant properties of Mucuna pruriens seeds naturally restore erectile function and reduce diabetic-induced sperm damage caused by oxidative stress [12]. A significant and common medical problem, the ED is characterized by extreme or complete erections. There are more than 150 million men worldwide who suffer from ED in some form or another. It is estimated that by 2025, 322 million men will suffer from ED. The incidence of ED in the United States is 25.9 cases/1,000 people, and it increases with age, with more than 70% of men over 70 having ED [13]. Phosphodiesterase-5 (PDE5) is an enzyme involved in preventing the breakdown of cyclic guanosine monophosphate (cGMP) in the cGMP signaling pathway, and is directly associated with penile erections. A penile erection is regulated primarily by the signaling pathways nitric oxide (NO) and cGMP. As a result of the downstream effect of cGMP caused by NO, PDE5 inhibitors can improve the erectile response and help treat ED [14]. In T2D patients, the incidence of severe ED increases with age. Age, duration of diabetes, and cholesterol are positively associated with ED [15]. Preclinical studies and clinical trials were reviewed to update the knowledge of the potential therapeutic effects of Mucuna pruriens on diabetes, PD, and ED. Research on reactive oxygen species (ROS) is gaining popularity in medical science due to their involvement in metabolic related disorders like T2D, PD, and ED. As a notable source of hypoglycemic compounds, Mucuna pruriens activates the antioxidant defense mechanism, helping to prevent diabetes, PD, and ED [16]. Despite the multipurpose healing capabilities of Mucuna pruriens, there is still a lack of scientific evidence and clinical trials to support the efficacy of the herb.
This is the first study to investigate the potential benefits of Mucuna pruriens for PD, diabetes prevention, and most importantly ED. There is no standard method for diagnosing PD, and ED. All of these metabolic disorders are directly affected by glucose and insulin metabolism. Scientific advancements have made it easier for us to understand inflammation and endocrine pathways, which affect brain functions [17, 18].
Literature review
A literature search was carried out using the PubMed, MEDLINE, and Mendeley databases. For the database searches, terms related to Mucuna pruriens and/or Diabetes, PD, and ED were used. The following data were evaluated for eligibility in each study: first author’s surname, publication year, country of study, study design, sample size, follow-up duration, characteristics of participants, disease targeted, and major results.
This study considered the following: (1) clinical studies; (2) prospective observational studies conducted with participants at least 18 years of age; (3) prospective observational studies (animal model); (4) studies published in English; (5) studies that included the following end-points: Mucuna pruriens, diabetes, PD, and ED; and (6) a study protocol, case report, comment, letter, editorial, duplicate study, and narrative or systematic review were excluded.
Clinical and preclinical trials
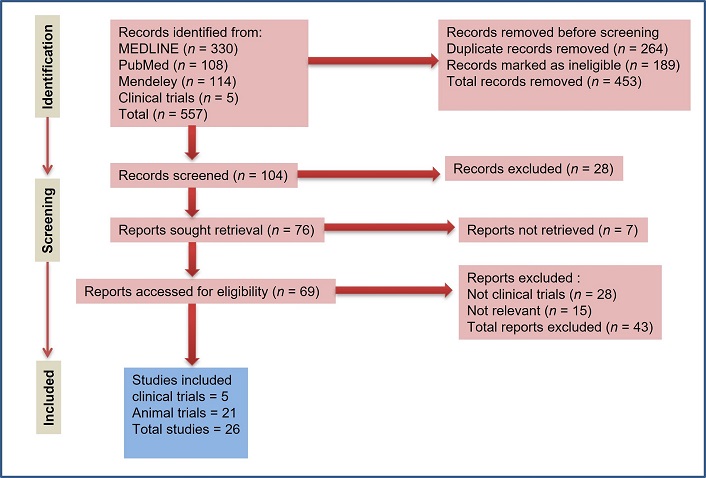
A total of 557 records were found in the initial search, and 453 reports were removed based on the title and abstract, leaving 104 for full-text consideration (Figure 1). Out of 104 reports, 78 reports were excluded for the different reasons outlined in Figure 1, and 26 studies were considered eligible for inclusion. Among these studies, five included clinical trials [19–23], and 21 included studies using animal models [9, 11, 24–42].
The main characteristics of the 26 selected articles are summarized in Table 1 [19–23] and Table 2 [9, 11, 24–42]. Five studies (n = 5) reported PD clinical trials in humans [19–23], thirteen studies reported PD in animal models [24–36], two (n = 2) studies reported diabetes in animal models [9, 37], and six (n = 6) reported ED [11, 38–42]. Diabetes and ED have not been studied in humans. Twenty-six of these studies (n = 26) were published in English in the last twenty years (2002–2022), with the exception of [23]. Human trials have been conducted in Bolivia [19, 20], Italy [21], and the UK [22], while one was multicenter [23]. The majority of animal trials have been conducted in India [27, 30, 31, 37, 39–41], and the USA [26, 32–36]. The remaining studies were carried out in Italy [24, 25, 28], Thailand [11, 38], and Nigeria [8]. There were 13 preclinical trials conducted on animal models of PD, of which (n = 5) were conducted on the Drosophila melanogaster fruit fly model [24–26, 28, 29], (n = 4) on the Sprague-Dawley rat model [33–36], (n = 3) on the Swiss albino rat model [27, 30, 31], and one (n = 1) was conducted on the monkey [32].
Effects of MP in PD (human model)
| S.No. | First citation | Year of publication | Country | Targeted disease | Design of the study | Number of participants and age group | Duration of follow-up | Dose | Adverse event | Major finding |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cilia et al. [19] | 2018 | Bolivia | PD | Randomized order and crossover design | 14< 21 years | 16 weeks | 18.7 mg/kg/day | Gastrointestinal side-effects | Clinical response to MP was similar to LD/CD |
| 2 | Cilia et al. [20] | 2017 | Bolivia | PD | Double-blind, randomized, controlled, and crossover | 18, (13 male, 5 female)< 21 years | 16 weeks |
| Fewer dyskinesias | MP-Hd induced greater motor improvement |
| 3 | Contin et al. [21] | 2015 | Italy | PD | Not specified | 1 | One day | 100 mg LD from Mucuna capsules in 2 different sessions | Impaired LD bioavailability from MP | No significant subacute LD motor response was observed |
| 4 | Katzenschlager et al. [22] | 2004 | UK | PD | Randomized, controlled, and double blind crossover trial | 9 (5 women and 4 men) | 4 hours | 15 g/day (500 mg of LD) and 30 g/day (1,000 mg of LD) | Gastrointestinal side-effects, mild and short lasting nausea | Natural sources of LD might possess advantages over conventional LD preparations in the long term management of PD |
| 5 | HP-200 in Parkinson’s Disease Study Group [23] | 1995 | Not specified (multicenter clinical trial) | PD | Open study | 60 (46 male and 14 female)< 59 ± 9 years | 12-week treatment | 7.5 g/day | Mild gastrointestinal in nature | Found to be an effective treatment for patients with PD |
CD: carbidopa; DDCI: dopa-decarboxylase inhibitor; Hd: high dose; LD: leva-dopa; Ld: low dose; MP: Mucuna pruriens; PD: Parkinson’s disease
Effects of MP in diabetes, PD, and ED (animal model)
| S.No. | First citation | Year of publication | Country | Targeted disease | Experimental model | Dose | Major finding |
|---|---|---|---|---|---|---|---|
| 1 | Baroli et al. [24] | 2019 | Italy | PD | Drosophila melanogaster model (fruit fly) | Not applicable | Beneficial effect on the oxidative stress conditions, rescues the mitochondrial functioning from oxidative stress |
| 2 | Solari et al. [25] | 2018 | Italy | PD | Drosophila melanogaster model (fruit fly) | Not applicable | MPE treatment rescued the crop muscle parameters and also the mitochondrial morphology |
| 3 | Johnson et al. [26] | 2018 | USA | PD | Drosophila melanogaster model (fruit fly) | 12.5–50 g/mL | MPE contains bioactive compounds, beyond leva-dopa, which may impart neuroprotective effects against PD |
| 4 | Singh et al. [27] | 2016 | India | PD | Swiss albino male mice (8–10 week old, 30–45 g) | 48 mg/kg body wt | Significantly decreased the elevated levels of oxidative stress found in Parkinsonian mice |
| 5 | Poddighe et al. [28] | 2014 | Italy | PD | Drosophila melanogaster model (fruit fly) | Not applicable | Suggesting that its effects cannot only depend upon its leva-dopa content but support the clinical observation of MPE as an effective medication with intrinsic ability to delay the onset of chronic leva-dopa-induced long-term motor complications |
| 6 | Jansen et al. [29] | 2014 | UK | PD | Drosophila melanogaster model (fruit fly) | Not applicable | No significant effects were observed |
| 7 | Yadav et al. [30] | 2014 | India | PD | Swiss albino male mice, 25 ± 5 g wt | 25, 50, 100, 150, and 200 mg/kg body-wt | Decreasing oxidative stress and possibly implementing neuronal and glial cell crosstalk |
| 8 | Yadav et al. [31] | 2013 | India | PD | Swiss albino male mice weighing 25 ± 5 gram | 25, 50,100, 150, and 200 mg/kg body-wt | Significantly reduced the PQ induced neurotoxicity as evidenced by decrease in oxidative damage, physiological abnormalities, and immunohistochemical changes in the parkinsonian mouse |
| 9 | Lieu et al. [32] | 2012 | USA | PD | Fourteen adult (6–9 kg) rhesus (Macaca mulatta) and two cynomolgus (Macaca fascicularis) monkeys | MPEP + CD (4.5 g/25 mg) | Distinctive neurophysiological findings in the basal ganglia and the ability to ameliorate parkinsonism without causing dyskinesias |
| 10 | Lieu et al. [33] | 2010 | USA | PD | Female Sprague-Dawley rats (250–400 g) | 80 mg/kg MPE (group 2), and 40 mg/kg MPE (group 3) with 15 mg/kg of BZ (MPE + BZ) | MP contains water-soluble ingredients that either have an intrinsic DDCI-like activity or mitigate the need for an add-on DDCI to ameliorate parkinsonism |
| 11 | Dhanasekaran et al. [34] | 2008 | USA | PD | Male Sprague-Dawley rats (200–225 g) | Varying doses with different combinations | Inhibited the oxidation of lipids and deoxyribose sugar by exhibiting antioxidant and metal chelating effects |
| 12 | Tharakan et al. [35] | 2007 | USA | PD | Male Sprague-Dawley rats (200–225 g) | Varying doses with different combinations | MPCP has shown anti-parkinson and neuroprotective effects in animal models of PD that are superior to synthetic levo-dopa. The copper chelating property may be one of the mechanisms by which MPCP exerts its protective effects on DNA |
| 13 | Manyam et al. [36] | 2004 | USA | PD | Sprague-Dawley rats | 2.5, 5.0, or 10.0 g/kg/day | Antiparkinson effect may be due to components other than levo-dopa or that it has a levo-dopa enhancing effect |
| 14 | Majekodunmi et al. [9] | 2011 | Nigeria | Diabetes | Wistar rats (180–240 g) and albino mice (16–20 g) of both sexes | Methanol and ethanol fractions of MP | A single dose of the ethanolic extract of MP resulted in a significant reduction in the blood glucose level, reducing the weight loss associated with diabetes |
| 15 | Rathi et al. [37] | 2002 | India | Diabetes | Rats | 100, 200, and 400 mg/kg/day | MP had no significant effect |
| 16 | Choowong-In et al. [38] | 2021 | Thailand | ED | Ninety-six adult male (n = 48) and female (n = 48) ICR mice (10 weeks, weighing 35–40 g) | 600 mg/kg | Improve the sexual performances and reproductive parameters especially functional proteins in testis, epididymis, and sperm |
| 17 | Seppan et al. [39] | 2020 | India | ED | Mouse model | 200 mg/kg body weight | Restoring aging induced structural and functional impairment in dorsal nerve of the penis and ED |
| 18 | Duangnin et al. [11] | 2017 | Thailand | ED | Male Wistar rats of 250–300 g, 6–8 weeks old | 20 mg/kg and 200 mg/kg body weight | Polar fraction of MP is able to upregulate the expression of ED-related genes including eNOS and nNOS in vitro which subsequently promotes nitric oxide production and maintains intracellular cyclic guanosine monophosphate levels |
| 19 | Goswami et al. [40] | 2012 | India | ED | Male Wistar rats weighing 200–250 g | 50 ug/mL | Methanolic extract of MP failed to inhibit Rho-kinase 2 |
| 20 | Suresh et al. [41] | 2012 | India | ED | Albino rats of Wistar strain twelve-week-old male rats around 225–250 g bw | 200 mg/kg | Improve male sexual behavior with androgenic and antidiabetic effects |
| 21 | Suresh et al. [42] | 2011 | India | ED | Albino rats of Wistar strain twelve-week-old male rats around 225–250 g bw | 200 mg/kg bw | Significantly protected the erectile tissue and also improved penile reflex |
bw: body weight; BZ: benserazide; CD: carbidopa; DDCI: dopa-decarboxylase inhibitor; DNA: deoxyribonucleic acid; ED: erectile dysfunction; eNOS: endothelial nitric oxide synthase; ICR: institute for cancer research; MP: Mucuna pruriens; MPCP: Mucuna pruriens cotyledon powder; MPEP: Mucuna pruriens endocarp powder; nNOS: neuronal nitric oxide synthase; PD: Parkinson’s disease; PQ: paraquat; wt: weight
PD (human clinical trials)
A crossover study of fourteen patients with PD was conducted over 16 weeks with Mucuna pruriens powder and commercial dopamine/carbidopa (LD/CD) to measure quality of life, symptoms of motor and nonmotor impairments, and good mobility without excessive dyskinesias. It was found that Mucuna pruriens intake could control motor and nonmotor symptoms in patients with advanced PD. The clinical outcome of Mucuna pruriens powder was similar to that of commercial LD/CD powder. However, gastrointestinal effects were also observed in some patients [19]. Mucuna pruriens seed powder shows superior results to conventional LD in long-term treatment of PD for a short period of time [22]. Mucuna pruriens powder extracted from roasted seeds was tested for efficacy and safety in 18 PD patients with advanced disease. Compared to LD plus dopa-decarboxylase inhibitor (LD + DDCI), Mucuna pruriens high dose (Hd) (17.5 mg/kg) showed similar clinical effects with a better tolerability profile [20]. The effectiveness and tolerability of combinations of herbal compounds (Datura stramonium, Vicia faba, Banisteria caapi, and Mucuna pruriens) in PD patients were examined. In a 12-week open study, sixty PD patients (46 males and 14 females) were treated. The adverse effects were mild gastrointestinal effects [23]. The co-administration of Mucuna preparation with aromatic amino acid decarboxylase inhibitors results in impaired bioavailability, which explains why Mucuna has a lower dyskinetic potential than standard LD formulations [21]. The results of all five studies support the fact that Mucuna pruriens is capable of treating PD, although each study used different dose combinations. The side effects are few, and include dyskinesias and gastrointestinal problems. All five studies on human trials confirmed the efficacy of Mucuna pruriens in treating PD.
PD (animal model clinical trials)
A standard diet was used to feed PTEN-induced putative kinase 1 (PINK1B9) mutant larvae and adults, which were tested on various days. When PINK1B9 flies were treated with 0.1% Mucuna pruriens extract, their olfactory response to 1-hexanol was completely restored and their climbing behavior improved. However, LD (0.01%, percentage present in Mucuna pruriens extract 0.1%) improved climbing only for PINK1B9 flies aged 10–15 days. The levels of Mucuna pruriens extract were restored to those of the wild type (WT) through the use of both damaged mitochondria and thiobarbituric acid reactive substances (T-bars) in the PINK1B9 antennal lobes, as determined via transmission electron microscopy. In a whole-brain analysis, Mucuna pruriens extract was found to restore Bruchpilot (BRP) expression and tyrosine hydroxylase (TH) expression, but not LD expression alone. According to these findings, the effects of Mucuna pruriens are not solely determined by its LD content. These findings support the clinical observation that Mucuna pruriens extract is a medication that delays the onset of chronic LD-induced motor complications [28]. Similarly, another study examined the glutathione (GSH) system and superoxide dismutase (SOD) levels, as well as telomere length, using a Drosophila melanogaster model of PD (PINK1B9). Evaluations were performed both before and after treatment with phytoextracts. Mutant PINK1B9 flies presented lower GSH and SOD activity and longer telomeres than WT flies. Mucuna pruriens treatment improved oxidative stress resistance [24]. When Mucuna pruriens extract was administered to larvae and adults, crop muscle parameters and mitochondrial morphologies were rescued [25]. Using cellular models of PD (BV-2 microglia in mice and SH-SY5Y cells in humans), Caenorhabditis elegans, and Drosophila melanogaster, an LD reduced Mucuna pruriens seed extract was prepared and evaluated for its anti-PD effects. It has been suggested that Mucuna pruriens contains more bioactive compounds than LDs and may have a neuroprotective effect against PD [26]. There was no significant difference between climbing ability in treated and LD-treated flies administered Zandopa (31.7%), which contains Mucuna pruriens (for which 7.5 g of Zandopa contains 6.525 g of Mucuna pruriens standardized processed seed powder). Even though the LD content was approximately the same as that in the LD treatment, the treatment did not result in a significant effect [29]. Treatment with Mucuna pruriens seed extract improved health outcomes in PD mouse brains by enhancing catalase activity, reducing malondialdehyde (MDA) levels, and reducing nitrite levels. Compared to those of untreated Parkinsonian mouse brains, the hanging times were significantly greater, the narrow beam walk times were significantly lower, and the foot printing errors were significantly lower. Mucuna pruriens seed extract significantly improved TH immunoreactivity in the paraquat-treated substantia nigra (SN) and striatum [31]. One study used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) as a neuroprotectant to evaluate the neuroprotective effects of Mucuna pruriens seed extract. Tests such as rotarod, foot printing, and hanging were used to determine whether Mucuna pruriens and estrogen-treated mice exhibited behavioral recovery. A TH immunostaining technique was used to evaluate the regeneration of dopamine neurons in the SN. In addition, oxidative damage and glial activation were assessed using the immunoreactivity of inducible NO synthase (iNOS) and glial fibrillary acidic protein (GFAP). Using high-performance liquid chromatography (HPLC), the nigrostriatal region was analyzed for dopamine and its metabolites. TH-positive cells were restored to both the striatum and SN region after Mucuna pruriens treatment, while iNOS and GFAP levels were reduced in the SN region. Compared to those in MPTP-intoxicated mice, dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid in Mucuna pruriens-treated mice were significantly greater than estrogen. By reducing oxidative stress and possibly integrating neuronal-glial cell communication, Yadav et al. [30] concluded that Mucuna pruriens has therapeutic potential for neurodegenerative diseases, especially PD. Several in vitro studies have shown that LDs strongly damage deoxyribonucleic acid (DNA) when combined with copper ions. A substantial amount of Mucuna pruriens cotyledon powder (MPCP) chelated copper ions in a dose-dependent manner, which may have contributed to the DNA protection properties of the MPCP [35]. To study the long-term effects of Mucuna pruriens endocarp in HP-200 on monoaminergic neurotransmitters and metabolites, different regions of the rat brain were examined. In animal models and human studies, 6-hydroxydopamine was found to have antiparkinson effects resulting from components other than LDs or enhancing LDs [36]. Mucuna pruriens seed powder water extract was studied as part of chronic parenteral administration, as was treatment with DDCIs, and benserazide. According to their study, Mucuna pruriens contains water-soluble ingredients that are either similar to DDCI or reduce its need [33]. PD pathophysiology is closely linked to oxidative stress. In addition to scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, Mucuna pruriens also scavenges 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals and ROS. On plasmid DNA, Mucuna pruriens showed no genotoxic/mutagenic activity and significantly inhibited lipid and deoxyribose sugar oxidation [34]. After treatment with Mucuna pruriens extract for one month, the increase in oxidative stress in PD patients significantly decreased. In behavioral tests, Mucuna pruriens extract was demonstrated to significantly increase spontaneous locomotion and grip strength [23]. Mucuna pruriens endocarp powder (MPEP) without and with CD, as well as LD + CD was used to evaluate the mechanistic basis of Mucuna pruriens effects in hemiparkinsonian (HP) monkeys. Both treatments decreased the SN reticulata (SNR) firing rate compared to that in the HP group. There were no significant changes in subthalamic nucleus (STN) firing properties. Mucuna pruriens water extract alleviates symptoms without causing dyskinesia. As such, Mucuna pruriens has a different effect on the basal ganglia than LDs when treating parkinsonism and dyskinesia [32]. Several clinical trials on animal models confirm the efficacy of Mucuna pruriens seed extract in PD.
ED
It has been demonstrated that Mucuna pruriens prevents damage to sexual behavior and reproductive parameters, including essential proteins, in chronic unpredictable mild stress (CUMS) mice. A dose of 600 mg/kg led to an increase in sperm quality and testosterone levels, and a decrease in corticosterone levels, which improved sexual performance and seminiferous epithelium degeneration. Mucuna pruriens-CUMS co-treatment also improved the expression of A-Kinase Anchoring Protein 4 (AKAP4), androgen receptor (AR), and tyrosine-phosphorylated (TyrPho) proteins in the testis, epididymis, and sperm. Mucuna pruriens increased steroidogenic acute regulatory protein (StAR) and cytochrome P450 family 11 subfamily A Member 1 (CYP11A1) expressions in the testis. It also suppressed testicular apoptosis via decreased expression of heat shock protein 70 (Hsp70) and caspases 3, and 9 [38]. Mucuna pruriens seed extracts without LDs were also studied for their biological effects. In vitro, polar fractions of Mucuna pruriens seeds upregulate endothelial NO synthase (eNOS) and neuronal NO synthase (nNOS), which in turn stimulate the production of NO and maintain intracellular levels of cGMP. In penile tissue, the extract may stimulate eNOS and nNOS expression, which results in NO production, and maintains cGMP levels. Furthermore, this extract has been shown to prevent the deterioration of penile tissue as a result of diabetes [11]. In vitro and in vivo, Rho-kinase 2 (ROCK-II) activates smooth muscle in the corpus cavernosum, and ROCK-II inhibitors relax smooth muscles, so Mucuna pruriens seed extracts that inhibit this process may prove useful for treating ED. Mucuna pruriens, as aphrodisiacs and for male sexual disorder (MSD) might be involved in this process, in part due to the inhibitory potential of ROCK-II [39]. The effect of the ethanolic seed extract of Mucuna pruriens on damaged dorsal nerve of the penis (DNP) in aged rats in relation to penile erection was studied. A significant disturbance in the hypophysial gonadal axis was observed in aged rats. The number of myelinated fibers was reduced, and the diameter, vacuolization, indentation of the myelin sheath, and degeneration were observed. A decrease in nNOS and its cofactor nicotinamide adenine dinucleotide phosphate (NADPH diaphorase) was observed in aged DNP rats. The nerve conduction velocity was slow in aged rats, and a concomitant poor penile reflex was also noted. AR expression was lower in aged rats in the DNP group than in the young and control groups. The number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive cells was increased in aged rats with DNP [39]. Compared with diabetes-induced rats, animals with diabetes had significantly lower daily sperm production (DSP), luteinizing hormone (LH) levels, follicle-stimulating hormone (FSH) levels, and testosterone levels, whereas animals administered seed extracts of Mucuna pruriens showed significant improvements in sexual behavior, libido and potency, sperm parameters, DSP, and hormone levels [41]. A high level of oxidative stress and a low level of antioxidants in penile tissue may contribute to an increase in collagen deposition and fibrosis. Mucuna pruriens overcome oxidative stress and preserve penile histoarchitecture [42]. Studies show that Mucuna pruriens seeds extract are found effective in the treatment of ED along with other disorders.
Diabetes
In a study (plasma glucose > 180 mg/dL, 21 days) and a chronic study in alloxanized rats (plasma glucose > 280 mg/dL, 120 days) in streptozotocin mice (plasma glucose > 400 mg/dL, 60 days), Mucuna pruriens-ethanol extracts (100, 200, and 400 mg/kg/day) were evaluated. At week 6, at a dose of 200 mg/kg/day, an aqueous extract of an alcohol extract of Mucuna pruriens demonstrated the greatest antihyperglycemic effect [37]. In another study, crude ethanolic extracts of Mucuna pruriens seeds were administered to diabetic rats induced by alloxan (plasma glucose > 450 mg/dL) at doses of 5, 10, 20, 30, 40, 50, and 100 mg/kg. After 8 hours of treatment, the blood glucose levels of the diabetic rats were reduced by 18.6%, 24.9%, 30.8%, 41.4%, 49.7%, 53.1%, and 55.4%. It is also important to note that even though several adverse effects have been observed at higher doses (8–31 mg/kg), these findings confirm the safety of Mucuna pruriens extracts at lower doses [9]. Only a few studies are observed in diabetes, still more scientific evidences are required to elaborate on the effects of Mucuna pruriens on diabetes.
Discussion
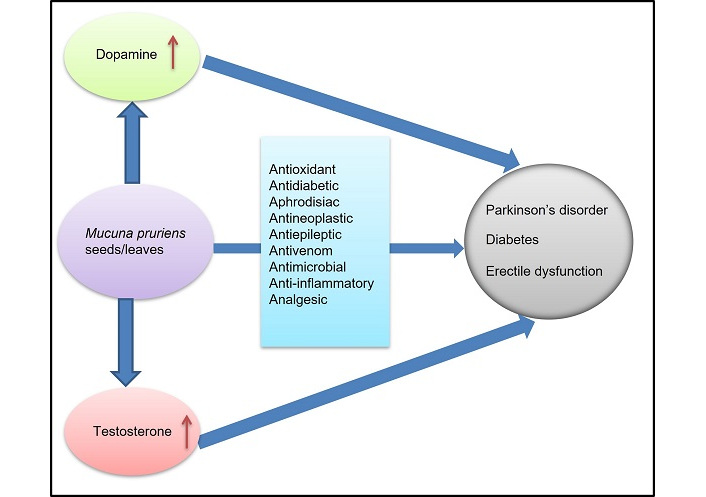
The tropical legume Mucuna pruriens is indigenous to Africa and tropical Asia, but has become widely naturalized and cultivated throughout the world. The most common names for these plants are cowage, cowitch, Florida velvet bean, lacuna bean, Lyon bean, Mauritius velvet bean, monkey tamarind, velvet beans, and Yokohama velvet bean [43]. Mucuna pruriens seed extract contains alkaloids, flavonoids, glycosides, saponins, steroids, tannins, terpenoids, and other plant secondary metabolites, as well as other secondary metabolites, which are responsible for its medicinal properties [44] or their health disorders. The use of Mucuna pruriens-based therapeutics as a source of LD medication could have a positive impact on the market. It is widely available in local markets at an affordable price in many countries. It is well tolerated and beneficial for brain function and well-being. There is clinical evidence that it has a positive effect on libido and testosterone levels. It also has a positive effect on ED and diabetes [16]. Researchers can learn how to prevent, treat, and cure human diseases through animal testing in clinical research since humans and animals are biologically similar. The Missouri Medicine Institute reported that rats, mice, and humans all have approximately 30,000 genes in common, approximately 95% of which are shared by all three [45]. This review therefore reviewed clinical and preclinical studies to obtain more accurate results. A major disadvantage of using Drosophila to investigate human diseases is the difference between the brain anatomy of flies and that of humans. However in flies expressing mutant α-synuclein, inclusions of the protein were also found in non dopaminergic neuronal cells, as in human brains affected by PD [46]. It is pertinent to note, however, that mice and rats are not suitable models for humans in many significant areas, for example, in brain function, and reproduction, where human biology is remarkably different from that of rodents [47]. Research on monkeys led to the development of life-changing PD treatments, such as dopaminergic therapies, deep brain stimulation for tremor reduction, and constraint-induced movement therapy. ED is well understood in animal models. While human sexual function cannot be reproduced perfectly, these animal models provide a reproducible paradigm for investigating and evaluating ED. While there is some scientific research investigating the potential benefits of Mucuna pruriens for these conditions, it is important to note that the evidence is limited, and further studies are needed to establish its efficacy.
To determine the link between EDs and PD, researchers conducted a study on 32,616 health professionals from 1986 to 2000. They observed that ED was associated with a higher risk of developing PD [48]. Diabetes inhibits the firing of SN dopaminergic neurons, leading to PD. Insulin receptors are relatively abundant in SN neurons. The toxicity of MPTP, which causes parkinsonism in many patients, has also been linked to mitochondrial dysfunction. A key component of diabetes pathophysiology is mitochondrial dysfunction, a key regulator of glucose-stimulated insulin secretion in pancreatic epithelial cells [49, 50]. Several studies have confirmed that men with EDs are more likely to have diabetes mellitus than men without EDs [51, 52].
In diabetes mellitus, oxidative stress is increased and ROS are produced at an elevated rate, resulting in a cascade of events: reduced NO increased prothrombotic factors including tissue factor, and plasminogen activator inhibitor-1 increased endothelin-1, resulting in thrombosis and vasoconstriction, and increased nuclear factor kappaB and activation protein 1, which leads to ED [52]. Diabetes-related ED is closely associated with adenosine triphosphate (ATP) levels in the mitochondria. Specifically, long-term hyperglycemia impairs the function of smooth muscles in the corpus cavernosum by reducing mitochondrial biogenesis, increasing adenosine diphosphate (ADP)/ATP ratios, altering ultra structural changes, and triggering ROS [53]. It is important to note that the relationship between these conditions is complex. The exact mechanisms linking PD and ED are not fully understood, but several factors may contribute to these findings (Figure 2). Not all people with diabetes or PD experience ED.
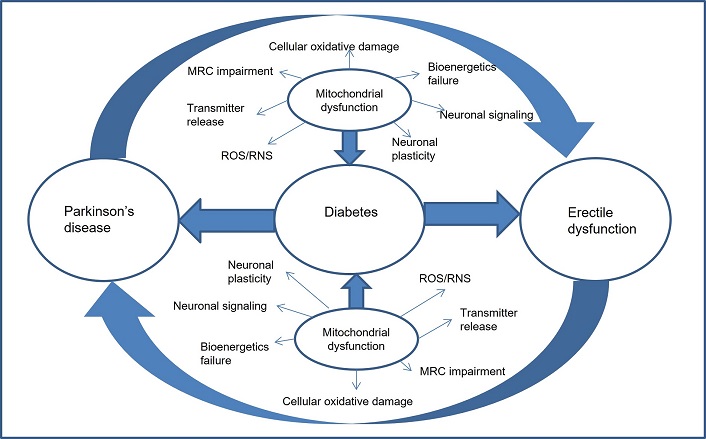
Relationships between diabetes, Parkinson’s disease, and erectile dysfunction. MRC: Medical Research Council; RNS: reactive nitrogen species; ROS: reactive oxygen species
Evidence supports the hypothesis that Mucuna pruriens seed extract containing LDs and antioxidants can enhance sperm quality and essential testicular proteins under cesarean section (CS) conditions by enhancing testosterone levels while reducing caspase proteins involved in apoptosis [54, 55]. It improves sperm count, motility and alleviates psychological stress and seminal plasma lipid peroxide levels. Additionally, treatment restored the amount of SOD, catalase, GSH, and ascorbic acid in the seminal plasma of infertile men [56]. In PD, the ethanolic Mucuna pruriens extract improves immunoreactivity and protects dopaminergic neurons from NO injury. It has been shown that the ethanolic extract from Mucuna pruriens seeds naturally restores erectile tissue from oxidative stress, and reduces diabetes-induced sperm damage [16]. As “first-line” drugs for treating ED, PDE inhibitors prevent PDE5 enzymes from neutralizing cGMP in a way that prolongs penile erection. PDE5 inhibitors can cause major side effects, so researchers are investigating natural bioactive compounds that can alleviate and cure ED [57]. The extract of pruriens free of LDs contained catechols and polyphenols, which upregulated the expression of NO-producing genes. This extract improved sexual behavior and prevented diabetes-induced penile tissue deterioration. By inhibiting ROCK-II, Mucuna pruriens relaxes erectile tissue, improving sexual function. ROCK-II is responsible for contracting penile smooth muscle and impairing erections [40]. The PDE5 inhibitory constituents of medicinal plant extracts include alkaloids, phenolics, and polycyclic aromatic hydrocarbons. Isoflavones and biflavones are the major active constituents of isoflavones, and the prenyl groups in isoflavones and the methoxy groups at the C-5 position in flavones are considered essential for their inhibitory action [58]. Mucuna pruriens contains polyphenolic compounds that are pharmacological precursors and contribute to antioxidant activity. Mucuna pruriens has a high phenolic content (3,730.1 ± 15.52 mg gallic acid equivalent/g; flavonoids = 63.03 ± 1.95 mg quercetin equivalent/g), which may be responsible for its antioxidant activity and therapeutic properties [59]. Mucuna pruriens contains isoflavanones, isoflavones, and pterocarpans that inhibit α-glucosidase [60]. There is preliminary evidence that Mucuna pruriens may improve erectile function and sexual desire in men with ED. However, additional well-designated studies are needed to verify the efficacy and safety of these herbs.
Diabetes complications may develop due to the production of ROS in mitochondria by hyperglycemia. AMP-activated protein kinase (AMPK) works through peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) to promote mitochondrial biogenesis. However, the mechanism by which AMPK upregulates PGC-1α activity remains unclear [61]. Blockade of mitochondrial ROS production through the induction of AMPK or PGC-1α could be useful in the design and development of drugs to prevent diabetic complications [62]. Diabetes patients could benefit from Mucuna pruriens because it has an antihyperglycemic effect [63]. However, further research is needed to determine efficacy, optimal dosage, and safety of Mucuna pruriens in humans.
A rich source of LD, a precursor to dopamine in PD, Mucuna pruriens could ease symptomatic symptoms with fewer side effects. In PD, dopamine deficiency results in motor symptoms and Mucuna pruriens may alleviate these symptoms by increasing dopamine levels. Protein aggregation, oxidative stress, excitotoxicity, neuroinflammation, and genetic mutations all contribute to the development of PD [64, 65]. Additional neuroprotective effects of Mucuna pruriens beyond LD supplementation have been suggested [36, 66]. A number of antioxidant compounds as well as bioactive substances may protect neurons from inflammation and oxidative stress. A key mediator of neurodegeneration in PD is synuclein overexpression, inflammation, mitochondrial dysfunction, and apoptosis, which are protected from Mucuna pruriens and its constituent compound, ursolic acid. A recent study revealed that the possible mechanism involved the regulation of glycogen synthase kinase 3β (GSK-3β)/α-synuclein/Ca2+ signaling [67]. A recent study indicated that neurons with mutations in glucosylceramidase beta 1 (GBA1) exhibit extended mitochondria-lysosome connections [68]. A recent study demonstrated that seed extract and LD can potentially alleviate neuronal apoptosis by regulating the PI3K/Akt signaling pathway and affecting both forkhead box transcription factor and GSK-3β [69]. These studies support the protective effects of Mucuna pruriens in PD. However, additional clinical trials are necessary to prove its effectiveness.
Possible molecular mechanism of Mucuna pruriens phytocompounds for treating T2D, PD, and ED
Mucuna pruriens seeds contain a large amount of bioactive compounds. Its phytochemical composition is largely responsible for its efficacy in treating diabetes, PD, and ED. In-depth review of the key phytochemicals in Mucuna pruriens and how they work on a molecular level to offer therapeutic benefits for these conditions are mentioned in Table 3.
Mucuna pruriens phytocompounds and its mechanism of action in related disorders
| S.No. | Phytocompounds | Mechanism of action | Disorders | Reference |
|---|---|---|---|---|
| 1 | Leva-dopa | Leva-dopa is a direct precursor to dopamine and is critical for its potential in treating PD | PD and ED | [70] |
| 2 | Alkaloids (prurienine, mucunine, and mucunadine) | Anti-inflammatory, antioxidant, and neuroprotective effects | Diabetes, PD, and ED | [71] |
| 3 | Flavonoids (quercetin, kaempferol, and rutin) | Strong antioxidant, anti-inflammatory, and cardioprotective properties | Diabetes and ED | [72, 73] |
| 4 | Tannins (astringent properties) | Antioxidant, anti-inflammatory, and antibacterial effects | Diabetes and ED | [74] |
| 5 | Saponins | Anti-inflammatory, immunomodulatory, and antidiabetic effects | Diabetes, PD, and ED | [75] |
| 6 | Phenolic acids (chlorogenic acid and caffeic acid) | Antioxidants that help to neutralize oxidative stress | Diabetes, PD, and ED | [76] |
| 7 | Proteins and peptides (lectins and enzymes) | Modulate immune responses and have a role in managing glucose metabolism | Diabetes | [77] |
ED: erectile dysfunction; PD: Parkinson’s disease
Mucuna pruriens may influence several key molecular pathways involved in glucose regulation. Diabetes is characterized by chronic hyperglycemia, insulin resistance, and impaired insulin secretion. In insulin secretion and glucose metabolism, dopamine plays an important role. Mucuna pruriens increases dopamine levels, which may boost insulin sensitivity and glucose uptake. Insulin secretion and sensitivity are affected by dopamine receptors (especially D2 receptors) [78, 79]. Increasing dopamine signaling may improve insulin sensitivity and pancreatic beta-cell function [9]. In Mucuna pruriens, flavonoids and phenolic compounds inhibit the enzyme alpha-glucosidase, reducing carbohydrate breakdown and slowing the absorption of glucose [80]. This can help prevent postprandial blood sugar spikes. By neutralizing free radicals, flavonoids, phenolic acids, and saponins in Mucuna pruriens protects pancreatic beta cells from damage caused by oxidative stress. PD is characterized by the progressive loss of dopaminergic neurons, particularly in the SN. The alkaloids and phenolic compounds in Mucuna pruriens help mitigate oxidative stress by neutralizing ROS that damage neuronal cells. Chronic inflammation is a hallmark of neurodegenerative diseases like PD. Phytochemicals in Mucuna pruriens, particularly alkaloids and flavonoids, reduce the levels of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), potentially slowing the progression of PD [81]. Mucuna pruriens may also have a role in improving mitochondrial health, which is crucial for maintaining neuronal integrity and function. Saponins in Mucuna pruriens may modulate mitochondrial activity, supporting cell survival. Clinical studies have shown that Mucuna pruriens can be as effective as synthetic LD in improving motor scores, and also improves the quality of life in PD patients by reducing symptoms like motor fluctuations and “off” periods [22]. ED is a condition that affects sexual health and is often linked to hormonal imbalances, stress, poor circulation, and oxidative stress. Dopamine is a key neurotransmitter involved in sexual arousal and erectile function. By increasing dopamine levels through LD, Mucuna pruriens may help improve sexual drive and erectile function [38]. The dopaminergic pathways are crucial for initiating and maintaining an erection. Saponins and flavonoids are believed to stimulate the production of testosterone, potentially improving libido and sexual function [82]. NO is critical for vasodilatation and proper erectile function. Study observed that Mucuna pruriens may increase NO production, promoting better blood flow to the penis and supporting erection [11]. Chronic stress is a common cause of ED, and Mucuna pruriens may help reduce cortisol levels [55, 56]. The alkaloids and flavonoids in Mucuna pruriens have anxiolytic (anxiety-reducing) properties, which may improve ED caused by psychological factors.
Limitation of clinical trials
It is unlikely to determine a safe dose of Mucuna pruriens based on the available literature and trials. No studies were found in this review on the genotoxicity, development toxicity, chronic toxicity, and carcinogenicity of Mucuna pruriens seeds. Only limited toxicological data are available for the seed extracts of Mucuna pruriens seeds. All aspects of toxicity have not been studied in available human and animal clinical trials.
This is the first systematic review of its kind, and because several databases and key terms were included, the authors are confident that they have not missed much information. Because the studies had a cross-sectional design, quality assessment was not possible. Since different approaches are used to measure quality and quantity insecurity, it is difficult to compare the outcomes of different studies. This review therefore does not attempt to conduct a meta-analysis.
Conclusions
Various clinical and preclinical studies confirm that Mucuna pruriens powder has similar clinical outcomes to commercial LD/CD powders. However, some patients reported gastrointestinal side effects. Long-term treatment of PD with Mucuna pruriens seed powder is more effective than treatment with conventional LD. Mucuna pruriens has been shown to alleviate PD symptoms by increasing dopamine levels in the brain. There are several antioxidant compounds as well as bioactive compounds in Mucuna pruriens that may prevent inflammation and oxidative stress in neurons. Clinical evidence suggests that Mucuna pruriens enhances libido and testosterone levels. It also reduces blood sugar levels and improves glucose tolerance. The results of numerous clinical trials (human) and preclinical trials (animal) suggest that Mucuna pruriens may be effective at treating ED, PD, and diabetes, although additional clinical evidence is still needed. However, more rigorous clinical trials are needed to establish the efficacy, optimal dosage, and long-term safety of Mucuna pruriens for the treatment of diabetes, ED, and PD.
Abbreviations
| AMPK: | AMP-activated protein kinase |
| AR: | androgen receptor |
| ATP: | adenosine triphosphate |
| CD: | carbidopa |
| cGMP: | cyclic guanosine monophosphate |
| CUMS: | chronic unpredictable mild stress |
| DDCI: | dopa-decarboxylase inhibitor |
| DNA: | deoxyribonucleic acid |
| DNP: | dorsal nerve of the penis |
| DSP: | daily sperm production |
| ED: | erectile dysfunction |
| eNOS: | endothelial nitric oxide synthase |
| GFAP: | glial fibrillary acidic protein |
| GSH: | glutathione |
| GSK-3β: | glycogen synthase kinase 3β |
| HP: | hemiparkinsonian |
| iNOS: | inducible nitric oxide synthase |
| LD: | leva-dopa |
| MPCP: | Mucuna pruriens cotyledon powder |
| MPTP: | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| nNOS: | neuronal nitric oxide synthase |
| NO: | nitric oxide |
| PD: | Parkinson’s disease |
| PDE5: | phosphodiesterase-5 |
| PGC-1α: | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PINK1B9: | PTEN-induced putative kinase 1 |
| ROCK-II: | Rho-kinase 2 |
| ROS: | reactive oxygen species |
| SN: | substantia nigra |
| SOD: | superoxide dismutase |
| T2D: | type 2 diabetes |
| TH: | tyrosine hydroxylase |
| WT: | wild type |
Declarations
Author contributions
RV: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing. VM and PSB: Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The author declares that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.