Affiliation:
Department of Vaccine Technology, Vaccine Institute, Hacettepe University, Ankara 06230, Turkey
Email: sezerokay@gmail.com
ORCID: https://orcid.org/0000-0003-0355-6672
Explor Immunol. 2023;3:433–441 DOI: https://doi.org/10.37349/ei.2023.00111
Received: February 09, 2023 Accepted: May 11, 2023 Published: October 10, 2023
Academic Editor: Vladimir N. Uversky, University of South Florida, USA
The article belongs to the special issue Old and New Paradigms in Viral Vaccinology
Vaccines are prophylactic medical products effectively used against infectious diseases. Although a high amount of vaccine studies are conducted at the preclinical stage, the number of approved vaccines is less than 10%. Development of vaccines from the research stage to the approval of administrative institutions takes about 5 years to 10 years conventionally. However, this period of time for vaccine development is not convenient during public health emergencies because an effective vaccine is required in a short time to restrict the speed of high mortality and morbidity. The pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had its catastrophic effects worldwide quickly. Therefore, an atypical process was followed for the development of COVID-19 vaccines. Great effort was spent in terms of cooperation among the governmental institutions, academia, and medical companies as well as a high amount of budget was allocated to develop effective vaccines against COVID-19. As of March 2023, the numbers of COVID-19 vaccines in clinical and preclinical development were 183 and 199, respectively. An emergency use authorization (EUA) process was applied to accelerate the approval of the vaccines. Consequently, vaccinations could be started in less than a year, which decelerated the speed of the pandemic. Although EUA caused hesitancy among some people questioning the safety and efficacy of the vaccines, the vast majority of the population was vaccinated. Currently, more than 5.5 billion people (about 70% of the world population) have received 13.38 billion doses of 11 different COVID-19 vaccines, and 73% of the doses were Comirnaty manufactured by Pfizer/BioNTech.
Vaccines are the most effective tools for the prophylaxis of infectious diseases. Attenuated pathogens were used for vaccination initially to cope with diseases such as smallpox, anthrax, tuberculosis, and pasteurellosis. Later, at the end of the 19th century, the inactivation of microorganisms to use them for vaccination was invented, and many vaccines were produced using this technique against various diseases. Technological improvements especially in molecular genetics facilitated the development of innovative vaccines such as recombinant hepatitis B vaccine or more recently the messenger RNA (mRNA) vaccines [1, 2].
The development process is different for varying types of vaccines. Therefore, launching a new vaccine, including preclinical studies, clinical trials, approval procedures, and mass production takes 5 years to 10 years generally. The number of preclinical studies conducted for the development of vaccines is high. However, many of them fail in the clinical phases. The rate for getting approval from administrative institutions such as the Food and Drug Administration (FDA) or European Medicines Agency (EMA) is less than 10% [3]. Therefore, certain steps are followed during the development of a vaccine from the research phase to the post-market period. A vaccine candidate should pass the following steps to come onto the market [4–6]:
Exploratory phase: In this stage, components of the vaccine formulation are studied. For subunit vaccines, antigenic proteins are determined or epitope peptides can be identified using in silico methods. The adjuvant to be used in the vaccine formulation is selected. For live vaccines, the pathogen is attenuated. For killed vaccines, the inactivation methodology of the pathogen is optimized. The vaccine candidate is formulated to be tested in the preclinical phase.
Preclinical phase: The aim of this stage is to have an idea about the safety and efficacy of vaccine candidate using cell cultures and experimental animals. The disease in humans is mimicked in an appropriate animal model. Single and repeated-dose toxicity, pharmacokinetics, pharmacodynamics, immunogenicity, and local tolerance of the vaccine candidate are tested. If the animal model is not appropriate for the disease type, preclinical results would not reflect the situation in humans.
Clinical phase: In this stage, the candidate vaccine is tested in healthy human volunteers. Safety, immunogenicity, and clinical efficacy profiles are evaluated in three distinct phases for approval, and one more stage (phase 4) is included for post-market surveillance.
Phase 1: The safety of the vaccine candidate is evaluated in a small number of healthy humans ranging from 20 to 100. Different doses of vaccine are administered to volunteers to find out any possible side effects, and finally, an optimal dosage for the vaccine is determined. This stage takes several months, and approximately 70% of the candidates move to phase 2.
Phase 2: The safety and immunogenicity of the vaccine are evaluated by randomized controlled studies in a higher number of humans ranging from 100 to 1,000. The immune responses are compared between the volunteers who received the vaccine and placebo controls. Sometimes efficacy data might be obtained in this phase. This stage takes several months to 2 years, and approximately 33% of the candidates move to phase 3.
Phase 3: The safety and clinical efficacy of the vaccine are tested in this final phase in a large population of volunteers (several thousand). Most of the safety data including long-term or rare side effects are provided in this phase. This stage takes 1 year to 4 years. If the vaccine provides successful safety and efficacy data in phase 3, the manufacturer applies for a biologics license application to administrative institutions such as FDA or EMA to get approval for marketing. Approximately 25–30% of the vaccines move to phase 4.
Phase 4: The vaccines with approval after phase 3 come onto the market. However, potential safety and efficacy issues of the vaccine are still monitored during the post-market period, which is called phase 4.
The conventional vaccine development paradigm spreads over a wide period of time, which is not appropriate for emergency situations such as pandemics. Therefore, a new paradigm for the “emergency” vaccines has been applied to develop coronavirus disease 2019 (COVID-19) vaccines in a shorter time. Although shortening the process created hesitancy about the safety and efficacy of vaccines in some people, the majority of the world population was fully vaccinated with COVID-19 vaccines. The aim of this article is to discuss the vaccine development paradigm during emergency situations, the effect of COVID-19 on this process, and the hesitancy against vaccines developed using a new paradigm.
Over the past two decades, many epidemics including influenza, Ebola, Zika, measles, and recently the COVID-19 pandemic threatened human health seriously. New outbreaks urge us to be prepared for combating new or reemerging diseases. Since vaccines have a pivotal role in this combat, preparedness for vaccine development is crucial in the era of epidemics and pandemics [7].
In the spring of 2009, a novel influenza A (H1N1) virus pandemic strain pdm09 emerged, which was composed of an unusual repertoire of influenza genes different than the ones identified previously. Therefore, the cross-protection provided by seasonal influenza vaccines against A(H1N1)pdm09 was little [8, 9]. However, cross-neutralization was demonstrated using sera from mice immunized with DNA vaccines encoding hemagglutinin (HA) proteins of A/California/04/2009 or A/South Carolina/1/1918 [10]. Following the identification of 2009 H1N1, companies from the vaccine industry cooperated with public health and regulatory agencies for the development of a vaccine against A(H1N1)pdm09. In mid-September 2009, FDA approved four monovalent A(H1N1)pdm09 vaccines including inactivated ones produced by CSL Biotherapies, Novartis, and Sanofi Pasteur as well as a live attenuated vaccine produced by MedImmune [11, 12]. It was estimated that the vaccination from October 2009 to April 2010 in the United States (US) prevented up to 1.5 million clinical cases and 500 deaths [8]. Development of A(H1N1)pdm09 vaccines was relatively rapid because the technology and key regulators needed for influenza vaccines were well-established [7].
The rapidness of influenza vaccine development was not valid for the works on Ebola, Zika, and severe acute respiratory syndrome (SARS). In November 2002, an outbreak of SARS coronavirus (SARS-CoV) started and rapidly spread worldwide. The disease resulted in a relatively small number of deaths. However, the mortality and transmissibility rates of the virus were high. Therefore, work on the vaccine development against SARS-CoV was started immediately but the epidemic ended approximately 8 months later. Eventually, the funds were cut, and the development of the SARS-CoV vaccine was interrupted. Only two candidates, a DNA vaccine and an inactivated vaccine, were evaluated in the phase 1 trial [13].
In December 2013, the Ebola epidemic started when a 1.5-year-old boy from a village in Guinea was supposed to be infected by bats, and soon five more cases of fatal diarrhea were reported in the same area. A disease alert was issued officially in January 2014 in the district and the disease spread to Conakry, the capital city of Guinea. Next, a medical alert was issued by Guinea’s Ministry of Health for an unidentified disease in March 2014. The illness was identified by the Pasteur Institute (France) as Ebola virus disease (EVD) caused by Zaire ebolavirus, and the World Health Organization (WHO) declared an EVD outbreak officially on March 23, 2014 [14]. The epidemic continued for more than 24 months with widespread transmission in Guinea, Liberia, and Sierra Leone. There was sufficient time for vaccine development, and WHO impelled acceleration for it. Although Ebola vaccines had been developed and tested in non-human primates previously, these candidates were not evaluated in clinical trials during the epidemic [13]. Merck was funded by the US government for the development of an Ebola vaccine but the vaccine could not be produced before the epidemic ended. The company did not stop the work, and the vaccine (Ervebo) was approved by the EMA and FDA at the end of 2019, 70 months after the epidemic started. In 2018, Ervebo was used as an investigational vaccine against the world’s second largest EVD outbreak occurred in the Democratic Republic of the Congo [7, 15, 16].
The first case of COVID-19 caused by the SARS-CoV-2 virus was reported in December 2019 in Wuhan, China [17]. Shortly after, the disease spread worldwide rapidly, and the WHO declared a public health emergency of international concern on January 30, 2020. There were discussions about the pandemic potential of the outbreak [18, 19], and the WHO declared COVID-19 as a global pandemic on March 11, 2020 [20, 21]. More than 753 million confirmed cases and at least 6.8 million deaths have been reported globally as of January 29, 2023 [22].
The spread of COVID-19 at high speed worldwide created pressure on vaccine developers to accelerate the process because vaccination is the most appropriate way to obtain herd protection [1]. A wide variety and a vast amount of COVID-19 vaccines started to be developed quickly. At least 183 vaccine candidates passed the clinical trial phases [23]. Although the development of a vaccine takes more than five years conventionally, some of the COVID-19 vaccines showed success in an extremely short time and got approval in less than a year. Preclinical studies of mRNA vaccine BNT162b2 (Comirnaty), by Pfizer/BioNTech, were finalized in less than three months, and its phase I/II clinical trials started in April 2020 [24]. According to the data obtained from a multinational phase III clinical trial, an emergency use authorization (EUA) was issued to Comirnaty first time in the United Kingdom (UK) on December 2, 2020 [24]. In December 2020, Comirnaty received EUA in many countries including the US and the European Union (EU). EUA is granted to medical products when there is a public health emergency [4].
For acceptable COVID-19 vaccines, the WHO requested a minimum of 50% efficacy against the development of the disease, its progression to severe disease, and/or viral shedding/transmission [25]. The mRNA vaccine Comirnaty also obtained the emergency use listing (EUL) by the WHO on December 31, 2020. The EUL is given by the WHO to the vaccines that can be recommended for use according to their safety and efficacy data based on clinical trials, manufacturing and quality control processes, and vaccines’ feasibility in low- and middle-income countries. As of December 2, 2022, 11 COVID-19 vaccines (9 formulations) obtained the EUL (Table 1) [26, 27].
| No. | Vaccine | Platform | Company | Number of approved countriesa | EUL date |
|---|---|---|---|---|---|
| 1 | Comirnaty | mRNA | Pfizer/BioNTech | 149 | December 31, 2020 |
| 2 | Covishieldb | Non-replicating viral vector | Serum Institute of India | 49 | February 15, 2021 |
| 3 | Vaxzevria | Non-replicating viral vector | AstraZeneca | 149 | February 15, 2021 |
| 4 | Jcovden | Non-replicating viral vector | Johnson and Johnson | 113 | March 12, 2021 |
| 5 | Spikevax | mRNA | Moderna | 88 | April 30, 2021 |
| 6 | Covilo | Inactivated | Sinopharm | 93 | May 7, 2021 |
| 7 | CoronaVac | Inactivated | Sinovac | 56 | June 1, 2021 |
| 8 | Covaxin | Inactivated | Bharat Biotech | 14 | November 3, 2021 |
| 9 | Covovaxc | Protein subunit | Serum Institute of India | 6 | December 17, 2021 |
| 10 | Nuvaxovid | Protein subunit | Novavax | 40 | December 20, 2021 |
| 11 | Convidecia | Non-replicating viral vector | CanSino | 10 | May 19, 2022 |
a The vaccine was approved, authorized, licensed, granted EUA, or made available for use by a regulatory agency, a national authority, or another entity; b Covishield has the same formulation of Vaxzevria; c Covovax has the same formulation of Nuvaxovid
The typical vaccine development timeline is useless in public health emergencies such as COVID-19. A protective vaccine was needed in a short time to get over the devastating effects of the pandemic. A vaccine was also needed quickly because of the high mutation rate of the virus. If the vaccine is developed too late, its efficacy could be limited against new variants [28]. The process of vaccine development has many stages from research to marketing. Therefore, various local and international organizations such as government agencies, academia, and pharmaceutical companies should cooperate to accelerate the process [29]. Rapid information sharing through this cooperation can enable diversification, risk sharing, and the efficient use of resources by preventing unnecessary repetitions [30].
As an example of this cooperation, a program known as Operation Warp Speed (OWS) was announced in the US on May 15, 2020. Within the scope of OWS, the Department of Defense (DOD) and the Department of Health and Human Services (HHS) cooperated with private companies for the development of COVID-19 vaccines, therapeutics, and diagnostics to control the pandemic [31]. More than 13 billion US dollars (USD) were committed for the COVID-19 vaccines through OWS. Roughly 2.5 billion USD of this budget was allocated for vaccine research and development, and the rest for purchase agreements. Other high-income countries such as the UK and the EU were also allocated high budgets for COVID-19 vaccines [30].
Technological preparedness also accelerated COVID-19 vaccine development. The high speed for the RNA-based vaccines was not surprising because this platform has been used for the development of vaccines against various diseases such as cancer for more than 30 years, and some of them entered clinical trials. Although previous RNA-based vaccine candidates have not been granted approval for use in humans until COVID-19, this technology showed its success against SARS-CoV-2 [1]. The process for the development of mRNA vaccines against COVID-19 is depicted in Figure 1.
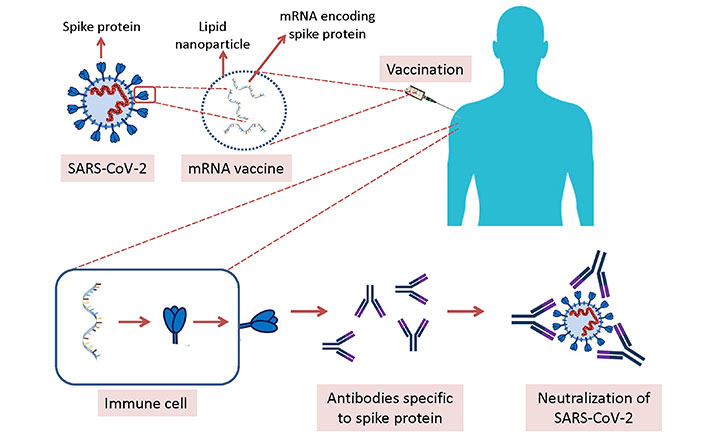
Development of mRNA vaccines against COVID-19. mRNA molecules encoding spike protein of SARS-CoV-2 are formulated with lipid nanoparticles, and injected intramuscularly. Immune cells in the body receive the mRNA molecules, and the spike protein is produced by these immune cells followed by the production of antibodies specific to spike protein. If the vaccinated person is infected with SARS-CoV-2, the virus is neutralized by the specific antibodies
Improvements in omics sciences, bioinformatics, and synthetic biology also sped up the development of COVID-19 vaccines. Determination of potential antigens and epitopes, the interaction between virus and human cells, schedules for vaccination, and issues related to vaccine safety and efficacy are laborious and costly taking too much time in conventional vaccinology. However, in silico prediction methods following computational and mathematical approaches help for the elimination of vaccine candidates showing low potential for safety and efficacy [25].
EUA was widely recognized by the public during the COVID-19 pandemic, however, it was first introduced in 1938 by the US Federal Food, Drug, and Cosmetic Act and revised in 2004 by the Project BioShield Act, authorizing the FDA to grant EUA to medical products including vaccines when a public health emergency such as pandemic occurs [4]. At the time of such a condition, unapproved medical countermeasures or their uses for the prevention, treatment, or diagnosis of life-threatening health conditions may be allowed by FDA on condition of certain statutory criteria [29, 32]. For example, FDA evaluated the EUA application of Comirnaty considering the safety data including side effects after the first and second dose, and effectiveness data including the rate of prevention from COVID-19 following vaccination. Serious side effects were not reported, and the vaccine was found to be 95% effective in COVID-19 prevention, the data obtained from 18,198 and 18,325 volunteers received vaccine or placebo, respectively. Therefore, FDA granted EUA to the COVID-19 vaccine of Pfizer/BioNTech [33].
Vaccine hesitancy is the reluctance to receive a vaccine, and it is a significant threat to public health in the prophylaxis of infectious diseases [34]. Hesitancy to the childhood vaccines has been observed for a long period of time. The main reasons for this hesitancy were found as religious or personal beliefs, safety concerns, and demand for additional information about the vaccines from healthcare providers [35].
Mass vaccination became a critical issue in COVID-19, and the EUA was useful to start vaccination of populations earlier to restrict the mortality of this disease. However, vaccination rates could not be reached to the desired levels especially at the beginning because a wide skepticism of EUA-granted COVID-19 vaccines was generated about their safety. The shorter time for the clinical phases caused questioning the safety of vaccines and many people hesitated to receive COVID-19 vaccines.
Many factors affect vaccine hesitancy, such as age, gender, race/ethnicity, politics, religiosity, education, and income [4, 36, 37]. The rate of COVID-19 vaccination was reported to be higher for women in the US [3]. However, according to the meta-analysis studies, vaccine acceptance was lower among women [36, 38].
Having a democratic and liberal political view was found to be associated with vaccine acceptance [36, 38]. Vaccination rates were reported to be higher among Whites and Asians compared to Blacks and Hispanics in the US, being the lowest among the black population [4]. As to the age groups, young people between 18–29 years old were less likely to accept vaccination compared to the ones aged 30 years and above [4] probably because the course of COVID-19 was more fatal for the elderly. EUA also affected the COVID-19 vaccination among children. The rate of acceptance for vaccination was only 31.3% for the parents of children under five years old in the US [39]. However, a study conducted in Zambia reported that the willingness of parents to have their child vaccinated against COVID-19 was 92% while the rate of receiving the vaccine themselves was 66% [40]. The rate of hesitancy to receive COVID-19 vaccines was higher in African countries, from 6% in Ethiopia to 41% in the Democratic Republic of Congo. The reason for this hesitancy was declared as low confidence in the safety and efficacy of the vaccines [34].
Vaccine hesitancy was also reported to be associated with low education, low income, and high religiosity [36]. Another study showed that internet search queries about the COVID-19 vaccines were mostly related to their influence on fertility, which was a factor for vaccine hesitancy [41]. These reports show that the vaccine hesitancy due to EUA was probably because some groups of people could not understand adequately the idea behind the EUA, and its dependence on scientific data.
Vaccines are critical countermeasures in public health emergencies such as pandemics. Like other medical products for use in human health, the development of vaccines requires critical steps in preclinical and clinical studies, which takes at least five years. However, this duration is too long at the time of the pandemic, and an efficient vaccine is highly desired in a shorter time than the traditional process. Therefore, an atypical process can be followed in pandemics, such as shortening the duration of clinical phases normally taking a long time in vaccine development. Approval of the vaccines for pandemics is also different than normal times, considered in the context of EUA. COVID-19 had devastating effects with high rates of mortality and morbidity worldwide. Therefore, the development of vaccines was supported, and EUA was granted to some vaccines in less than a year. These vaccines restricted the speed of the COVID-19 pandemic lowering the number of deaths. However, the shortened time for vaccine development and EUA increased vaccine hesitancy, especially among people with low education, low income, high religiosity, and at a young age.
COVID-19: coronavirus disease 2019
EMA: European Medicines Agency
EUA: emergency use authorization
EUL: emergency use listing
EVD: Ebola virus disease
FDA: Food and Drug Administration
mRNA: messenger RNA
OWS: Operation Warp Speed
SARS-CoV: severe acute respiratory syndrome coronavirus
US: United States
WHO: World Health Organization
SO: Conceptualization, Visualization, Writing—original draft, Writing—review & editing.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Marc H.V. Van Regenmortel
Christine Jacomet
Vladimir N. Uversky
Brent Brown ... Ingo Fricke
Chittaranjan Baruah ... Bhabesh Deka
Brent Brown ... Enrique Chacon-Cruz
Om Saswat Sahoo ... Subhradip Karmakar
Mikolaj Raszek ... Alberto Rubio-Casillas
Ankit Kumar ... Vijay Mishra
Yulia Desheva ... Irina Isakova-Sivak
