Affiliation:
Department of Public Health and Applied Nutrition, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima 770-8503, Japan
Affiliation:
Department of Public Health and Applied Nutrition, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima 770-8503, Japan
Affiliation:
Department of Public Health and Applied Nutrition, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima 770-8503, Japan
Affiliation:
Department of Public Health and Applied Nutrition, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima 770-8503, Japan
Affiliation:
Department of Public Health and Applied Nutrition, Institute of Biomedical Sciences, Tokushima University Graduate School, Tokushima 770-8503, Japan
Email: sakai@tokushima-u.ac.jp
Explor Immunol. 2024;4:333–340 DOI: https://doi.org/10.37349/ei.2024.00144
Received: December 15, 2023 Accepted: March 29, 2024 Published: May 29, 2024
Academic Editor: Lucia Malaguarnera, Università degli Studi di Catania, Italy
The article belongs to the special issue The Nutritional Influence on Immune Functionality
Aim: Antigen (Ag) presentation by Ag-presenting cells (APCs) is the first step in the generation of adaptive humoral and cellular immune responses. However, there have been few studies on the effects of flavonoids on APC function. In this study, we examined the effects of five polymethoxyflavones, two isoflavones, and one flavanol on CD11c+ dendritic cell function.
Methods: CD11c+ dendritic cells were differentiated from bone marrow cells by culturing with granulocyte macrophage-colony stimulating factor. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The Ag-presenting ability was determined by a mixed lymphocyte reaction assay. Expressions of MHC class II, CD40, CD80, and CD86 molecules on CD11c+ cells were determined by flow cytometric analysis. Lipopolysaccharide-induced inflammatory cytokines productions were determined by enzyme-linked immunosorbent assay.
Results: The flavonoids used in the study did not show strong toxicity to CD11c+ cells. Nobiletin, heptamethoxyflavone, and genistein enhance Ag-presenting function. Nobiletin and heptamethoxyflavone increased the expression of MHC class II and CD80 molecules. A direct correlation between APC function and lipopolysaccharide-induced cytokine production was not found.
Conclusions: The results of the in vitro study indicate that flavonoids, nobiletin, heptamethoxyflavone, and genistein regulate innate dendritic cell function.
Vaccination is a useful strategy for preventing infectious diseases. When individuals are immunized with an exogenous antigen (Ag), the administered Ag is taken up by Ag-presenting cells (APCs) such as dendritic cells and the Ag is presented to helper T cells via MHC class II molecules, resulting in the generation of Ag-specific humoral and cellular immune responses [1].
Polymethoxyflavones (PMFs) are flavonoid compounds that contain more than two methoxyl groups and are almost extensively found in citrus peel [2]. PMFs have been shown to possess several biological properties including anti-inflammation [3–5], anti-obesity [6], and anti-cancer effect [7]. We have been examining the effects of PMFs on immune functions. Sudachitin (SUD) is a PMF found in Citrus sudachi. Treatment of ovalbumin (OVA)-immunized mice with SUD enhanced OVA-specific interleukin-4 (IL-4) and IL-10 production, resulting in enhancement of humoral immunity. These responses have been shown to contribute to the enhancement of APC function [8]. We have also examined the effect of PMF nobiletin (NOB) in OVA-immunized mice. NOB enhanced OVA-specific T cell and B cell responses, and the enhancement of their responses might be reflected by the function of APCs [9].
The effects of flavonoids on Ag-presenting function have not been fully studied. In this study, we examined the effect of five PMFs, two isoflavones, and one flavanone on the function of Ag presentation and expression of surface molecules in CD11c+ dendritic cells.
NOB, natsudaidain (NAT), and heptamethoxyflavone (HMF) were provided by Ushio-Chemix Co. (Shizuoka, Japan). SUD was provided by Ikeda-Yakusou Co. (Tokushima, Japan). Quercetin (QER), hesperidin (HES), genistein (GEN), and daidzein (DAI) were purchased from Funakoshi Co. (Tokyo, Japan). The chemical structures of each flavonoid are shown in Figure 1.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Tokyo Chemical Ind. Co., Ltd. (Tokyo, Japan). Lipopolysaccharide (LPS) and granulocyte macrophage-colony stimulating factor (GM-CSF) were obtained from Sigma Co. (MA, USA).
Eight-week-old female C57BL/6 and BALB/c mice (Japan SLC, Shizuoka, Japan) were maintained under specific pathogen-free conditions with a 12-h light:dark cycle at (25 ± 2)°C and (55 ± 10)% relative humidity. Weight of mice was 20 g to 23 g. All studies were performed in accordance with the ethical guidelines for animal experimentation by the Institute of Biomedical Sciences, Tokushima University, Japan and were approved by the institution review board of the animal ethics committee (T2020-107). Mice were treated with CO2 for euthanasia.
Bone marrow (BM) cells were collected from the femurs of BALB/c mice. BM cells (1 × 106 cells/mL) were cultured in the medium supplemented with 10% fetal bovine serum, 50 mmol/L 2-mercaptoethanol, 100 µg/mL streptomycin, 100 U/mL penicillin, and 10 ng/mL GM-CSF for 7 days at 37°C under 5% CO2. We confirm that differentiated cells were CD11c+ cells over 97%. We have evaluated the optimal cell number for MTT assay. The cells (1 × 105 cells) were cultured in a 96-well plate with LPS (100 ng/mL) and flavonoids (0, 5, or 10 µmol/L) in a total volume of 100 µL for 24 h. For the last 4 h, 10 µL of MTT solution (5 mg/mL) was added to the wells. After the culture, 100 mL of 10% SDS solution was added and then incubated overnight. The OD value at 550–630 nm was determined by Multiskan GO (Thermo Fisher, MA, USA).
For analysis of the expression of co-stimulatory molecules, cells were cultured with LPS and/or 5 µmol/L flavonoids in a 48-well plate for 24 h at 37°C under 5% CO2. The cells were stained with phycoerythrin (PE)/Cy5-conjugated anti-mouse CD40 monoclonal antibody (mAb), PE-conjugated anti-mouse I-A/I-E mAb, APC-conjugated anti-mouse CD86 mAb, and FITC-conjugated anti-mouse CD80 mAb for 30 min on ice in the dark. All of the Abs were purchased from eBioscience (CA, USA). Flow cytometric analysis was performed on Guava easyCyte using Guava Incyte software ver 2.7 (Merck Millipore, Darmstadt, Germany). The fluorescence intensity of each molecule was analyzed by gating live cells according to FSC and SSC parameters.
CD4+ cells were purified from C57BL/6 mouse spleen by CD4+ T Cell Isolation Kit according to the manufacturer’s instructions (Miltenyi Biotec Inc., Auburn, CA, USA). For determination of the Ag presentation ability, CD11c+ cells derived from BALB/c mice (2 × 104 cells) were cultured with CD4+ T cells derived from C57BL/6 mice (2 × 105 cells) in a 96-well round-bottom plate for 72 h at 37°C under 5% CO2. For the last 8 h of culture, 7.2 kBq of [3H]TdR was added to the wells, and the amount of [3H]TdR incorporated was measured by a scintillation counter (Aloka, Tokyo, Japan).
CD11c+ cells were cultured with LPS and/or flavonoids (5 µmol/L) in a 48-well plate for 24 h at 37°C under 5% CO2. Tumor necrosis factor-α (TNF-α) and IL-6 in the supernatants were quantified using a mouse TNF-α (eBioscience) and IL-6 (eBioscience) enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions.
Data were normal distribution and homogeneity of variance. Data were analyzed using Student’s t-test for comparison to the control group. Data are expressed as means ± SD. Differences were considered significant at P < 0.05.
First, we determined the viability of CD11+ cells cultured with each type of flavonoid using the MTT assay. As shown in Figure 2, some flavonoids significantly decreased the OD value but did not show strong toxicity when cells were cultured with flavonoids at the concentration of 5 µmol/L or 10 µmol/L.
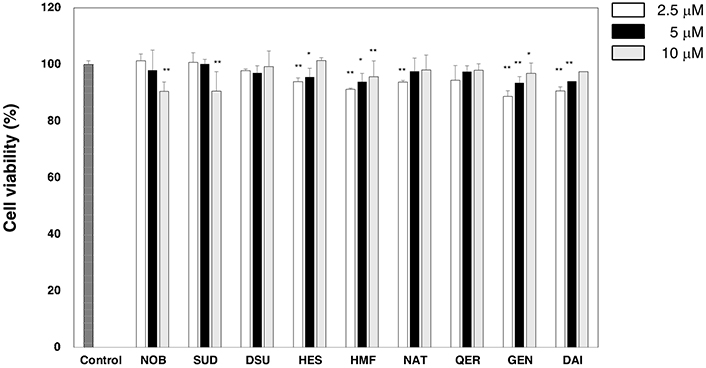
Viability of dendritic cells treated with flavonoids. Dendritic cells were treated with LPS with or without a flavonoid for 24 h. Cell viability was evaluated by an MTT assay. The values of OD at 550–630 nm are shown. Data are shown as means ± SD. We did same experiment at least three times and representative result is shown in Figure. * P < 0.05; ** P < 0.01. NOB: nobiletin; SUD: sudachitin; HES: hesperidin; HMF: heptamethoxyflavone; NAT: natsudaidain; QER: quercetin; GEN: genistein; DAI: daidzein; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; LPS: lipopolysaccharide
We evaluated the Ag-presenting ability of CD11c+ cells by a mix lymphocyte reaction (MLR) assay. CD4+ cells derived from C57BL/6 mice recognize allo-Ag on BALB/c mouse CD11c+ cells and then induce proliferation. As shown in Figure 3A, NOB, HMF, and GEN enhanced the Ag-presenting ability on CD11+ dendritic cells. An APC presents Ag to CD4+ cells via MHC class II molecules, and co-stimulatory molecules, CD40, CD80, and CD86, on APCs enhance these signals. A significant increase in the expression of MHC class II molecule was observed when the cells were cultured with NOB or HMF, while a significant reduction in expression was observed when the cells were cultured with GEN. NOB and HMF also enhanced the expression of CD80. Treatment with a flavonoid tended to decrease expression of CD80 and CD86. Significant decreases in the expression of CD80 and the expression of CD86 were observed when CD11c+ cells were cultured with HES, NAT, QER, or GEN and with NOB, HMF, NAT, or GEN, respectively (Figure 3B).
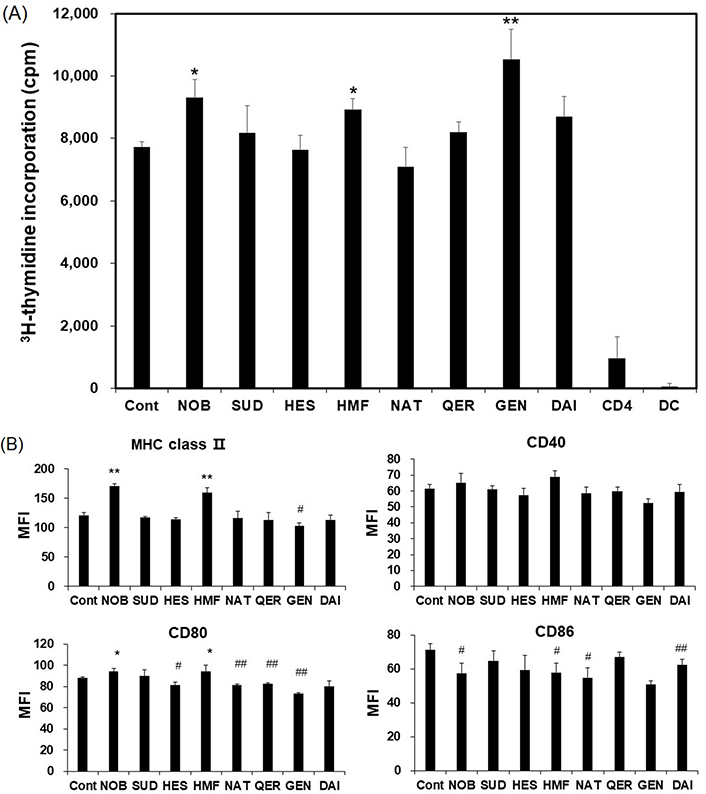
Effects of flavonoids on APC function. Dendritic cells were treated with LPS with or without a flavonoid for 24 h. The Ag-presenting ability was determined by a mix lymphocyte reaction (MLR) assay as described in the Materials and methods section. (A) CD4 and DC in Figure 3A show response of cells cultured only in each cell population. Expression of MHC class II and co-stimulatory molecules (CD40, CD80, CD86) were determined by flow cytometric analysis; (B) data are shown as means ± SD. We did the same experiment at least three times and the representative result is shown in Figure. * significantly increased compared to control (P < 0.05); ** significantly increased compared to control (P < 0.01); # significantly decreased compared to control (P < 0.05); ## significantly decreased compared to control (P < 0.01). MFI: mean fluorescence intensity; APC: antigen-presenting cell; LPS: lipopolysaccharide; MHC: major histocompatibility complex ; NOB: nobiletin; SUD: sudachitin; HES: hesperidin; HMF: heptamethoxyflavone; NAT: natsudaidain; QER: quercetin; GEN: genistein; DAI: daidzein
Finally, we evaluated LPS-induced signal transduction when cells were treated with NOB, HMF, and GEN by determining the production of inflammatory cytokines. GEN enhanced TNF-α production and NOB decreased IL-6 production in CD11c+ cells after LPS stimulation (Table 1).
TNF-α and IL-6 secretion in LPS-stimulated CD11c+ cells treated with NOB, HMF, or GEN
| Group | TNF-α (ng/mL) | IL-6 (pg/mL) |
|---|---|---|
| Control | 234 ± 4* | 471 ± 5 |
| NOB | 250 ± 20 | 390 ± 28# |
| HMF | 198 ± 13 | 431 ± 40 |
| GEN | 327 ± 18** | 432 ± 39 |
* Mean ± SD; ** significantly increased compared to control (P < 0.01); # significantly decreased compared to control (P < 0.05). IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; NOB: nobiletin; HMF: heptamethoxyflavone; GEN: genistein; LPS: lipopolysaccharide
Flavonoids in plants possess a broad spectrum of biological activities including antioxidant and anti-inflammatory actions [2]. The anti-inflammatory effects of flavonoids have been extensively studied [3–5]. Full protective immunity against a pathogen should be induced by adoptive immunity followed by innate immunity. Presentation of an Ag by dendritic cells to T cells is the first and crucial step for the induction of adaptive immunity [1]. However, there have been few studies on the effects of flavonoids on APC function. Although we previously examined the effect of PMF SUD and NOB on APC function [8, 9], we did not comparison of their action to other PMF. To our knowledge, this study is the first study in which the effects of many types of flavonoids on APC function in vitro were examined.
We found that NOB, HMF, and GEN enhance the APC function of CD11c+ dendritic cells (Figure 3A). We previously found in an in vivo study that mice that had been treated with NOB had higher levels of Ag-specific Th2 type cytokines and Ag-specific Abs than those in control mice [9]. This study raises the possibility that the enhanced Ag-specific immunity in vivo is correlated the regulation of the Ag-presenting function by NOB. The enhancement of Ag-presenting function by GEN is unexpected because Ag-immunized mice that were administered GEN showed a weak Ag-specific immune response [10]. It has been shown that GEN tends to inhibit or suppress immune responses. GEN completely inhibits LPS-induced TNF-α production from macrophages in vitro [11]. In another study, ex vivo TNF-α production from alveolar macrophage was lower in GEN-treated mice than in control mice [12]. In a dextran sodium sulfate-induced in vivo colitis mouse model, administration of GEN inhibited polarization into inflammatory M1 macrophages and attenuated colitis [13]. We found that HMF enhances the Ag-presenting ability of CD11c+ cells (Figure 3A). NOB, SUD, DSU, NAT, and HMF are classified in PMFs and have many methoxy groups binding flavonoid structures. A number of methoxy groups might contribute to the biological function of PMFs. The numbers of methoxy groups in SUD, NOB, NAT, and HMF are three, six, six, and seven, respectively. The number of methoxy groups is not directly correlated with the function of Ag presentation.
Expression of MHC class II and CD80 molecules has been shown to be crucial for Ag presentation [1]. Flow cytometric analysis showed that the expression of MHC class II and CD80 molecules is increased by both NOB and HMF but not by GEN (Figure 3B). We cannot explain the dissociation between the Ag-presenting ability and the Ag-presenting-related molecule observed in GEN. Examination of cytokine production showed that GEN enhances TNF-α production in CD11c+ cells (Table 1). Therefore, it is possible that GEN positively regulates DC function. Augmentation of immune cell response by GEN has been reported. Administration of GEN in DO11.10 mice that carried an OVA-specific T cell receptor gene has been shown to enhance OVA-specific IFN-γ and IL-4 production ex vivo [14]. Although it was shown in that study that DO11.10 mouse splenocytes were stimulated by OVA323–339 peptide on MHC class II molecules on APCs, it was not examined whether the expression of MHC class II and co-stimulatory molecules changed.
A relationship between Ag-presenting ability and MHC class II/CD80 molecule have been shown in both HMF and NOB but not in GEN (Figure 3B). In co-stimulatory molecules CD40 and CD86, flavonoids tested in this study tended to decrease their expressions (Figure 3B). We cannot explain why effects of flavonoids on co-stimulatory molecule expressions is different between MHC class II/CD80 and CD40/CD86 (R1, 3).
Suppressive effects of flavonoids on APC function have been shown in some studies [15–17]. In this study, we used each of the flavonoids at a dose of 5 µmol/L. That dose is less than the doses used in other studies that show suppressive effects of flavonoids on APC function.
In this study, we found that some flavonoids can up-regulate APC function in vitro.
Ag: antigen
APCs: antigen-presenting cells
GEN: genistein
HMF: heptamethoxyflavone
IL-4: interleukin-4
LPS: lipopolysaccharide
mAb: monoclonal antibody
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
NAT: natsudaidain
NOB: nobiletin
OVA: ovalbumin
PMFs: polymethoxyflavones
SUD: sudachitin
TNF-α: tumor necrosis factor-α
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1003144_sup_1.fcs.
We thank Ushio-Chemix Co. providing nobiletin, heptamethoxyflavone, and natsudaidain and Ikeda-Yakusou Co. for providing sudachitin.
YT: Investigation, Visualization, Writing—original draft. HO: Investigation, Methodology. AN: Writing—original draft, Writing—review & editing. MN: Formal analysis, Writing—original draft. TS: Funding acquisition, Investigation, Supervision, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
All studies were performed in accordance with the ethical guidelines for animal experimentation by the Institute of Biomedical Sciences, Tokushima University, Japan, and were approved by the institution review board of the animal ethics committee (T2020-107). All studies were also performed in accordance with the Declaration of Helsinki.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study was supported, in part, by JPSS KAKENHI [JP19K1176800, JP22K11701]; by Kieikai Research Foundation; by Fuji Foundation for Protein Research; and by Tojuro Iijima Foundation for Food Science and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hebe Mendez, Ger Rijkers
Beatriz Elina Martínez-Carrillo ... Ana Laura Guadarrama-López
