Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
2Laboratory of Immunology, Abu Dhabi Stem Cells Center, Abu Dhabi, United Arab Emirates
Email: gisela.suarez@adscc.ae
ORCID: https://orcid.org/0000-0001-8883-4197
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0000-0003-1426-3162
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0000-0001-9164-0238
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0009-0002-8397-7935
Affiliation:
5Clinical Direction, National Center for Biopreparations, Bejucal, Mayabeque 32600, Cuba
ORCID: https://orcid.org/0000-0002-8374-9011
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0000-0003-3284-0541
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
2Laboratory of Immunology, Abu Dhabi Stem Cells Center, Abu Dhabi, United Arab Emirates
ORCID: https://orcid.org/0000-0003-3812-1767
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0000-0002-2550-7292
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0000-0001-9472-9158
Affiliation:
2Laboratory of Immunology, Abu Dhabi Stem Cells Center, Abu Dhabi, United Arab Emirates
ORCID: https://orcid.org/0000-0002-6209-0393
Affiliation:
1Clinical Immunology Department, Center of Molecular Immunology, Havana 11600, Cuba
ORCID: https://orcid.org/0000-0002-6614-3819
Explor Immunol. 2024;4:433–445 DOl: https://doi.org/10.37349/ei.2024.00150
Received: July 18, 2023 Accepted: May 30, 2024 Published: August 05, 2024
Academic Editor: Tamas Fulop, Université de Sherbrooke, Canada
Aim: Currently, malignant diseases represent a health issue worldwide. Among these, lung cancer is of growing importance, due to its high incidence and mortality. Chemotherapy, one of the most frequently used treatments, has shown its ability to induce accelerated immunosenescence in classic and as well non-classic lymphocyte compartments, being less described in the latter. The immune restoration strategies have demonstrated their ability to reverse immunosenescence and exhaustion markers in conventional lymphocyte subpopulations after chemotherapy. However, the possible immunorestorative effect on non-classical lymphocytes has not been widely reported. The aim of this study was to evaluate the effect of chemotherapy and the administration of a thymic polypeptide factor on non-classical lymphocyte populations in patients with advanced lung cancer.
Methods: Eighteen patients with advanced lung cancer, were evaluated at baseline before and after platinum-based chemotherapy (4–6 cycles). All patients could complete treatment with a thymic polypeptide factor [Biomodulina T (BT)] at the end of chemotherapy. Blood from patients was collected by venipuncture in heparinized tubes before and after chemotherapy and at the end of BT treatment to analyze the frequencies of non-classical immune subpopulations by flow cytometry.
Results: Natural killer (NK), natural killer T cells (NKT), and double-positive T lymphocyte (DPT) proportions reached normal values in patients diagnosed with advanced lung cancer before receiving cytotoxic treatment. Chemotherapy did not induce modifications in the total percent of NK, NKT, and DPT populations in these patients. However, the administration of BT decreased DPTs and NK cells expressing the cluster of differentiation (CD)57 molecule, which is considered a marker of immunosenescence.
Conclusions: These results suggest a lower influence of platinum-based chemotherapy on non-classical lymphocytes and the potential to generate a reconstitution of lymphocyte subpopulations in patients with advanced lung cancer by using the thymic factor BT, which reveals a new possibility for improving the response to cancer immunotherapies [Cuban Public Registry of Clinical Trial (RPCEC, https://rpcec.sld.cu/en/trials/RPCEC00000358-En) identifier: RPCEC00000358].
Cancer represents one of the major health problems in the world. In 2020, the tumor site with the highest incidence (over 2 million new cases) and mortality rate (around 1.8 million deaths), was lung cancer [1].
Among the lung malignancies, non-small cell lung cancer (NSCLC) is the most frequent histological type, representing approximately 85% of all lung tumors [2, 3], and more than 60% of patients are diagnosed in advanced stages of the disease [4].
Cancer and aging share common characteristics and are closely interrelated [5]. Aging is recognized as the most important risk factor for common malignancies, including lung cancer [6], where the median age at diagnosis is 70 years [4]. Recently, it has been proposed that senescent cells are emerging as hallmarks of cancer [7]. With increasing age, gradual immune remodeling occurs; a decrease in the naive T cell compartment, accumulation of memory T cells, and reduced expression of immune co-stimulatory molecules, a process commonly called “immunosenescence”, which is associated with a chronic low-grade inflammation named “inflammaging” [8, 9]. Considering the role of immunity in the elimination of damaged cells and the maintenance of homeostasis in an organism, it is not surprising that immunosenescence, in addition to being a consequence of aging, is an additional accelerator of functional deterioration in other tissues.
The cluster of differentiation (CD)57 antigen (alternatively Leu-7, HNK-1, or L2) is commonly used to identify terminally differentiated “senescent” cells with altered functional properties and reduced proliferative capacity. The functions associated with this antigen have been described in classical lymphocytes (αβ-TCR T cells and B cells) as well as in non-classical cells (falling between the classical characteristics of innate immune cells and adaptive T and B cells) [10]. In general terms, the expression of CD57 in NK, CD8+ T, and CD4+ T cells reveals the history of more cell divisions and telomeric shortening [11]. Its expression increases with age and is associated with chronic infections, particularly human cytomegalovirus infection [12], cancer, and chronic pulmonary and autoimmune diseases [13].
Likewise, a transition from lymphocytes with less differentiation state to late stages of maturation has been described [14]. The most differentiated lymphocytes, including terminally differentiated ones, are a source of proinflammatory cytokines in cancer patients receiving chemotherapy [14]. These molecules support chronic low-grade inflammation in aging and tumor diseases [6]. Therefore, reversing chemotherapy-accelerated immunosenescence, through immune system restoration strategies, represents a promising intervention in cancer patients. The administration of Biomodulina T (BT), a polypeptide fraction obtained in Cuba from bovine thymus and registered in 1994 for the treatment of recurrent infections in the elderly, has demonstrated its ability to reverse markers of immunosenescence and enhance the response to immunotherapies against malignant tumors [15, 16].
In studies involving healthy older adults, BT was able to expand naive CD4+ T cells, recently migrated thymus lymphocytes, and memory CD8+ T cells with stem cell characteristics. In addition, this product decreased CD4+ and CD8+ T cells that express programmed cell death protein-1 (PD-1) and did not expand regulatory T lymphocytes [16]. These changes in cellular subpopulation are crucial when considering immunosuppression as a hallmark of cancer [7, 16].
Recently, our group demonstrated the ability of BT to increase naive CD4+ T cells, decrease terminally differentiated populations, and decrease CD4+ and CD8+ T lymphocytes expressing PD-1 in patients with advanced non-cell lung cancer after chemotherapy [15].
In this study, we evaluated profiles of immune cells in patients with advanced lung cancer, before and after receiving platinum-based chemotherapy and also the effects of Biomodulina T treatment following chemotherapy, to determine whether the administration of these therapies affects the distribution of non-classical lymphocytes and markers of immunosenescence. By using flow cytometry, the expression of the senescence marker CD57 was evaluated in double-positive T lymphocytes (DPTs) and natural killer (NK) cell subsets at different study time points.
Immune restoration by using a thymic factor in patients diagnosed with advanced malignant diseases could reverse changes induced by chemotherapy and improve the response to cancer immunotherapies. In future studies, the non-classical lymphocyte profile could be monitored in patients with various types of advanced cancers and its relevance in clinical evaluation and therapeutic response should be addressed.
Eighteen patients with histologically confirmed stage IIIB or IV NSCLC aged 53 to 82 years were evaluated at baseline before and after platinum-based chemotherapy (4–6 cycles). All patients were able to complete treatment with BT, 3 mg three times a week for 4 weeks (Figure 1).
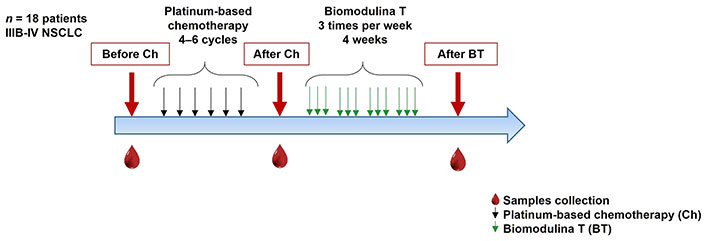
Chemotherapy and Biomodulina T administration in patients diagnosed with advanced non-small cell lung cancer. NSCLC: non-small cell lung cancer
The patients were seen in the Oncology consultation units at the Medical & Surgical Research Center in Havana. The research protocol is registered in https://rpcec.sld.cu/en/trials/RPCEC00000358-En, the Cuban Public Registry of Clinical Trial (Spanish acronym: RPCEC), a WHO-validated Public Registry, Trial Number RPCEC00000358 and was approved by the institution’s ethics committees. Informed consent was obtained from each patient before participating in the research protocol. The investigation was done in compliance with the principles of Good Clinical Practices according to the International Conference of Harmonization and the Declaration of Helsinki (2013). Demographic and clinical characteristics are summarized in Table 1.
Demographic and clinical characteristics of NSCLC patients
| Demographic and clinical data | Number and percentage (%) |
|---|---|
| Total of patients | 18 (100%) |
| Sex | |
| Male | 12 (66.7%) |
| Female | 6 (33.3%) |
| Histologic subtype | |
| Adenocarcinoma | 11 (61.1%) |
| Squamous cell carcinoma | 4 (22.2%) |
| NSCLC | 3 (16.7%) |
| ECOG status | |
| ECOG status 0 | 5 (27.8%) |
| ECOG status 1 | 11 (61.1%) |
| ECOG status 2 | 2 (11.1%) |
| Response to first-line treatment | |
| Complete response | 2 (11.1%) |
| Partial response | 4 (22.2%) |
| Stable disease | 11 (61.1%) |
| Progressive disease | 1 (5.6%) |
The proportion of patients in each category indicated was calculated from the total absolute number of patients in this study. ECOG: Eastern Cooperative Oncology Group; NSCLC: non-small cell lung cancer
Note. Adapted from “Thymic Polypeptide Fraction Biomodulina T Decreases Exhausted and Terminally Differentiated EMRA T Cells in Advanced Lung Cancer Patients Treated With Platinum-Based Chemotherapy” by Suárez GM, Catalá M, Peña Y, Portela S, Añé-Kourí AL, González A, et al. Front Oncol. 2022;12:823287 (https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2022.823287/full). CC BY.
Blood from patients was collected by venipuncture in heparinized tubes before (before Ch), after chemotherapy (after Ch), and at the end of BT treatment (end BT). Peripheral blood mononuclear cells (PBMC) were purified by Ficoll-Paque PLUS centrifugation (Amersham Biosciences). The cells were immediately cryopreserved in Roswell Park Memorial Institute medium (RPMI) 1640 supplemented with 40% fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO) until the flow cytometry assays were performed.
The anti-human antibodies used were anti-CD3 [phycoerythrin (PE)-Cy7, clone UCHT1, Biolegend], anti-CD4 (Alexa Fluor 700, clone RPA-T4, Biolegend), anti-CD8 (APC AF 700, clone B4918, Beckman Coulter), anti-CD57 [fluorescein isotiocyanate (FITC), clone TB01, Bio-Rad], anti-CD56 (PE, clone BG1398 AA, Beckman Coulter) and anti-CD16 (Percp-5.5, clone B61376AA, Beckman Coulter). Monoclonal antibodies were used to perform immunophenotyping as follows:
DPTs: CD3+, CD56–, CD4+, CD8+
NK cells and subsets:
CD3–CD56bright CD16neg
CD3–CD56dim CD16pos
CD3–CD56neg CD16pos
Natural killer T cells (NKT) and subsets:
CD3+CD56+CD4+CD8–
CD3+CD56+CD4–CD8+
CD3+CD56+CD4–CD8–
All steps were performed at 4°C. After thawing, the cells were washed three times with phosphate buffered saline (PBS), resuspended in RPMI medium and 10% bovine serum albumin (BSA), surface staining was performed by using 1 × 106 cells, and respective antibodies in fluorescence-activated cell sorting (FACS) buffer [PBS with 5 mM ethylenediaminetetraacetic acid (EDTA) and 0.2% BSA] in the dark for 30 min at 4°C. Subsequently, the cells were washed twice with FACS buffer and fixed by treatment with Formaldehyde 2%/1× PBS/BSA/sodium azide solution for 30 min at room temperature. Data acquisition was performed with a Gallios flow cytometer (Beckman Coulter, 3-laser configuration), with a minimum of 50,000 acquired events.
To perform multiparametric analysis and characterization of several phenotypes of cell populations in a single tube, two-dimensional analyses were hierarchically concatenated. In Figure 2, the first dot plot was generated to relate cell size to complexity, distinguishing cells from artifacts or detritus; the second and third dot plots reflect a window through which individual cells passed through the laser beam for cell-by-cell analysis and the fourth dot plot allows us to identify lymphocyte populations based on the expression of the CD3 molecule. The data was processed with FlowJo software (Tree Star Inc., v 10(2)) and exported as tabulated results for statistical analyses.
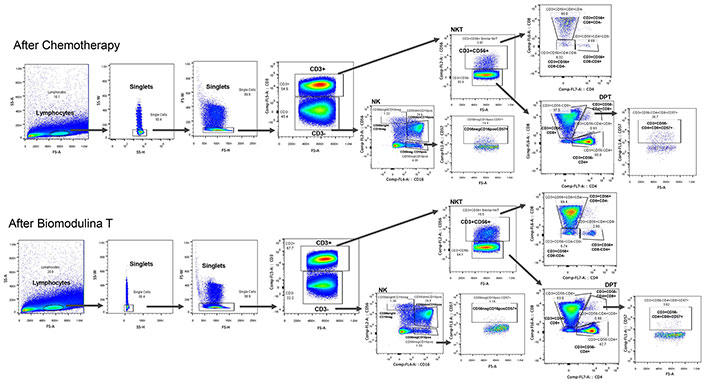
Cell definition strategy: NKT CD3+CD56+ and its subsets (CD3+CD56+CD4+CD8–, CD3+CD56+CD4–CD8+, CD3+CD56+CD4–CD8–), NK and its subsets (CD56bright CD16–, CD56dim CD16+, CD56–CD16+ and CD56–CD16+CD57+) and double-positive T lymphocytes expressing CD57 (CD3+CD4+CD8+CD57+), after first-line platinum-based chemotherapy and at the end of Biomodulina T administration in patients diagnosed with non-small cell lung cancer. Black arrows indicate that within the selected populations, the subsets indicated by the arrow were searched. NK: natural killer; NKT: natural killer T cells; DPT: double-positive T lymphocyte; pos: positive; neg: negative
Note. Adapted from “Thymic Polypeptide Fraction Biomodulina T Decreases Exhausted and Terminally Differentiated EMRA T Cells in Advanced Lung Cancer Patients Treated With Platinum-Based Chemotherapy” by Suárez GM, Catalá M, Peña Y, Portela S, Añé-Kourí AL, González A, et al. Front Oncol. 2022;12:823287 (https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2022.823287/full). CC BY.
Descriptive statistics were calculated for relative frequencies, medians, and interquartile range of percentage for each cell population, before and after platinum-based chemotherapy.
The Shapiro–Wilk normality test was used to determine the normal distribution of variables. Statistical significance among the groups: Before chemotherapy (before Ch) and After chemotherapy (after Ch) as well as; After chemotherapy (after Ch) and at the end BT administration (end BT) were evaluated using t-tests and Wilcoxon, for paired values tests, when data passed or failed normality test, respectively. These statistical analyses were performed by using GraphPad Prism 7. The statistical data were considered significant if P < 0.05.
Proportions of NK, NKT, and DPTs as well as the expression of CD57 molecule as a senescence marker were studied in patients with advanced lung cancer, before receiving platinum-based chemotherapy.
Percentages refer to relative frequencies of NK, NKT, and DPTs in relation to total lymphocytes. The percentages of cell subtypes and cells expressing CD57+ were referred to as the percentage of cell populations NK, NKT, and DPTs as appropriate (Table 2).
Distribution of lymphocyte subpopulation percentages in patients diagnosed with advanced non-small cell lung cancer
| Phenotype | Before Ch | After Ch | P Value | ||
|---|---|---|---|---|---|
| Median | Percentiles (25–75%) | Median | Percentiles (25–75%) | ||
| Double-positive T lymphocytes (%) | 0.74 | (0.50–1.43) | 0.75 | (0.62–1.02) | 0.6406a |
| CD3+CD4+CD8+CD57+ (%) | 0.09 | (0.06–0.17) | 0.08 | (0.04–0.24) | 0.0781a |
| NK lymphocytes (%) | 9.38 | (4.47–17.71) | 9.37 | (4.47–17.71) | 0.3144b |
| CD56bright CD16neg (%) | 0.82 | (0.46–1.45) | 1.12 | (0.56–1.45) | 0.8438a |
| CD56bright CD16neg CD57+ (%) | 1.27 | (0.48–4.79) | 1.4 | (0.37–3.50) | 0.3828a |
| CD56dim CD16pos (%) | 29.35 | (9.37–48.25) | 24.64 | (11.50–49.52) | 0.2006b |
| CD56dim CD16pos CD57+ (%) | 21.23 | (4.89–48.29) | 38.62 | (8.62–61.66) | 0.1626b |
| CD56neg CD16pos (%) | 3.82 | (1.67–5.0) | 3.93 | (2.43–9.24) | 0.2500a |
| CD56neg CD16pos CD57+ (%) | 4.29 | (1.84–8.0) | 4.43 | (1.92–10.79) | 0.8125a |
| NKT Lymphocytes (%) | 8.87 | (5.84–19.60) | 5.38 | (2.69–12.24) | 0.3125a |
| CD3+CD56+CD4+CD8– (%) | 20.07 | (4.86–33.37) | 12.84 | (3.39–27.22) | 0.0876b |
| CD3+CD56+CD4+CD8–CD57+ (%) | 1.98 | (0.30–8.41) | 1.86 | (0.34–5.25) | 0.2188a |
| CD3+CD56+CD8+CD4– (%) | 66.25 | (44.89–80.93) | 60.05 | (43.73–86.38) | 0.2357b |
| CD3+CD56+CD8+CD4–CD57+ (%) | 25.84 | (3.95–41.34) | 6.49 | (1.0–52.25) | 0.0313a* |
| CD3+CD56+CD4–CD8– (%) | 9.10 | (6.45–13.86) | 7.97 | (3.38–32.46) | 0.6406a |
| CD3+CD56+CD4–CD8–CD57+ (%) | 1.86 | (1.06–3.45) | 1.50 | (0.99–8.41) | 0.5781a |
Distribution of lymphocyte subpopulation percentages in patients diagnosed with advanced NSCLC before and after receiving platinum-based chemotherapy. Percentages refer to relative frequencies of natural killer (NK), natural killer T cells (NKT), and double-positive T lymphocytes (DPTs) in relation to total lymphocytes. The percentages of cell subtypes and cells expressing CD57+ were referred to as the percentage of cell populations. NK, NKT, and DPT as appropriate. Ch: chemotherapy. a Wilcoxon test; b paired t-test. The asterisks indicate significant differences between groups: * P < 0.05
The median percentage of DPTs and NKT were normal according to the reference values in Cuban healthy adults, ranging between 0.1–5.1% and 0.8–23.0%, respectively [17]. Likewise, the median of the percentages of NK cells was found among normal values (Table 2) according to reference ranges (3.7–28.0%) [18].
NK, NKT, and DPTs expressing CD57 were studied in these patients before and after receiving cytotoxic treatment.
One week after first-line platinum-based chemotherapy (after Ch), a significant decrease in the percentage of CD3+CD56+CD8+CD4–CD57+ cells was found (P = 0.0313; Wilcoxon test; Table 2). No other changes concerning the expression of CD57 were observed in the rest of the immune cell populations at this study time point (Table 2).
Additionally, chemotherapy did not induce modifications in the total percent of NK, NKT, and DPT populations in patients diagnosed with advanced lung cancer (Table 2).
The relative percentages of non-classical lymphocyte populations (NK, NKT, and DPTs) were evaluated in patients diagnosed with NSCLC after chemotherapy and at the end of treatment with BT. The gating strategy is shown in Figure 2.
Total percentages of DPTs did not change after treatment with BT in patients diagnosed with advanced lung cancer who received chemotherapy (Figure 3a). However, when evaluating the expression of the senescence marker CD57 in DPTs, a significant decrease in the DPTs CD57+ subset was observed (P = 0.0420; Wilcoxon test; Figure 3b) within five to seven days after the last dose of BT.
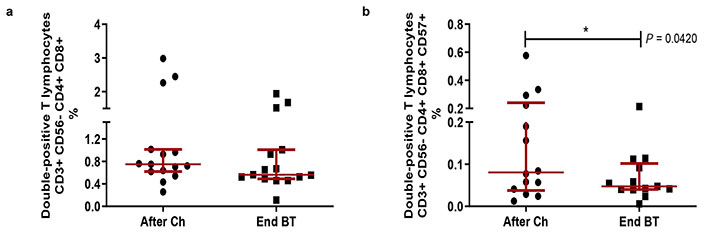
Double-positive T lymphocyte frequencies and CD57+ expression in patients diagnosed with non-small cell lung cancer, after receiving first-line platinum-based chemotherapy and at the end of Biomodulina T administration. (a) Relative percent of CD4+CD8+ T cells; (b) relative percentage of CD57+ double-positive T lymphocytes. The median and interquartile range are represented. The asterisks (*) indicate significant differences between groups: P < 0.05, using the Wilcoxon test. After Ch: after chemotherapy; End BT: at the end of the administration of Biomodulina T
When administered after first-line chemotherapy, BT did not influence the total NK cell population or most NK subsets (P > 0.05; Wilcoxon test; Figure 4a–f). Nevertheless, a decrease in CD56–CD16+CD57+ NK subset was evident (P = 0.0010; Wilcoxon test; Figure 4g) at the end of treatment with BT.
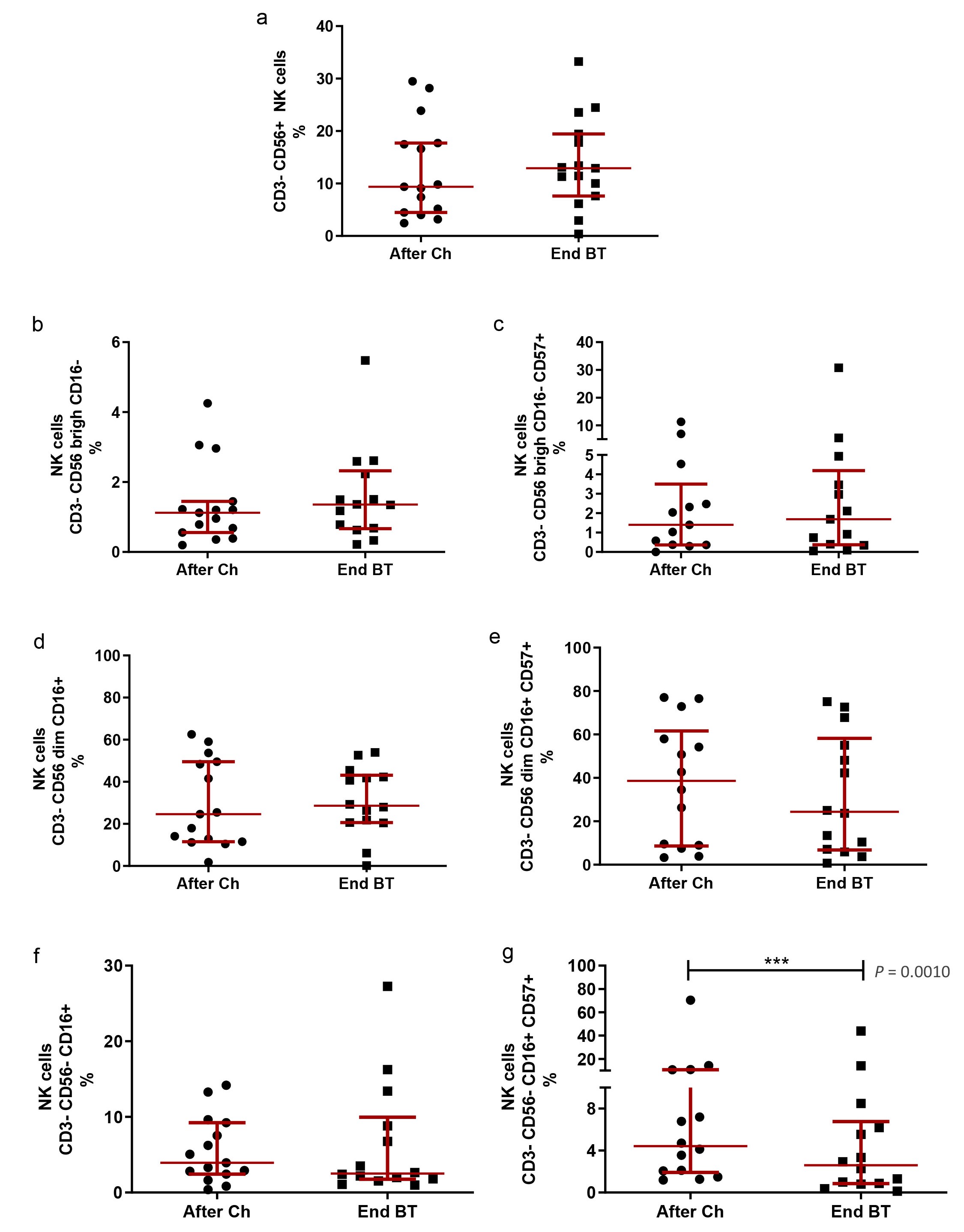
NK cell subpopulations after first-line platinum-based chemotherapy and at the end of Biomodulina T administration in patients diagnosed with non-small cell lung cancer. (a) Relative percentage of NK cells; (b) relative percentage of CD3–CD56bright CD16– NK cells; (c) relative percentage of CD3–CD56bright CD16–CD57+ NK cells; (d) relative percentage of CD3–CD56dim CD16+ NK cells; (e) relative percentage of CD3–CD56dim CD16+CD57+ NK cells; (f) relative percentage of CD3–CD56–CD16+ NK cells; (g) relative percentage of CD3–CD56–CD16+CD57+ NK cells. The median and interquartile range are represented. The asterisks (***) indicate significant differences between groups (P < 0.001), using the Wilcoxon Test. NK: natural killer; After Ch: after chemotherapy; End BT: at the end of the administration of Biomodulina T
The relative percentages of NKT were evaluated in patients after chemotherapy and at the end of treatment with BT. In our study, the median percentage of NKT lymphocytes was 5.38% after chemotherapy (2.69–12.24%) and 8.65% (3.55–31.10%) at the end of treatment with BT, without significant differences. The administration of BT did not modify the CD3+CD56+CD4+CD8–, CD3+CD56+CD8+CD4–, CD3+CD56+CD4–CD8– NKT or the expression of CD57 within these cell compartments (P > 0.05; Wilcoxon test; Figure 5).
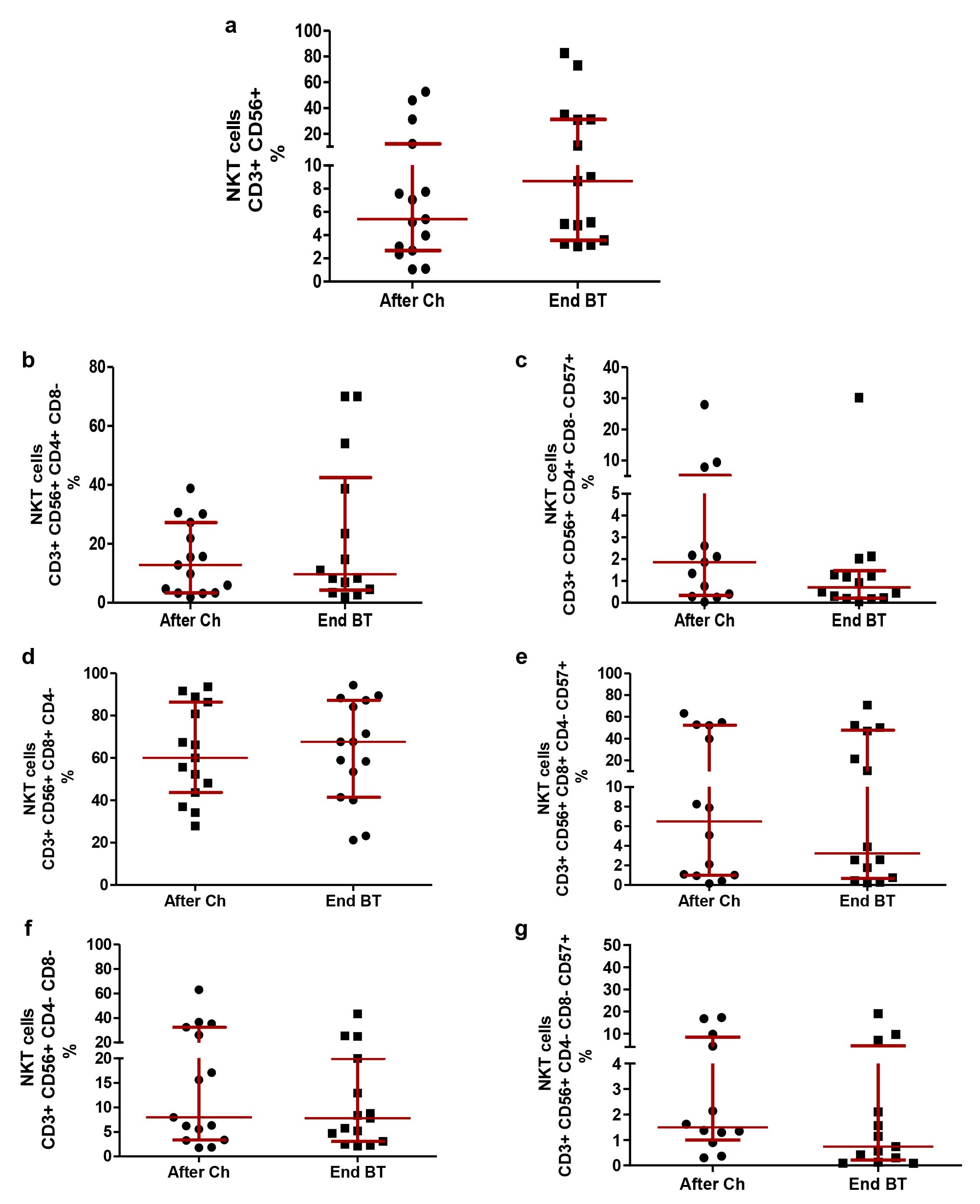
NKT subpopulations after first-line platinum-based chemotherapy and at the end of Biomodulina T administration in patients diagnosed with non-small cell lung cancer. (a) Relative percentage of NKT; (b) relative percentage of CD3+CD56+CD4+CD8– NKT; (c) relative percentage of CD3+CD56+CD4+CD8–CD57+ NKT; (d) relative percentage of CD3+CD56+CD8+CD4– NKT; (e) relative percentage of CD3+CD56+CD8+CD4–CD57+ NKT; (f) relative percentage of CD3+CD56+CD4–CD8– NKT; (g) relative percentage of CD3+CD56+CD4–CD8–CD57+ NKT. The median and interquartile range are represented. There were no significant differences between groups (P > 0.05), using the Wilcoxon Test. NKT: natural killer T cells; After Ch: after chemotherapy; End BT: at the end of the administration of Biomodulina T
Platinum-based first-line chemotherapy, frequently administered in patients with NSCLC, has different effects on immune cells; as immunostimulatory effects by promoting antitumor immune responses induced by cytotoxic activity, and immunosuppressive effect [19]. Previous studies suggest that changes in immune parameters associated with chemotherapy should be taken into consideration during the treatment and clinical management of patients [20].
In this study, no significant differences in NK, NKT, and DPT population frequencies were observed in patients with advanced lung cancer prior to receiving first-line platinum-based chemotherapy when compared to age-matched healthy donors. The advanced disease did not appear to have a significant impact on these peripheral non-classical lymphocyte populations in patients with NSCLC. However, chemotherapy has demonstrated its ability to modify classical lymphocyte populations [14] as well as non-classical lymphocyte populations in patients with advanced lung cancer [21]; suggesting the wide susceptibility of immunological cell populations to cytotoxic treatment. Thus, an immunological restoration strategy seems to be appropriate in patients with advanced cancer after receiving platinum-based chemotherapy.
Previous experiments reported the immunomodulatory capacity of BT by increasing the populations of naive T lymphocytes and decreasing the cell populations that express PD-1 as an exhaustion marker [15, 16]. In this study, we show for the first time the effects of the thymic factor BT on non-classical lymphocyte populations in patients with advanced lung cancer after receiving chemotherapy.
BT treatment significantly reduced DPTs and CD56–CD16+ NK subsets expressing CD57. These findings could be another example of the immunological restoration capacity of BT, considering the CD57 marker as an indicator of telomere shortening associated with the loss of proliferation and cellular memory capacities in lymphocyte subpopulations [22, 23].
Lopez-Vergès et al. [22] reported an increase in the populations of DPTs and CD57+ NK cells during aging and associated the expression of this marker with a decrease in cell proliferative capacity, and in the case of CD56dim CD16+CD57+ NK cells to a greater cytotoxic potential. More recently, Forconi et al. [24] reported an accumulation of CD56–CD16+ NK cells in children exposed to plasmodium falciparum and in patients diagnosed with Burkitt’s lymphoma. These cell populations appear to be the result of an immune adaptation influenced by chronic inflammatory diseases. It retains antibody-dependent cytotoxicity but presents a lower expression of genes encoding inflammatory cytokines compared to CD56dim CD16+ NK cells, suggesting an immunoregulatory role [24]. In this context, the decrease in CD57 expression in an immunoregulatory NK subpopulation, after the administration of Biomodulina could be beneficial in the context of a tumor disease favored by chronic inflammation.
In addition, an increase in DPTs is reported during aging [25] and controversial functions are attributed to these immune cells, with cytotoxic and immunosuppressive functions, indicating that DPTs have pleiotropic functions and are heterogeneous [26]. Bohner and colleagues [26] studied these populations in bladder, prostate, and kidney cancers. They reported elevated levels of circulating DPTs in hospitalized patients with urological cancer. DPTs favored the polarization of naive CD4+ T cells to a Th2 profile, which was exacerbated in these patients compared to healthy donors.
In our study, the frequencies of DPTs were found in similar ranges described in healthy adults [17] and when exploring the expression of CD57 in this small subset of T lymphocytes, a decrease in double-positive CD57+ T lymphocytes was observed at the end of treatment with BT. Little has been reported on these populations in non-small cell lung cancer. We believe that more research needs to be carried out to clarify its role in lung cancer and explore whether the expression of CD57+ interferes with its proliferative and cytotoxic capacity.
Additionally, we evaluated the effect of the administration of BT in NKT lymphocyte subpopulations. King et al. [27] recently reported the ability of Thymosin-α-1 (Tα1), a polypeptide generated by thymic epithelial cells, to increase the number of T cells, promoting their differentiation and maturation. Sugahara and colleagues [28] reported an increase in the number of intrahepatic NKT in 7 patients (between 25 and 41 years old) with chronic hepatitis B who received treatment with Tα1. Interestingly, Ramos et al. [29] reported an increase in NKT and B lymphocyte populations in older patients who received only BT, at 3 mg three times a week for one week, which is lower than the standard effective dose reported previously [16]. In our study, we did not observe changes in the NKT lymphocyte subpopulations of our patients after BT administration. We can hypothesize that different effects can be observed in the lymphocyte subpopulations depending on BT dose and treatment period.
This research, along with others regarding BT intervention during aging and after chemotherapy [15, 16, 29], suggests the possibility of reversing some features of immunosenescence and chemotherapy-induced effects on the immune system. This immunomodulatory treatment could be a proper way to induce immune restoration in cancer patients and get a better response to cancer immunotherapies.
The limitation of this study is the lack of a group of healthy controls matched by age and sex, as well as the lack of a control group that receives chemotherapy without BT.
For future research efforts involving BT as a cancer therapy complement, it is recommended to increase the number of patients with different stages of the disease, evaluate changes in lymphocyte subpopulations in tumor tissue (tumor microenvironment) as well as correlate immune cell subpopulations with the survival of patients suffering from advanced cancer.
BSA: bovine serum albumin
BT: Biomodulina T
CD: cluster of differentiation
Ch: chemotherapy
DPT: double-positive T lymphocyte
NK: natural killer
NKT: natural killer T cells
NSCLC: non-small cell lung cancer
PBS: phosphate buffered saline
PD-1: programmed cell death protein-1
The authors are extremely thankful to patients and their relatives who so valuably supported this research. The authors are also grateful for the participation of physicians, nurses, and colleagues at the Medical & Surgical Research Center. Thanks to Dr. Mays Abuhantash, Aya Zeid Zakaria, and Dr. Syed Mazher Hussain for their contributions in reviewing this manuscript and providing their valuable comments. The authors also thank the Center for Molecular Immunology for supporting the completion of this study.
GMS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. MC, YP, and SP: Methodology, Project administration. ALA-K and AL: Conceptualization, Writing—review & editing, Supervision. AG: Resources. PL-L: Software. MD and MM: Methodology, Project administration. KP: Resources, Investigation. JH: Investigation. MCR: Conceptualization, Writing—review & editing. NL, ZM, and TC: Supervision, Writing—review & editing. AB-H: Writing—review & editing. DS: Conceptualization, Writing—original draft, Writing—review & editing, Methodology, Project administration, Resources, Investigation, Software, Supervision, Writing—review & editing. All authors read and approved the submitted version.
The author declares that there are no conflicts of interest.
The Biomodulina T- CIMAvax-EGF-Advanced Non-Small Cell Lung Cancer-Adults was approved by the Institutional Review Boards of the participating hospitals and by the National Regulatory Authority in Cuba. The research protocol is registered in https://rpcec.sld.cu/en/trials/RPCEC00000358-En, the Cuban Public Registry of Clinical Trial (Spanish acronym: RPCEC). The study was done in compliance with the principles of Good Clinical Practices (according to the International Conference of Harmonization) and the Declaration of Helsinki (2013).
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. The research protocol is available at https://rpcec.sld.cu/en/trials/RPCEC00000358-En, the Cuban Public Registry of Clinical Trial (Spanish acronym: RPCEC).
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1253
Download: 25
Times Cited: 0
