Abstract
Interleukin-6 (IL-6) plays a critical role in the pathogenesis of various chronic inflammatory diseases, tracked across numerous fields of research. Despite this, a crucial aspect often overlooked in studies is the differentiation between IL-6 classic-signaling and trans-signaling, leading to potential confounding of their findings; less than half of selected IL-6 studies included differentiation. This review delves into the distinction between IL-6 classic- and trans-signaling and their role in chronic inflammatory diseases and endometriosis. The unique pro-inflammatory nature of IL-6 trans-signaling, contrasted with the anti-inflammatory character of IL-6 classic-signaling, presents significant implications for research methodology, particularly in studies investigating anti-inflammatory interventions or interleukin inhibitors. Diagnostic failure to account for these distinct pathways may inadvertently misrepresent beneficial immune responses or the efficacy of interventions, posing significant challenges in predicting health outcomes. Interventions that do not differentiate these pathways could face reduced efficacy or safety. This review proposes adjustments to research methodologies and stresses the importance of careful interpretation of inflammatory markers in IL-6-related research. Examples of differentiation issues are discussed across the topics of endometriosis and multiple inflammatory diseases. By addressing this methodological issue, researchers could potentially improve patient outcomes, enhance the efficacy of interventions, and contribute to public health advancements.
Keywords
Inflammation, interleukin-6, endometriosis, cytokines, biomarkers, inflammatory diseasesIntroduction
Interleukin-6 (IL-6), a pleiotropic cytokine, has drawn considerable attention for its central role in the pathogenesis of various chronic inflammatory diseases [1]. Its dual functionality can be attributed to the existence of two distinct signaling mechanisms: IL-6 classic-signaling and IL-6 trans-signaling. IL-6 classic-signaling has been recognized for its regulatory and protective roles, contributing to immune resilience [2]. Conversely, IL-6 trans-signaling has been implicated in instigating and perpetuating inflammatory responses, exacerbating disease progression. Each of these pathways carries unique physiological and pathological implications, however, much of the extant literature investigating the efficacy of anti-inflammatory interventions fails to differentiate between them. This lack of specificity potentially confounds our understanding of the therapeutic effects and hinders the development of precision interventions [3].
The study of endometriosis, where conventional therapies are often dependent on early and accurate diagnostics, offers a valuable perspective toward the importance of the differential effects of IL-6 classic- and trans-signaling [4]. From a clinical perspective, the reliance on IL-6 levels for diagnosing endometriosis is fraught with challenges. The heterogeneity of endometriosis manifestations means that IL-6 levels can vary widely among patients, influenced by disease stage, lesion location, and individual immune responses. Unreliable IL-6 measurements may lead to misdiagnosis or underdiagnosis, particularly in cases where IL-6 levels are incongruent with clinical symptoms. This discrepancy complicates the development of standardized diagnostic criteria and can delay appropriate treatment interventions [5].
The treatment of endometriosis often involves managing symptoms and controlling disease progression through hormonal therapies and surgical interventions. Given IL-6’s involvement in inflammatory processes, therapies targeting IL-6 signaling have been proposed as potential treatments. However, without accurate and reliable measurements of IL-6 and an understanding of its signaling context, it is challenging to predict therapeutic outcomes or tailor treatments to individual patient profiles [6].
This review seeks to underscore the importance of differentiating between IL-6 classic- and trans-signaling in the study of chronic inflammatory diseases and in particular endometriosis. The subsequent sections will delve into the unique roles of these signaling pathways in inflammation and health, explore how their conflation could confound existing research, and propose potential directions for future studies in this area. Ultimately, the aim is to enhance our understanding of IL-6 signaling and facilitate the development of more targeted and efficacious therapeutic interventions for chronic inflammatory diseases.
IL-6 signaling: classic vs. trans
IL-6, a frequently tracked cytokine in immunological studies, operates through two distinct signaling pathways: IL-6 classic-signaling and IL-6 trans-signaling [7]. The division of these pathways, first identified in 1994, enables IL-6 to assume roles ranging from modulating inflammation to bolstering immune defenses, a balance that is gaining recognition as vital for human health [8].
IL-6 classic-signaling is limited to specific cells equipped with the membrane-bound IL-6 receptor (IL-6R), including hepatocytes and certain leukocytes. This signaling commences with IL-6 attaching to IL-6R, then pairing with the universally expressed signal-transducing receptor protein, glycoprotein 130 (gp130). This event triggers a succession of intracellular signals, culminating in the activation of diverse genes that drive the cellular response to IL-6. The selective character of IL-6 classic-signaling ensures its effects are predominantly on cells possessing the necessary receptor apparatus, thereby maintaining a restricted influence.
In contrast, IL-6 trans-signaling involves the soluble IL-6R (sIL-6R). This receptor is unattached to the cell membrane and can circulate freely and bind to IL-6. The resulting sIL-6R/IL-6 complex can engage with gp130 on the surfaces of practically all cells, instigating the same intracellular signal cascade as classic-signaling. This mechanism broadens IL-6’s influence on cells lacking the membrane-bound IL-6R, drastically augmenting the potential scope of its action.
These two signaling routes play contrasting roles within the immune system and inflammation, as demonstrated in Figure 1. IL-6 classic-signaling is tied to host defense functions, aiding the activation and differentiation of T cells and B cells-vital components of the immune response to infections that culminate in anti-inflammatory action [9]. Conversely, IL-6 trans-signaling has been linked to pro-inflammatory responses and is considered a major player in the pathogenesis of numerous chronic inflammatory diseases, such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD).
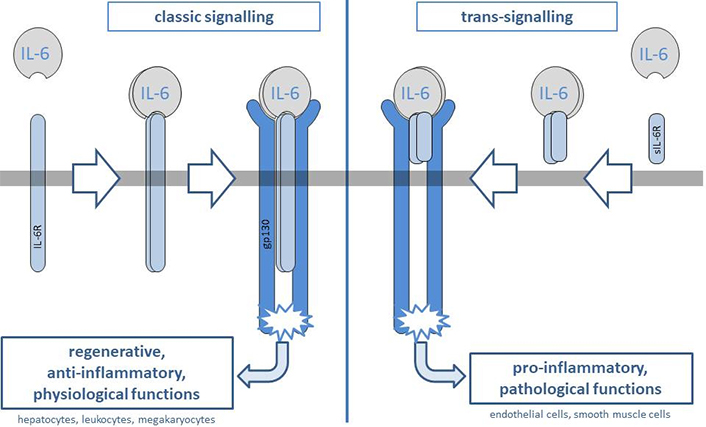
The different signaling pathways for IL-6
Note. Reprint with permission from “IL-6-induced classic and trans-signalling” by Schaper F. [cited 2024 Feb 16]. Available from https://www.systembiologie.ovgu.de/en/-p-176.html
Despite these crucial distinctions between IL-6 classic- and trans-signaling, many research investigations probing anti-inflammatory interventions often neglect this differentiation. Failing to distinguish between these signaling mechanisms when assessing IL-6 levels could lead to data misinterpretation, and subsequently an over- or under-estimation of the efficacy of anti-inflammatory interventions. Such an oversight could unintentionally compromise the evaluation of potential side effects and the overall effectiveness of therapeutic strategies [10].
For example:
If an anti-inflammatory IL-6 inhibitor suppresses both IL-6 classic- and trans-signaling, it may inadvertently impede beneficial immune responses, leading to unintended consequences like heightened susceptibility to infections.
Conversely, interventions that selectively inhibit IL-6 trans-signaling, could be successfully treating inflammatory symptoms but still present high IL-6 reports in undifferentiated biomarker tests [11].
Neglecting these differences can potentially obstruct progress in devising effective therapies for chronic inflammatory diseases, and may also complicate our understanding of the relationship between inflammation, IL-6 signaling, and the treatment of chronic inflammatory diseases. Hence, future research initiatives should make deliberate efforts to distinguish between these IL-6 signaling pathways when investigating anti-inflammatory interventions.
Lack of differentiation
The demarcation between IL-6 classic-signaling and IL-6 trans-signaling is essential to comprehending the multifaceted roles of IL-6 in health and disease. However, this distinction is frequently glossed over in biomedical research, especially in studies centered on chronic inflammatory disease and endometriosis.
A significant contributor to this oversight is the technical challenge of differentiating between these two signaling pathways in vivo. While in vitro studies can utilize techniques such as receptor blockade to distinguish between IL-6 classic- and trans-signaling, these methods are less practical in living organisms due to the universal expression of gp130 and the potential unintended effects of blocking agents [12].
Additionally, the most commonly employed assays for quantifying IL-6 levels in biological specimens do not differentiate between IL-6 bound to soluble or membrane-bound IL-6R, thus failing to encapsulate the distinction between the two pathways.
Another element contributing to this oversight is the relatively recent elucidation of the disparate roles IL-6 classic- and trans-signaling play. While IL-6 has been a point of interest in immunology for several decades, the concept of trans-signaling was introduced only in the mid 1990s [13]. Given this relatively recent evolution and the topic’s complexity, many researchers may not fully comprehend the significance of differentiating between these two signaling pathways when interpreting IL-6 measurements.
This failure to distinguish between IL-6 classic- and trans-signaling is especially relevant in behavioral medicine, which frequently employs anti-inflammatory interventions to enhance well-being and prolong healthspan. These interventions, encompassing mindfulness meditation, yoga, and acupuncture, among others, have been shown to affect IL-6 levels. However, the specific impacts of these interventions on IL-6 classic- vs. trans-signaling remain largely uncharted territory [14].
A meta-analysis compared measurements of IL-6 in response to both inflammatory diseases and anti-inflammatory interventions [15]. This analysis found that different collections of biomarkers labeled as undifferentiated “IL-6” were impacted with significant effect sizes in response to both pro-inflammatory and anti-inflammatory catalysts. The study proposed that these metrics were in fact capturing both IL-6 trans and classic, respectively, and had thus been underreporting the efficacy of the anti-inflammatory treatments. When IL-6 trans and classic were incorporated into the analysis, interventions expected to focus on IL-6 trans ranked higher in efficacy, as seen in Figure 2.
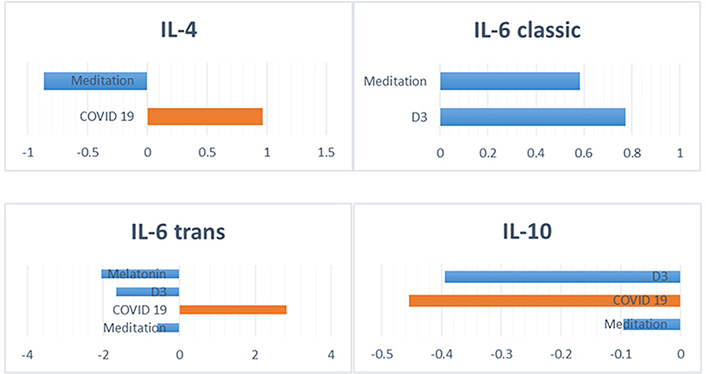
Biomarker measurements indicating an increase in pro-inflammatory action (orange) via IL-6 trans from COVID-19, while anti-inflammatory actions (blue) were associated with melatonin, vitamin D3, and meditation. Conversely, meditation and vitamin D3 were associated with anti-inflammatory action (blue) via IL-6 classic
Note. Adapted with permission from “Implications for Systemic Approaches to COVID-19: Effect Sizes of Remdesivir, Tocilizumab, Melatonin, Vitamin D3, and Meditation.” by Castle RD, Williams MA, Bushell WC, Rindfleisch JA, Peterson CT, Marzolf J, et al. J Inflamm Res. 2021;14:4859–76 (https://doi.org/10.2147/JIR.S323356). CC BY-NC.
Differentiating between IL-6 classic- and trans-signaling is a pivotal aspect of interpreting IL-6 measurements in research but is frequently overlooked, particularly in addressing complex, chronic conditions. The impacts of this lack of distinction can be found in the fields of endometriosis, neuroscience, and many chronic inflammatory diseases, though these are only examples of a far broader issue. Acknowledging and addressing this oversight is necessary to foster a more nuanced understanding of the effects of anti-inflammatory interventions, ultimately contributing to the formulation of more targeted and effective therapies.
Discussion
Unpredictable IL-6 metrics in endometriosis research
Endometriosis, a chronic inflammatory condition, affects approximately 10% of reproductive-aged women worldwide [16]. Characterized by the growth of endometrial-like tissue outside the uterus, endometriosis results in pain and fertility issues among the afflicted. The molecular and cellular mechanisms underpinning the pathology of endometriosis have been the subject of intensive study, with IL-6 emerging as a pivotal cytokine in this context. Research has suggested that IL-6, especially through the trans-signaling pathway, contributes to pain in endometriosis patients by promoting endometrial lesions [17]. Furthermore, IL-6 can also facilitate angiogenesis, supporting the survival and proliferation of ectopic lesions [18]. The nuanced roles of IL-6 classic- and trans-signaling in endometriosis bear implications for the diagnosis, treatment, and overall understanding of the condition.
The misinterpretation of inflammatory responses is a significant concern stemming from this lack of differentiation. The anti-inflammatory nature of IL-6 classic-signaling, particularly in tissue regeneration, contrasts sharply with the pro-inflammatory tendencies of trans-signaling [8]. Consequently, an inaccurate assessment of the inflammatory milieu in endometriosis patients could arise, potentially misleading clinicians in gauging the severity of the disease or the efficacy of therapeutic interventions. Given the centrality of IL-6 measurements in endometriosis research, such misinterpretations may also result in therapeutic strategies that inadvertently suppress the beneficial effects of classic-signaling, thereby potentially prolonging patient recovery and undermining tissue repair and regeneration [19]. Without precise differentiation, the potential for IL-6’s dual nature to serve as a nuanced biomarker for endometriosis regulation and dysregulation remains largely untapped, reducing treatment efficacy assessments [20].
This oversight is particularly visible in chronic inflammatory diseases like endometriosis. Figure 3 demonstrates the lack of studies incorporating differentiation between the signaling pathways of IL-6. Searches through PubMed were conducted on multiple search strings relating to endometriosis and IL-6, with an increasing focus on pathway differentiation. While endometriosis studies incorporating IL-6 were common, only a fifth of those studies mentioned signaling pathways. When specific pathways were articulated, results became negligible. There were only eight results for a search of endometriosis and IL-6 trans vs. classic, and only seven studies actually incorporated any differentiation between IL-6 trans and IL-6 classic.
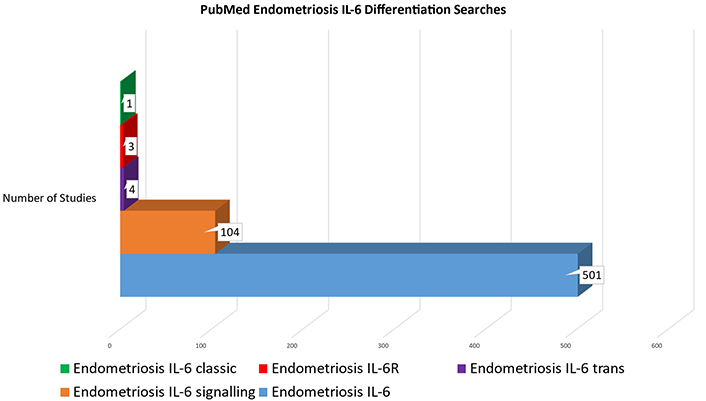
Results of searches through PubMed (accessed 2024 Feb 17) for studies on endometriosis and IL-6, with and without differentiation
This steep drop off reflects not only a significant gap in the state of research, but a disproportionate lack of focus in ongoing research. Table 1 consists of a network analysis of the preceding and proceeding citations of endometriosis studies relating to IL-6 examined the number of citations studies received and a similarity factor rating, based on how closely the studies list reflected the initial search parameter.
The top 40 studies in a network analysis of searches for endometriosis and IL-6 as well as endometriosis and IL-6 trans
| Endometriosis IL-6-trans | Endometriosis IL-6 | |||||
|---|---|---|---|---|---|---|
| Citations | Refs | Similarity | Citations | Refs | Similarity | |
| 7 | 0 | 100 | 42 | 31 | 100 | |
| 4 | 0 | 10.9 | 138 | 51 | ||
| 16 | 57 | 9.4 | 52 | 25 | ||
| 6 | 27 | 9.1 | 43 | 81 | ||
| 15 | 73 | 7.7 | 131 | 64 | ||
| 3 | 74 | 7.3 | 127 | 54 | 18.1 | |
| 11 | 61 | 6.8 | 85 | 49 | 18.1 | |
| 7 | 40 | 145 | 36 | 16.3 | ||
| 13 | 37 | 49 | 54 | 16.2 | ||
| 50 | 54 | 34 | 43 | 15.8 | ||
| 16 | 45 | 38 | 38 | 14.7 | ||
| 13 | 42 | 27 | 36 | 14.5 | ||
| 7 | 54 | 60 | 24 | 14 | ||
| 14 | 49 | 68 | 41 | 13.5 | ||
| 13 | 148 | 177 | 66 | 13.4 | ||
| 13 | 28 | 75 | 29 | 13.2 | ||
| 8 | 69 | 37 | 67 | 12.8 | ||
| 0 | 109 | 33 | 76 | 12.8 | ||
| 43 | 162 | 134 | 70 | 12.6 | ||
| 59 | 68 | 111 | 49 | 12.6 | ||
| 15 | 48 | 32 | 89 | 12.4 | ||
| 108 | 37 | 230 | 65 | 12.4 | ||
| 9 | 43 | 41 | 28 | 12.2 | ||
| 73 | 36 | 90 | 173 | 12.1 | ||
| 52 | 44 | 228 | 24 | 11.9 | ||
| 25 | 57 | 141 | 63 | 11.8 | ||
| 27 | 128 | 137 | 57 | 11.8 | ||
| 18 | 135 | 41 | 49 | 11.6 | ||
| 239 | 125 | 47 | 49 | 11.6 | ||
| 38 | 190 | 35 | 43 | 11.4 | ||
| 50 | 46 | 53 | 54 | 11.3 | ||
| 9 | 37 | 20 | 23 | 11 | ||
| 6 | 46 | 282 | 271 | 11 | ||
| 1 | 0 | 22 | 65 | 11 | ||
| 1 | 45 | 63 | 28 | 11 | ||
| 1 | 0 | 152 | 150 | 11 | ||
| 1 | 87 | 57 | 21 | 10.9 | ||
| 1 | 54 | 95 | 60 | 10.9 | ||
| 13 | 227 | 64 | 114 | 10.8 | ||
| 1 | 46 | 35 | 79 | 10.6 | ||
| 1 | 21 | 81 | 160 | 10.6 | ||
| 1,007 | - | 228.7 | 3,552 | - | 539.3 | |
Multiple search terms were used to identify synonyms and largest returns were utilized. Relative similarities were color coded from strong to weak on a respective blue-red spectrum. Bold numbers at the bottom indicate total sums of the fields above. -: blank cell
The number of citations for papers related to endometriosis and undifferentiated IL-6 was 235% higher than for endometriosis IL-6 trans.
The similarity factor rating for undifferentiated IL-6 was 352% higher than for endometriosis and IL-6 trans.
There is a clear and significant difference in number of citations for the top 40 articles related to either differentiated or undifferentiated IL-6 and endometriosis. It is likely this reflects far less activity and ongoing research into the differentiation of IL-6 signaling pathways within endometriosis diagnosis and care. The low numbers of similarly clustered studies relating to endometriosis and differentiated IL-6 suggests problematic heterogeneity in the different studies related to the topic. Diagrams using weighted clustering can visually depict the distance between studies with or without differentiation. Figure 4 and Figure 5 illustrates how a network analysis of IL-6 trans and endometriosis produced distant, disparate clustering, whereas the network analysis of undifferentiated IL-6 and endometriosis is close and evenly clustered.
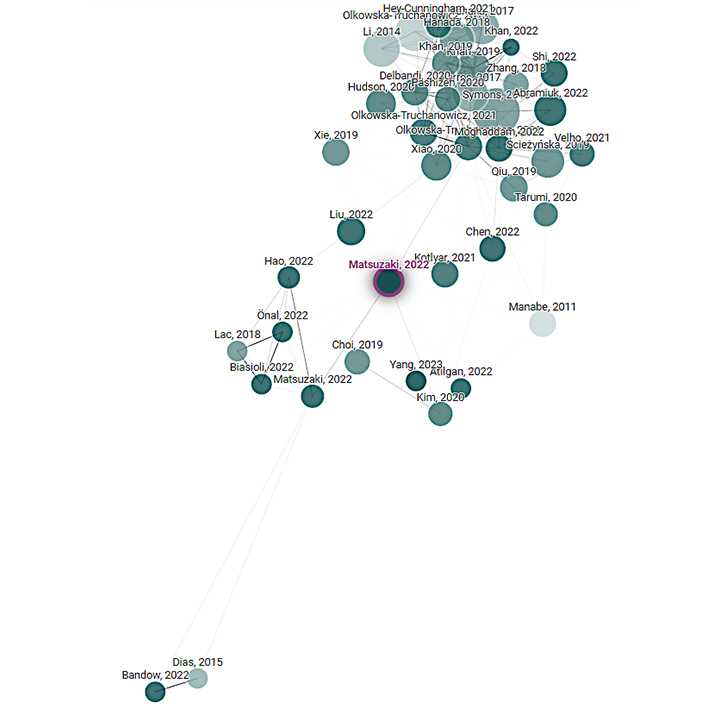
A visualization of the network analysis of search parameter “endometriosis IL-6-trans” as seen in Table 1, organized by citation. Clustering is by weighted similarity factor, studies that are more closely linked are more closely clustered. Created with Connected Papers accessed 2024 Feb 18, which is connected to the Semantic Scholar Paper Corpus (licensed under ODC-BY)
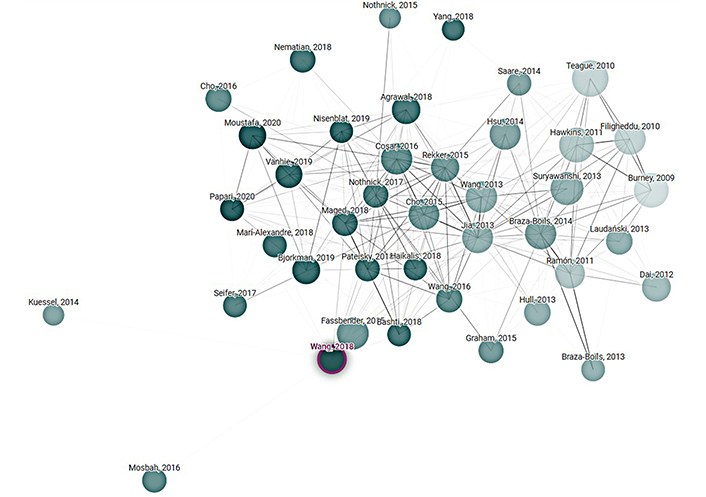
A visualization of the network analysis of the search parameter “endometriosis IL-6” as seen in Table 1, organized by citation. Clustering is by weighted similarity factor, studies that are more closely linked are more closely clustered. Created with Connected Papers accessed 2024 Feb 18, which is connected to the Semantic Scholar Paper Corpus (licensed under ODC-BY)
Taken together, there is a scarcity of endometriosis studies specifically incorporating IL-6 differentiation, a relatively low number of citations related to endometriosis and any focus on IL-6 trans, and a low network analysis rating of IL-6 differentiated studies. These factors strongly suggest the study of endometriosis diagnostics and treatments do not commonly incorporate differentiation of IL-6 signaling pathways. It is believed endometriosis is indicative of similar disparities relating to many other chronic inflammatory diseases.
There are several potential consequences for this disparity. From a clinical perspective, the reliance on IL-6 levels for diagnosing endometriosis is common and highly influential. The heterogeneity of endometriosis manifestations means that IL-6 levels can vary widely among patients, influenced by disease stage, lesion location, and individual immune responses, requiring precise measurement. Unreliable IL-6 measurements may lead to misdiagnosis or underdiagnosis, particularly in cases where undifferentiated IL-6 levels are incongruent with clinical symptoms [21]. This discrepancy complicates the development of standardized diagnostic criteria and can delay appropriate treatment interventions. Timely diagnosis of endometriosis is critical for proper care, with serious health impacts resulting from misdiagnosis or underdiagnosis [22].
Moreover, the treatment of endometriosis often involves managing symptoms and controlling disease progression through hormonal therapies and surgical interventions. However, without accurate and reliable measurements of IL-6 and an understanding of its signaling context, it is challenging to predict therapeutic outcomes or tailor treatments to individual patient profiles. For instance, indiscriminate blockade of IL-6 could dampen its beneficial effects in tissue protection and repair, leading to unclear side effects and obscured therapeutic outcomes. For example, two different studies into the use of tocilizumab as an IL-6 inhibitor in rats produced comparable clinical results, but starkly different IL-6 measurements. One study, which did not differentiate IL-6 trans and classic, reported no discernible IL-6 changes after the intervention [23]. The other study, which included signal differentiation in its measurements, reported sharp reductions in IL-6 trans [24]. If IL-6 measurements are not consistent, inflammation measurements can be unreliable.
The challenges extend to monitoring disease progression and treatment efficacy. IL-6 has been investigated as a biomarker for disease activity, however, variable IL-6 levels, influenced by both biological fluctuations and measurement inaccuracies, can obscure the correlation between cytokine levels and disease state [25]. This uncertainty complicates the clinician’s ability to make informed decisions about treatment adjustments or to accurately predict disease prognosis.
Given IL-6’s involvement in inflammatory processes, narrow inhibitor therapies targeting IL-6 signaling have been proposed as potential treatments [26]. Research into olamkicept has focused on targeting IL-6 trans-signaling while leaving IL-6 classic-signaling intact [27]. The results have been promising for IBD and ulcerative colitis, reducing clinical inflammatory symptoms without the immunosuppressant side effects of total IL-6 inhibition. The success of precise targeting indicates the value of widespread differentiation.
A practical dimension where greater differentiation of IL-6 pathways might prove valuable is in symptom management. Pain, a predominant symptom of endometriosis, has been linked to IL-6 mediated nerve fiber growth in ectopic lesions [28]. By understanding and targeting the specific IL-6 signaling pathways responsible, pain management could see substantial advancements, enhancing patient quality of life. Differentiated IL-6 inhibitors have been widely explored for the treatment of COVID-19, and a similar focus may be warranted for endometriosis and other inflammatory diseases.
By addressing the oversight in differentiation several benefits could emerge that may significantly improve endometriosis management. Tailored therapeutic strategies that specifically target IL-6 trans-signaling could mitigate the pro-inflammatory aspects of endometriosis more effectively without disrupting the beneficial effects of classic-signaling [29]. Such specificity in treatment could herald improved outcomes and reduced side effects. Furthermore, the diagnostic precision in endometriosis can be significantly enhanced. Accurately differentiated IL-6 signaling can offer clinicians insights into disease severity, progression, and earlier detection, a critical component of endometriosis care that can inform treatment decisions and prognostic evaluations [30].
Implications for chronic inflammatory illnesses
As seen in the focus on endometriosis, IL-6 plays a pivotal role in the immune response and has also been implicated in the pathogenesis of a wide range of inflammatory diseases, including rheumatoid arthriti, systemic lupus erythematosus (SLE), and IBD.
In the context of RA IL-6 trans is a key driver of inflammation and joint destruction. Some therapies that inhibit IL-6, such as monoclonal antibodies against IL-6R, have demonstrated significant efficacy in reducing disease activity and improving clinical outcomes. However, by inhibiting both signaling pathways, these therapies may also interfere with the protective roles of IL-6, such as its involvement in host defense and tissue regeneration. A more selective approach that targets only the trans-signaling pathway can preserve the cytokine’s homeostatic functions while effectively mitigating inflammation and disease progression [31]. Similarly, in diseases like IBD, where IL-6 levels correlate with disease severity and inflammation, selectively targeting the trans-signaling pathway could reduce intestinal inflammation and promote mucosal healing, offering a novel approach to treatment that minimizes the risk of systemic side effects [32]. Differentiated targeting of IL-6 trans inhibition has shown significant promise as a useful anti-inflammatory intervention, with awareness of differentiation being the limiting factor in adoption [33].
The differentiation of IL-6 signaling pathways also has profound implications for the development of diagnostic tools and the monitoring of disease activity in chronic inflammatory conditions. Inflammatory markers, including IL-6, play an essential role in diagnostics, and treatment decision-making. Elevated IL-6 levels have been associated with disease activity in several chronic inflammatory diseases, serving as a useful biomarker for disease progression and response to therapy. However, the common lack of specificity in distinguishing between the signaling pathways of IL-6 limits the utility of this cytokine as a biomarker. By encouraging diagnostic assays that can differentiate between classic- and trans-signaling activities of IL-6, clinicians could gain valuable insights into the underlying mechanisms driving disease activity, allowing for more personalized and precise treatment strategies. Such advancements in diagnostics could facilitate early intervention, improve disease management, and potentially lead to better patient outcomes by enabling the timely adjustment of therapies based on specific inflammatory profiles [34].
The precise differentiation of IL-6 signaling pathways holds significant promise for enhancing the treatment of chronic inflammatory diseases by providing a basis for the development of targeted therapies that minimize adverse effects while maximizing therapeutic efficacy [35]. Moreover, the differentiated measurement of IL-6 activity could improve the use of inflammatory markers in diagnostics and treatment monitoring, leading to more informed clinical decision-making and improved patient care.
Limitations
This review presents an examination of the role of IL-6 signaling in relation to chronic inflammatory diseases and the complexities inherent in the differentiation between IL-6 classic-and trans-signaling. This has significant implications, especially in the research of endometriosis, but there are several limitations worth noting.
While this review is comprehensive it is not exhaustive. Though it is among the most often measured, IL-6 is only one of many cytokines involved in immune response and inflammation. A focus solely on IL-6 does not provide a full picture of the dynamic nature of immune reactions and inflammation. The very problem addressed in this paper also limits the conclusions that can be derived from this paper. The involvement of other cytokines and signaling pathways with IL-6 signaling is both complex and unclear.
The review also relies heavily on published literature, which may be subject to publication bias. Studies with null or negative findings are less likely to be published, which may skew the body of literature toward studies that report significant effects. This bias could potentially impact the interpretation and conclusions of this review [36].
Finally, while this review highlights a methodological issue in the measurement of IL-6 and proposes potential solutions, the practical implementation of these solutions may be challenging. The differentiation between IL-6 classic- and trans-signaling in research settings is not straightforward and requires technical development [37]. Additionally, the proposed methodologies may not be feasible in all research settings due to cost, technical complexity, or lack of resources.
While this review provides a critical perspective on the role of IL-6 in endometriosis research, these limitations should be taken into consideration when interpreting the findings and suggestions put forth.
Conclusions
The examination of IL-6 signaling pathways and their distinct roles in the pathology of endometriosis highlights a growing issue in the study of chronic inflammatory diseases. This differentiation is not merely a molecular distinction but a pivotal factor that influences the direction of research, the development of diagnostic criteria, and the formulation of therapeutic interventions for endometriosis and potentially other chronic inflammatory diseases. The current research landscape, as indicated by the analysis of publications and citation patterns, demonstrates a pronounced disparity in focus, with a very limited number of studies addressing the different roles of IL-6 signaling pathways. This gap underscores a significant oversight in the field, limiting the understanding of IL-6’s dual roles in inflammation and tissue regeneration and its implications for disease management.
These gaps in research reveal critical vulnerabilities in the diagnosis and treatment of endometriosis, a condition marked by significant pain and fertility issues in women of reproductive age. The predominance of studies that fail to differentiate between IL-6 pathways may lead to a misinterpretation of the cytokine’s role in endometriosis, potentially delaying diagnosis or guiding therapeutic strategies that inadvertently suppress beneficial signaling mechanisms. This oversight, as highlighted in the tocilizumab studies, highlights a critical need for a shift towards incorporating IL-6 pathway differentiation in endometriosis research and inflammatory measurements.
The significance of IL-6 differentiation extends beyond the example of endometriosis, suggesting broader applications for the management of chronic inflammatory conditions. A more nuanced focus on IL-6 signaling such as that seen in olamkicept could inform biomarker interpretations for diseases like RA and IBD, where IL-6 plays a key role in pathogenesis. The potential for targeted inhibition of IL-6 trans-signaling, while preserving the protective effects of classic-signaling, offers a promising avenue for developing treatments that are both effective and exhibit fewer side effects. Progress in this field is unlikely unless the significance of IL-6-trans-specific inhibitors is discussed more widely.
Addressing the disparities in IL-6 research requires concerted efforts to prioritize studies that explore the distinct functions of IL-6 signaling pathways. This entails not only increased discussion and focus on research in this area but also the adoption of more advanced diagnostic tools capable of distinguishing between IL-6 signaling mechanisms. Such tools could provide clinicians with critical insights into the inflammatory status of patients, facilitating more informed treatment decisions and potentially improving patient outcomes.
The improved differentiation of IL-6 signaling pathways presents a critical step in the research and treatment of endometriosis and other chronic inflammatory diseases. Bridging the current research gap offers the promise of more accurate diagnostics, comprehensive therapeutic strategies, and a deeper understanding of inflammatory pathologies. As the scientific community moves forward it is imperative to embrace the complexity of cytokine signaling, recognizing its potential to transform the landscape of disease diagnosis and management. Through targeted research and clinical application, the nuanced roles of IL-6 trans and IL-6 classic in health and disease can be better understood, paving the way for advancements in patient care and treatment efficacy.
Abbreviations
| gp130: | glycoprotein 130 |
| IBD: | inflammatory bowel disease |
| IL-6: | interleukin-6 |
| IL-6R: | interleukin-6 receptor |
| RA: | rheumatoid arthritis |
| sIL-6R: | soluble interleukin-6 receptor |
Declarations
Acknowledgments
During the preparation of this work, the author used ChatGPT 4 in order to assess readability and identify typographical errors. After using this tool/service, the author reviewed and edited the content as needed and take full responsibility for the content of the publication. The author also thanks to Pubmed and Connected Papers.
Author contributions
RDC: Conceptualization, Analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
Conflicts of interest
The author declares that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.