Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
ORCID: https://orcid.org/0009-0001-0238-7945
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
ORCID: https://orcid.org/0009-0008-5556-2314
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
ORCID: https://orcid.org/0009-0004-6162-1174
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
ORCID: https://orcid.org/0009-0000-4812-1678
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
ORCID: https://orcid.org/0009-0009-1897-7120
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
ORCID: https://orcid.org/0009-0001-3263-0096
Affiliation:
1Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China
2Research Institute of Huazhong University of Science and Technology in Shenzhen, Shenzhen 518109, Guangdong, China
Email: jhsyyjs@126.com
ORCID: https://orcid.org/0000-0002-2291-2524
Explor Immunol. 2024;4:490–501 DOI: https://doi.org/10.37349/ei.2024.00154
Received: January 01, 2024 Accepted: May 20, 2024 Published: August 22, 2024
Academic Editor: Daishu Han, Chinese Academy of Medical Sciences & Peking Union Medical College, China
The article belongs to the special issue Immunobiology and Inflammation in the Male Reproductive System
The male reproductive immune system plays a pivotal role in safeguarding sperm from immune attacks and preventing the incursion of foreign pathogens. Nucleotide-binding and oligomerization domain-like receptors (NOD-like receptors, NLRs) family protein domain containing 3 (NLRP3) is a cytoplasmic sensor binding to the inflammasome and critically involved in inducing innate immunity in the testes. It also has a substantial impact on male reproductive immunity, which is closely associated with male infertility stemming from disorders related to the male reproductive immune system. This review introduces the distinct characteristics of the NLR family, elucidates the activation pathways and factors of NLRP3 inflammasomes, and discusses how they participate in male reproductive immune diseases such as bacterial orchitis, autoimmune orchitis, varicocele, and epididymitis. In bacterial orchitis, elevated levels of NLRP3 inflammasomes exacerbate the testicular inflammatory injury and lead to decreased testosterone, thus contributing to male infertility. In autoimmune orchitis, the NLRP3 inflammasomes inhibit testosterone synthesis by decreasing the expression of cytochrome P450, thereby impacting male reproductive function. Therefore, targeting NLRP3 could offer novel immunological strategies for the clinical treatment of male infertility.
The rising prevalence of male infertility has underscored the significance of male reproductive health. Inflammation is believed to contribute to 13–15% of male infertility, particularly conditions like epididymitis and epididymo-orchitis. In male reproductive immunity, the testes and epididymis are susceptible due to their non-renewable epithelial cells. The sequelae of infection and inflammation in these organs frequently lead to permanent damage, resulting in fertility loss [1].
It is worth noting that the testis is an immune-privileged site and one of the few organs with a significantly reduced systemic immune response, including the pregnant uterus, eyes, brain, etc. [2]. Such an immune-privileged environment not only tolerates testicular autoantigens but also protects vital tissues from immune aggression, significantly impacting both normal spermatogenesis and fertility protection [3]. An uncontrolled inflammatory response at the level of the epididymis, an accessory organ of the testis, can be considered an important cause of male infertility. Unlike the sophisticated design of the testis, the epididymis lacks the tight junction structure composed of adjacent Sertoli cells, yet immune cells are often observed within the epididymal epithelium and can even be found within the lumen of the epididymis. Crucially, there is little evidence that the epididymis is immunoprivileged, and its anatomical location makes it more susceptible to losing its immune tolerance [4]. Although the testis and epididymis are protected by innate immunity, they can still be infected by a wide range of pathogens, such as bacteria, viruses, protozoa, etc. The invasion of these pathogens can cause problems associated with poor sperm quality, low sperm concentration, and poor sperm motility [5]. Therefore, it is essential to clarify the immune features of the testis and epididymis. On the one hand, maintaining the immune environment of the testes and epididymis prevents the occurrence of autoimmune reactions, and on the other hand, preventing inflammation ensures normal sperm production, thus ensuring the normal operation of male reproduction [6].
NLRP3 is a central node of immune sensing within the innate immune system [7], and its inflammasome serves as a platform for perceiving danger stimuli from endogenous or exogenous environments, participating in both innate and adaptive immunity by regulating cytokine secretion and apoptosis [8]. NLRP3 inflammasomes are cytoplasmic protein complexes whose assembly leads to the activation of caspase-1, thereby promoting the maturation and release of inflammatory cytokines and interleukin (IL)-1β, and IL-18, and they also fulfill a crucial function in inflammatory cell death and apoptosis [9]. NLRP3 inflammasomes provide fuel for chronic and acute inflammation and are critical in the emergence of inflammation [10]. NLRP3 inflammasomes are primarily involved in the modulation of inflammatory responses and immune responses and are capable of sensing infections, tissue damage, and danger signals. They contribute to intracellular signaling cascades that ultimately lead to the production of inflammatory cytokines and the onset of inflammatory responses [11]. They have been found to be closely associated with the development of testicular varicocele [12], depression [13], neurodegenerative diseases [14], alcoholic liver disease [15], epididymitis, etc. Studies by Sutterwala FS et al. [16] found that NLRP3 inflammasomes in testicular immunity have the unique ability to recognize and activate pathogen-associated molecular patterns (PAMPs) on the surface of pathogenic microorganisms, which is the key to initiating the innate immune response in the testis. Minutoli L et al. [17] found that NLRP3 inflammasomes are triggered in the testis after ischemia/reperfusion (I/R), and this pathological cascade leads to enhanced apoptosis, vacuolization of spermatogonial epithelium, reduction of spermatozoa and neutrophil recruitment prompted by NLRP3 inflammasomes. The decreased number of germ cells induced by neutrophil recruitment, leading to sperm damage and testicular atrophy [17].
As innate immunity serves as the first line of defense for male reproductive immunity, elucidating the role of the NLRP3 inflammasome is imperative for the study of diseases associated with testis and epididymal immunity [18]. This paper will review the NLRP3 inflammasomes from the perspectives of their activation pathway, activating factors, and their relationship and function with male reproductive immunity.
NLRs, a family of cytoplasmic sensors that bind to inflammatory complexes, are gradually coming into the limelight; they are a representative class of receptors responsible for innate immune pattern recognition [19]. The NLR family possesses several distinctive structural features, including a central nucleotide-binding and oligomerization (NACHT) structural domain, which is usually surrounded by a C-terminal leucine-rich repeat (LRR) and an N-terminal caspase recruitment domain (CARD) or pyrin domain (PYD) [20]. The NLRP3 inflammasome is composed of the NLRP3 scaffold, apoptosis-associated speck-like protein containing CARD (ASC) junctions (PYCARD), and cystatinase-1 (caspase-1) together (Figure 1) [21]. Ratsimandresy RA et al. [22] found that NLRP3 is able to encode the PYD, which contributes significantly to the innate immune response pathways and is required for host defense against pathogens and the initiation of wound healing. Haneklaus M et al. [23] have shown that NLRP3 can provide an essential molecular mechanism in the induction of central IL-1β by activating caspase-1. IL-1β is a critical pro-inflammatory cytokine that drives the inflammatory process and restores homeostasis [23].
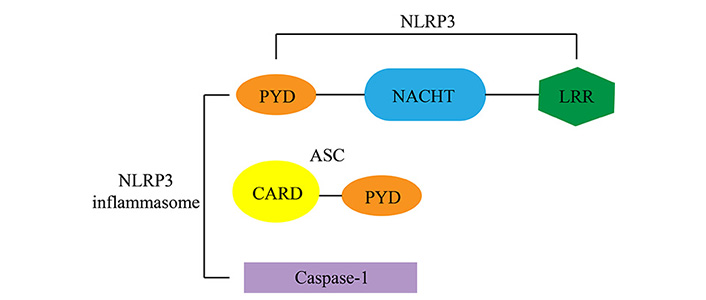
The structure of the NLRP3 inflammasome components. The NLRP3 inflammasome is composed of the NLRP3 scaffold, apoptosis-associated speck-like protein containing CARD (ASC) junctions (PYCARD), and cystatinase-1 (caspase-1) together
As a result of gene amplification from the last common ancestor, 22 human genes and many more mouse genes come together to form the NLR family. Among the structural domains found in NLR family members, the NACHT structural domain is the only structural domain common to all NLR family members and can activate the signaling complex through ATP-dependent oligomerization. LRR structural domains are thought to yield considerable influence on ligand perception and autoregulation, while the CARD and PYD structural domains may be instrumental in mediating homotypic protein-protein interactions for downstream signaling [19].
In general, the initiation of NLRP3 inflammasomes requires the realization of two signals. The first initiating stimulus, which can include any receptor signal (such as phosphorylated Toll-like receptor) leading to activation of the transcription factor NF-κB, the signal provided by NF-κB activators is essential for NLRP3 transcription [24]. However, under the premise of steady-state and sufficient concentrations, ASC and procaspase-1 are not required for transcriptional regulation to achieve activation of the inflammasome, nor is pro-IL-18, the substrate of caspase-1. They can promote caspase-1 activation through an acute mechanism that is independent of induction by inflammasome sensors [25]. Secondly, observations relying on pattern recognition receptor (PRR) signals have shown that free cytoplasmic NLRP3 remains in an inactive ubiquitinated state until the initiation signal stimulates its LRR structural domain ubiquitination, at which point NLRP3 is activated through a two-step deubiquitination mechanism initiated by Toll-like receptor signaling and mitochondrial reactive oxygen species (ROS) activation [26]. This discovery not only emphasizes the critical steps of the initiation program but also distinguishes it from the transcriptional step. Between the transcriptional up-regulation and post-translational modification of inflammasome components, PRR signal-dependent initiation establishes a high threshold; only by reaching this threshold can NLRP3 inflammasome activation be achieved. Another group of molecules that control the initiation of NLRP3 inflammasomes are the cellular inhibitor of apoptosis proteins (cIAPs), commonly considered programmed cell death. cIAP1 and cIAP2 have also been shown to be vital in the inflammasome pathway by acting on caspase-1 [27].
Research has shown that ROS, K+, Ca2+, and mitochondrial dysfunction are closely related to the activation of NLRP3 inflammasomes (Figure 2). NLRP3 inflammasomes can also be activated by many other molecules. In 2006, a study by Mariathasan S et al. [28] found that extracellular ATP released by damaged cells can activate caspase-1, thereby stimulating the activation of NLRP3 inflammasomes. In 2008, Halle A et al. [29] found that fibrillar peptide amyloid-β (aβ) also activated inflammasomes formed by the cytoplasmic receptor NLRP3, leading to the release of IL-1β; and in 2009, research by Yamasaki K et al. [30] found that hyaluronic acid could activate NLRP3 inflammasomes by stimulating IL-1β. Activation of the NLRP3 inflammasomes also monitors autometabolic stress. In 2006, Martinon F et al. [31] found that monosodium urate (MSU) and calcium pyrophosphate deposition (CPPD) were involved in the activation of NLRP3 inflammasomes, resulting in the production of active IL-1β. In 2009, Gasse P et al. [32] found that uric acid is released during cellular injury, and thus, uric acid-dependent pathways can also stimulate NLRP3 inflammasomes. In the same year, Griffith JW et al. [33] also demonstrated that the release of uric acid activates NLRP3 inflammasomes, which enhanced the host’s initial response to hemolysin. In 2010, Zhou R et al. [34] found that the activation of NLRP3 can improve glucose tolerance and insulin sensitivity. In addition, NLRP3 inflammasomes are activated by many factors. For example, trinitrophenyl chloride [35] and trinitro-chlorobenzene [36] are associated with pathologies related to contact hypersensitivity reactions; ultraviolet medium wave (UVB) [37] irradiation is linked to sunburn; SiO2 [38–40] is closely connected to silicosis; and asbestos [39] is related to the pathologies of asbestosis.
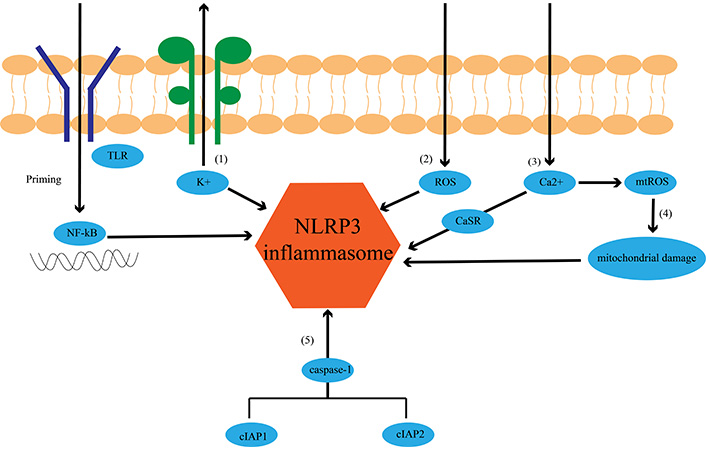
Schematic diagram of NLRP3 inflammasome activation. Toll-like receptor (TLR) is phosphorylated and subsequently activates NF-κB. NF-κB activates NLRP3 inflammasomes. NLRP3 inflammasomes activation (1) by inducing K+/potassium efflux, which leads to the assembly and activation of NLRP3 inflammasomes; (2) ROS production promotes the assembly and activation of NLRP3 inflammasome; (3) calcium, as a discrete activator, activates the NLRP3 inflammasomes using mouse calcium-sensing receptor (CaSR); (4) excessive or sustained Ca2+ uptake can increase mitochondrial ROS (mtROS), leading to mitochondrial rupture and ultimately activation of NLRP3 inflammasomes; (5) cellular inhibitor of apoptosis protein 1 (cIAP1) and cIAP2 activate NLRP3 inflammasomes by stimulating caspase-1
Orchitis usually refers to unilateral or bilateral orchitis caused by viruses and bacteria [41], mainly categorized into bacterial orchitis, autoimmune orchitis, and other testicular immune disorders. As for bacterial orchitis caused by prostate bacterial infection and urinary tract infection, its common pathogens include UroPathogenic Escherichia coli (UPEC), Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus and Streptococcus genera [42]. Autoimmune orchitis is mainly divided into two categories: primary and secondary. NLRP3 inflammasomes and testicular immunity are also inextricably intertwined. The study by Zhou Y et al. [43] confirmed that NLRP3 inflammasomes are paramount in destroying testicular structures. Activation of the typical inflammasome pathway of NLRP3/caspase-1 and IL-1β induces the secretion of inflammatory cytokine secretion and immune response in Sertoli cells. It is proven that NLRP3 inflammasomes engage in DBP (dibutyl phthalates)-induced inflammation of testicular Sertoli cells and provide a new reference point for the treatment of testicular inflammation. Walenta L et al. [44] concluded that NLRP3 may be a new participant in testicular immune regulation. To verify this claim, they performed immunostaining on human testicular sections from idiopathic infertility samples and found increased NLRP3 levels in Sertoli cells and peritubular cells, suggesting that NLRP3 expression is associated with idiopathic male infertility.
Su Y et al. [45] demonstrated that UPEC infection could induce testicular inflammatory responses by promoting NLRP3 inflammasome activation in testicular macrophages (TM). Li Y et al. [46] found that increased prokinetic 2 (PK2) in the TM and testicular interstitial fluid promoted this process. In addition, after UPEC infection, PK2 induces the activation of NLRP3 inflammasomes through a CaSR (calcium-sensing receptor) mechanism, facilitating the maturation of IL-1β. IL-1β is released from the TM into the testicular stroma, affecting the neighboring Leydig cells and inhibiting testosterone synthesis, thus decreasing sperm counts and anterograde motility [45]. Schaale K et al. [47] found that UPEC strains possess the ability to trigger lipopolysaccharide (LPS)-induced macrophage IL-1β release and can trigger NLRP3-dependent inflammasome activation and rapid cell death in macrophages. Sano M et al. [48] unearthed that NLRP3 inflammasomes were closely associated with testicular inflammation by investigating the effect of LPS-induced systemic inflammation on the role of NLRP3 inflammasomes in the mouse testis. It was discovered that modulation of NLRP3 inflammasomes exacerbates testicular inflammatory injury, reduced testosterone, and subsequent male infertility.
Autoimmune orchitis is one of the most important causes of decreased fertility in men, characterized by direct invasion of the testis and the presence of specific anti-sperm antibodies (ASA) [49]. Autoimmune orchitis can be divided into two categories. One is primary autoimmune orchitis, which refers to infertility and asymptomatic orchitis, associated with ASA (100%) in the basement membrane or seminiferous tubules of infertile males and without any systemic disease. Another type is secondary autoimmune orchitis, which is symptomatic orchitis or testicular vasculitis associated with systemic autoimmune disease [50]. According to consistent studies [51, 52], the activation marker IL-1β of NLRP3 inflammasomes significantly inhibits steroid production in Leydig cells. It does so by reducing the expression of cytochrome P450, which consequently inhibits testosterone synthesis. As of yet, the correlation between NLRP3 and autoimmune orchitis remains a relatively understudied area within the realm of immunological research.
The NLRP3 inflammasomes have been found to be closely associated with orchitis and autoimmune orchitis, and they have also been implicated in the pathogenesis of other diseases, such as cryopyrinopathies [53]. Cryopyrin is coded from NLRP3 (CIAS1), which encodes the protein called cryoprotein, hence its name. It manifests as aseptic inflammation [54] (changes in the wall of the male seminiferous tubules where spermatozoa are damaged, i.e., aseptic inflammation of the testes [55]), as well as male infertility. Mixed Atrophy (MA) is an autoimmune disease characterized by the simultaneous occurrence of intact and varying degrees of damaged seminiferous tubules; it is different from focal atrophy and typically involves atrophy in many areas of the testes, which can affect the formation of germ cells and the process of sperm production, resulting in infertility [56]. Enhancement of NLRP3 inflammasomes can be clearly seen in the Sertoli and peritubular cells of the seminiferous tubules in patients with MA, which results in a marked inflammatory response in the testicular interstitium, i.e., an increase in the number of macrophages and lymphocytes [19, 57].
Varicocele is an inflammatory syndrome caused by abnormal dilatation of the testicular veins, which is the most common abnormal condition in male reproductive diseases. It is characterized by a total tendon muscular injury and functional impairment of testicular interstitial cells. Varicocele is also defined as excessive dilation of the pampiniform plexus [58]. In the patient’s testicular tissues, elevated levels of ROS can lead to varicocele and low fertility [59]. Agarwal A et al. [60] have shown that ROS is negatively correlated with sperm concentration, viability, and sperm morphology. Desai N et al. [61] have also found that ROS causes sperm DNA damage and has adverse effects on fertilization potential and pregnancy rate. The above studies emphasize the outstanding contribution of ROS in activating NLRP3 inflammasomes, and the experimental model of varicocele also demonstrates the increase in NLRP3 inflammasome level expression [62, 63]. In this case, with the production of ROS and the activation of NLRP3 inflammasomes, an inflammatory state will occur in varicocele, damaging male reproductive tissues [59]. Whereas, the study of Sahin Z et al. [64] found that the levels of pro-inflammatory cytokines such as IL-1α and IL-1β also increased after varicocele induction in 11- and 13-week-old rats, and IL-1β is only expressed in the nuclei of spermatogonia and Sertoli cells. Increased IL-1α and IL-1β in varicocele alters homeostasis in favor of inflammation and immune response and adversely affects testicular tissue, leading to male infertility. At the current moment, more and more studies are linking immune disorders to the role of NLRP3 inflammasomes, providing new ideas and mechanisms for the disease [65]. Antonuccio P [63] and others have found that it is possible to combine seleno-L-methionine (Se) and polydeoxyribonucleotide (PDRN), an adenosine A2A receptor agonist, and that Se-PDRN improves all the renal tubular morphologic parameters and their ultrastructural features, increasing both testosterone levels and decreasing the expression of NLRP3, cysteinyl asparagin-1, and IL-1β, showing an overall positive effect on fertility. Given the many activators of NLRP3 inflammasomes, it remains to be further investigated whether we can turn our therapeutic attention to NLRP3 and provide a new approach to varicocele treatment while decreasing NLRP3 inflammasome expression.
Acute epididymitis is mostly an inflammation of the epididymis caused by an infection, the course of which is usually less than 6 weeks, and, in a few cases, accumulates to the testes, forming epididymo-orchitis [66]. In men under 35 years of age, the majority of cases are caused by sexually transmitted pathogens, such as Neisseria gonorrhoeae or Chlamydia trachomatis. Most cases in men older than 35 are infected by Escherichia coli or other gram-negative coliforms and usually occur in patients with urologic anomalies (e.g., benign prostatic hyperplasia), indwelling catheters, or recent urologic surgeries. And the main reason is a viral infection if it occurs in prepubertal boys [67]. Lin N et al. [68] found that sperm viability tended to decrease progressively in patients with chronic epididymitis, and the activation of NLRP3 inflammasomes promoted apoptosis and IL-1β production in the epithelial cells of the epididymis, thus leading to the progression of epididymal inflammation. Fan W et al. [69] found that a long-term high-fat diet leads to NLRP3 inflammasomes in reproductive tissues such as the epididymis. TNF-α and IL-6 expression levels are elevated, which are positively correlated with BMI and negatively correlated with sperm concentration and viability, causing a localized inflammatory response in the male reproductive tract and inducing epididymitis and other relevant diseases. Certainly, the effect of NLRP3 inflammasomes on infertility due to epididymitis deserves to be investigated in detail, and further efforts are needed.
In summary, the impression of NLRP3 inflammasomes in testicular, spermatic vein, and epididymal immune disorders is indispensable, and many testicular, spermatic vein, and epididymal immune disorders involve NLRP3 (Figure 3).
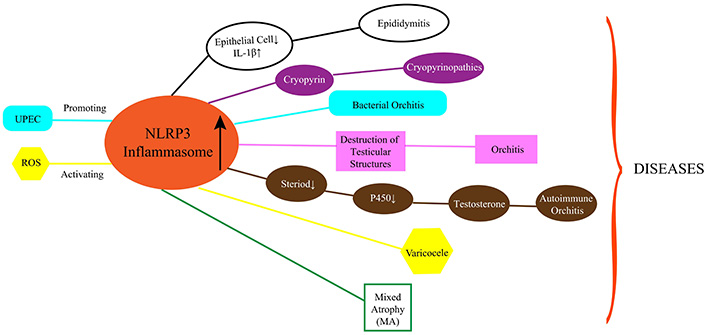
Schematic diagram of NLRP3 inflammasomes and related disorders. Changes in NLRP3 inflammasomes lead to Cryoglobinolinopathy, Mixed Atrophy (MA), Varicocele, Orchitis, Autoimmune Orchitis, Epididymitis, and more. UPEC: UroPathogenic Escherichia coli
This review includes the research progress of NLRP3 inflammasomes in male reproductive immunity and reveals their potential role in diseases such as testicular and epididymitis. NLRP3 inflammasomes are a driving force behind testicular and epididymal immunity and are closely bound up with various male reproduction-related diseases. Firstly, it was shown that the NLRP3 inflammasomes serve as a central node in male reproductive immunity. They act as platforms for sensing both endogenous and exogenous danger signals and are involved in regulating cytokine secretion and apoptosis, thus affecting the immune response of the male reproductive system. Activation of the NLRP3 inflammasomes triggers the production and release of inflammatory cytokines and ILs, which in turn affect the testicular and epididymal inflammatory responses, further confirming the importance of the NLRP3 inflammasomes in male reproductive immunity. Additionally, NLRP3 inflammasomes are closely related to many pathological processes and diseases of the male reproductive system. For instance, it has been shown that NLRP3 inflammasomes recognize and activate PAMPs that initiate testicular innate immune responses. This suggests that activation of NLRP3 inflammasomes is instrumental in diseases such as bacterial orchitis and autoimmune orchitis; furthermore, activation of NLRP3 inflammasomes is also correlated with the development of diseases such as varicocele and epididymitis, where its activation increases inflammatory response and testicular tissue damage.
This review illustrates the important role of NLRP3 inflammasomes in male reproductive immunity, which are involved in regulating inflammatory responses and are essential for male reproductive health. NLRP3 inflammasomes may be a promising target for the treatment of male reproductive immunity-related diseases and provide new therapeutic inspiration in the direction of immunomodulation of male infertility.
CARD: caspase recruitment domain
cIAPs: cellular inhibitor of apoptosis proteins
IL: interleukin
LRR: leucine-rich repeat
NLRP3: NOD-like receptors family pyrin domain containing 3
PYD: pyrin domain
ROS: reactive oxygen species
TM: testicular macrophages
UPEC: UroPathogenic Escherichia coli
XZ: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. XD: Writing—review & editing, Formal analysis. YK, XL, CZ, JZ, and SC: Investigation, Methodology. DH: Validation, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by the Guangdong Basic and Applied Basic Research Foundation [NO. 2021A1515220178]; and Independent Innovation Fund of HUST [5003519019].
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2548
Download: 28
Times Cited: 0
Xiaoyu Wu ... Xinzong Zhang
Yiming Zhang ... Ming Wang
Chuxiong Wang ... Donghui Huang
Prity Yadav, Pratap Chand Mali
