Affiliation:
1Department of Biology, Faculty of Sciences, University of Tunis El Manar, Tunis 2092, Tunisia
Email: abid_kalthoum@yahoo.fr
ORCID: https://orcid.org/0009-0006-2912-9678
Affiliation:
2Department of Medical Epidemiology, Laboratory of Transmission Control and Immunobiology of Infections (LR11IPT02), Pasteur Institute of Tunis, 1003 Tunis, Tunisia
ORCID: https://orcid.org/0000-0003-0056-452X
Affiliation:
3Department of Anatomo-Pathology, A. Mami Hospital, Lariana 2080, Tunisia
ORCID: https://orcid.org/0000-0002-3836-6572
Affiliation:
4Department of Medical Oncology, A. Mami Hospital, Lariana 2080, Tunisia
5Taoufik Clinic, Tunis 1082, Tunisia
ORCID: https://orcid.org/0000-0002-4131-8475
Explor Immunol. 2024;4:557–567 DOI: https://doi.org/10.37349/ei.2024.00159
Received: July 16, 2023 Accepted: July 09, 2024 Published: September 04, 2024
Academic Editor: Narinder K. Mehra, Indian Council of Medical Research (ICMR), India
The article belongs to the special issue Cytokines and Skin Diseases
Aim: Testing the feasibility of the determination to what extent the inability to repair DNA lesions in xeroderma pigmentosum (XP) patients, contributes to the alteration of immune responses, in the course of skin carcinogenesis.
Methods: Serum samples from 11 (five XP, six non-XP) bearing skin carcinomas and from three healthy donors, were available for the quantification of IL-2, IL-4, IL-10, IFN‐γ and TNF-α cytokines concentrations. We used kits for ELISA test, by a non-competitive sandwich method. Statistical analysis of the results was performed, using non-parametric Mann-Whitney U test, with an accuracy of 5%.
Results: Our results showed that the majority of XP and non-XP cancer patients showed a significant increase in the secretion of TNF-α cytokine above healthy individuals (controls). TNF-α was also found to be significantly high in the serum of XP patients above that reported for the studied non-XP cancer patients. At the same time, TNF-α was not detected in the serum of non-XP and of healthy controls. This increase in the expression level of TNF-α was statistically significant between XP and non-XP patients, and between XP patients and controls. In contrast, there were no significant differences between XP patients and healthy controls, as well as between XP and non-XP patients, for the level of serum IL-2, IL-4 and IL-10 cytokines. On the other hand, we found no detectable levels of IFN‐γ cytokine in the serum of all the studied subgroups.
Conclusions: In this study, we demonstrate a general tendency to secrete inflammatory cytokines, in the cancerous groups of patients (XP and non-XP), in comparison to healthy controls, while a significantly higher propensity to develop inflammation, in XP than in non-XP cancer patients.
Non-melanoma skin cancers (NMSCs) are the most common cancers in humans [1]. They occur as a result of chronic exposure to UV-B radiation that induces mutagenic DNA damage [2], and immune suppression [3].
Skin carcinomas are basal cell (70%) and squamous cell (20%) carcinomas that emerge from epidermal keratinocytes [4].
The human disease xeroderma pigmentosum (XP) is characterized by DNA repair and replication deficiencies, which have a 2,000-fold increased skin cancer risk [5]. Seven complementing groups have been described (XP-A to XP-G) to be involved in the nucleotide excision repair (NER) system [6], and a variant group XP-V. These groups have been illustrated by many studies, as a basis for the observed variability within XP patients [7].
Other studies have demonstrated impaired immune functions in XP patients, such as abnormal natural killer cell functions [8, 9], depletion of epidermal Langerhans cells [10], and other immune abnormalities [11, 12].
It has been shown by many clinical and epidemiological studies a strong interplay between various types of inflammation and cancer progression [13]. The recruitment of tumor-associated immune cells during inflammation, promotes tumor growth and the arising inflammatory cytokines have proven to be found in the tumor micro-environment [14]. Among inflammatory cytokines, TNF-α has been shown to play a major role in tumor progression. Initially, TNF-α was identified as a cytokine secreted by immune cells that have the capacity to suppress tumor cell proliferation. Later, research has revealed many roles for TNF-α [15], in physiological conditions such as in development, as well as in pathology, including tumor growth, inflammation, rheumatoid arthritis, and septic shock [16].
In a model using lab rats, it has been shown that long-term radio-therapy induced the production of inflammatory cytokines, such as TNF-α, at the mRNA level, and that short-term radio-therapy reduced the level of inflammatory cytokines, albeit to a lower extent for TNF-α [17]. Likewise, cancer patients treated by ionizing radiation showed an increase in pro-inflammatory cytokines (TNF-α) rather than immuno-modulatory cytokines (IL-2, IL-10, and IFN-γ). This increase was dose-dependent [18]. As serum cytokines are useful indicators of the type of immune response in the course of carcinogenesis, we investigated the levels of Th1 (IL-2, IFN-γ, and TNF-α) and Th2 (IL-4 and IL-10) cytokines in the serum of XP, non-XP patients and of healthy controls. Then we focused on the role of DNA repair deficiencies on UV-induced immune suppression, since it has been demonstrated there may be a possible role of serum cytokines, as biomarkers [19, 20], for evaluating the role of an altered DNA repair, in cutaneous carcinogenesis.
Cancer patients usually receive radiotherapy (RT) that activates both pro- and anti-proliferative signaling, altering the regulation of many processes involved in DNA repair, inflammation, cell death, and cell cycle progression [21].
Moreover, RT activates different mediators in tumor cells, as well as, in the tumor microenvironment [21, 22]. In contrast, 5-Fluorouracil (5-FU) chemotherapy has proven to be a good option for the treatment of skin cancers, without inflammation [23], unless when it is administered extensively [24]. Here, we hypothesize that DNA repair deficient patients develop more inflammation than DNA repair proficient ones, and we show at what extent, unrepaired DNA lesions promote inflammatory reactions in XP patients.
The study group comprised 11 patients with skin carcinomas (five XP and six non-XP) and three healthy controls. All of the 11 patients underwent surgical, radio- and chemotherapeutic treatments, in the Medical Oncology Department of A. Mami Hospital, Ariana, Tunisia. Informed consent to participate in the study was obtained from all the participants.
Our study investigated the level of some cytokines in the serum of XP and non-XP patients bearing skin carcinomas, and of healthy controls. Blood samples were taken from the antecubital vein of patients and controls by the physician of the chemotherapy department. Blood was processed within one hour of collection and serum was aliquoted and stored at –80°C in our laboratory, until analysis.
The assays were performed by means of kits for ELISA tests (kindly provided by Professor Armand Bensussan, Director of Immunology, Dermatology and Oncology Department formerly at INSERM Research Center 841, Henri Mondor Hospital, Créteil, Paris, France), by a non-competitive sandwich method, to investigate IL-2, IL-4, IL-10, IFN-γ and TNF-α in the studied groups.
We used a 96-well plate for each studied cytokine. The micro-titer plates are pre-coated for IL-4, but not for TNF-α (Immuno Tools, Germany), and for IL-2, IL-10, and IFN-γ (U-CyTech biosciences, The Netherlands). For IL-2, IL-10, and IFN-γ, we added 50 µL of the diluted coating antibody (capture antibody) solution to each well of the microtiter plate and filled up them to 100 µL with PBS (pH 7.4), we sealed the plate and incubated overnight at 4°C.
The day after, we washed the wells six times with 300 µL wash buffer (PBS containing 0.05% Tween 20) for each well. We added 200 µL of blocking buffer (PBS + Tween 20 + 0.1% BSA) to each well. We sealed the plate and incubated it on a horizontal plate shaker for one hour at room temperature.
The blocking buffer was removed without washing. Then we prepared the blank/standard/sample dilutions. For the standard, we made serial dilutions in diluent buffer (PBS + 0.5% BSA + 0.05% Tween 20). Then we added 100 µL of diluted standard solution, blank (without analyte), and samples to the wells.
We sealed the plate and incubated it for two hours at room temperature. Next, we added 300 µL wash buffer to each well and washed them six times. We added 100 µL of diluted biotinylated detection antibody to each well. Then the plates were sealed and incubated for one hour at room temperature. After washing, 100 µL of diluted SPP conjugate to each well. After sealing and incubation for one hour at room temperature (protected from light), followed by washing as described above. Then 100 µL of TMB substrate solution was added to each well. This step needed protection from light for 20 minutes and produced a blue color product. Finally, we put 100 µL stop solution (sulfuric acid) in each well, which stopped the enzymatic reaction, changing the color to yellow. The amount of the substrate transformed by the biotin-streptavidin complex was detected at 450 nm, in the micro-plate reader spectra count, with a “Plate Reader 3.0 for Windows” and was the measure for the quantity of the cytokine within the sample. The analyte concentrations in the samples can be determined by a standard curve which is generated from the results of the serial dilutions of the standard solutions. The standard dilutions were tested in triplicate. The concentrations of the cytokines in the samples can then be interpolated from the standard curve.
For TNF-α, nearly the same protocol was applied, with some differences, such as the number of washes that were five times instead of six times. The used conjugate was poly-HRP-streptavidin-HS, and the blocking buffer was (PBS + 2% BSA + 0.05% Tween 20). As for IL-4, the same protocol was also applied except that the plates were precoated.
As the data were not normally distributed, we used a non-parametric analysis and we applied the statistical Mann-Whitney U test, with an accuracy of 5% (i.e. the difference between the medians to compare is significant when the probability P ≤ 0.05). We compared pairs of groups XP/non-XP, XP/controls, and non-XP/controls for the concentrations of the serum cytokines cited above. Here, we describe an independent and dichotomic variable that matches patients and control groups. The concentrations of the circulating cytokines were entered as a dependent and continuous variable. We compared the median of the different groups of patients and controls.
The study group comprised 11 patients with skin malignancies (five XP and six non-XP) and three healthy controls. The 11 patients (six F, five M), the controls (three F, zero M). The mean age of XP patients was 26.60 years (they were aged 11 to 39 years). For non-XP patients, the mean age was 60.17 years (they were aged 42 to 74 years). The control group was aged 39 to 57 years, with an average of 47 years.
All of the 11 patients underwent surgical, radio- and chemotherapeutic treatments. The total amount of radiation used in photon radiation therapy is 70 Gy, the nuclide used is Cobalt 60. Topical chemotherapy consisted in two applications of 5-FU per day. This treatment can be extended for 12 months. While the systemic protocol consisted in the administration of 5-FU at a rate of 340 mg/m2, preceded by folinic acid, at a rate of 20 mg/m2, in perfusion and in many cycles of 5 days, every four weeks. It is important to note that chemotherapy was not successful for patient P9, who was prescribed a curie-therapy (that should begin after the sampling). In Table 1, we list the demographic, clinic, histologic, and therapeutic characteristics of the patients and the control groups, and also, the plasmatic concentrations from the assays, performed in triplicate, of the studied cytokines, as well as the comparisons between the three groups. In Table 1, it’s shown that the majority of XP and non-XP cancer patients had an increase in TNF-α secretion above healthy controls.
Demographic, clinic, and histologic characteristics of the patients, demographic characteristics of the controls, the cytokines’ concentrations in the systemic circulation, and comparisons of the three groups
| Individuals/Diagnostic | Age(year)/Gender | Type/Site of the tumor | IL-2 (pg/mL) | IL-4 (pg/mL) | IL-10 (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|---|---|
| P1 (XP) | 31/F | BCC (nose) | 0.78 | 244 | 0 | 47.17 |
| P2 (XP) | 35/F | BCC + SCC (cheek) | 4.35 | 0 | 0 | 0 |
| P3 (XP) | 17/M | Multiple carcinomas (BCC + SCC) + melanoma (ear) | 2.38 | 0 | 0.01 | 27.83 |
| P4 (XP) | 11/F | Multiple carcinomas (BCC + SCC) face | 0.08 | 0 | 8.33 | 47 |
| P5 (XP) | 39/F | BCC (nasogenian furrow) | 0 | 0 | 0 | 30.33 |
| P6 (non-XP) | 42/M | BCC (cheek) | 4.77 | 4.48 | 0 | 0 |
| P7 (non-XP) | 59/F | SCC in situ (left side) | 0.76 | 0 | 23.50 | 0 |
| P8 (non-XP) | 74/M | SCC (nasogenian furrow) | 1.37 | 0 | 0.60 | 0 |
| P9 (non-XP) | 64/M | SCC (nose slot + right eye + chest) | 0.47 | 4.33 | 0 | 0 |
| P10 (non-XP) | 55/M | BCC (cheek) | 1.67 | 0 | 0 | 0 |
| P11 (non-XP) | 67/F | Multiple carcinomas BCC + SCC (face, cheek, and eyelids) | 3.51 | 0 | 7.17 | 0 |
| C1 (Control) | 57/F | - | 0.77 | 0 | 0 | 0 |
| C2 (Control) | 39/F | - | 0 | 0 | 0.03 | 0 |
| C3 (Control) | 45/F | - | 1.43 | 4.17 | 0 | 0 |
| Median concentrations of the cytokines | XP | 0.78 | 0.00 | 0.00 | 30.33 | |
| Non-XP | 1.52 | 0.00 | 0.30 | 0.00 | ||
| Control | 0.77 | 0.00 | 0.00 | 0.00 | ||
| Comparisons of the median plasma concentrations of the cytokines in the three subgroups | XP vs non-XP (P value) | 0.465 | 0.816 | 0.619 | 0.011 | |
| XP vs Controls (P value) | > 0.999 | 0.845 | 0.864 | 0.050 | ||
| Non-XP vs Controls (P value) | 0.796 | 0.795 | 0.396 | > 0.999 | ||
It’s also shown that differences were found to be statistically significant for TNF-α. For IL-2, IL-4 and IL-10, the differences were not statistically significant. Our results showed no detectable levels of IFN-γ for all the studied subgroups.
As the level of serum cytokines is a variable that does not follow a normal distribution and data are presented as medians to compare different groups. A comparison of medians was performed by the Mann-Whitney U test. Statistical significance was set at P < 0.05, two tailed. The results of these comparisons are also shown in Table 1.
When compared, our data showed that for TNF-α, there was no significant difference between non-XP and controls (P > 0.999). However, there were significant differences between XP patients and controls (P = 0.05), as well as between XP and non-XP patients (P = 0.01). For IL-2, IL-4, and IL-10 cytokines, there were no significant differences between XP and non-XP patients, and between XP versus non-XP and controls.
The assays for the studied cytokines are presented in Figure 1, made by a GraphPad Prism version 7.0.
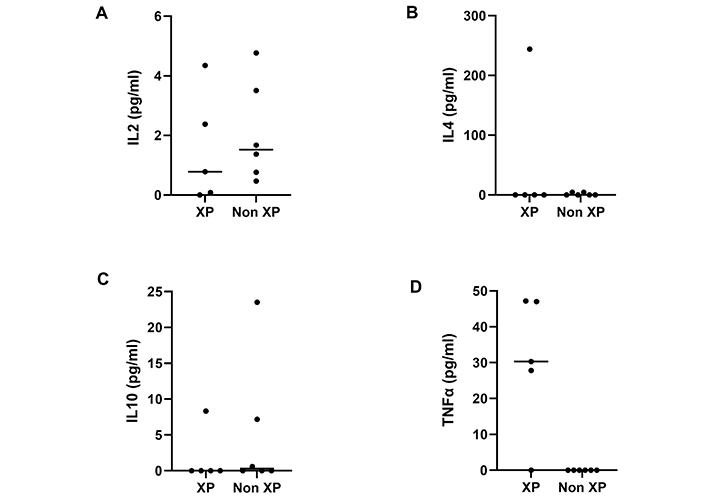
Distribution of the serum concentrations of the cytokines in cancer patients according to their pathological status (XP/non-XP). (A) There is no significant difference between XP and non-XP groups, in the rate of the IL-2 cytokine (P = 0.465); (B) there is no significant difference between XP and non-XP groups, in the rate of the IL-4 cytokine (P = 0.816); (C) there is no significant difference between XP and non-XP groups, in the rate of the IL-10 cytokine (P = 0.619); (D) there is a significant difference between XP and non-XP, in the rate of TNF-α (P = 0.011)
In the present study we investigated the rate of IL-2, IL-4, IL-10, IFN-γ and TNF-α cytokines of XP and non-XP patients, with skin carcinomas, and of a healthy control group of volunteers. We aimed at determining whether unrepaired UV-induced DNA damage was responsible for alterations of immune responses in XP, in comparison with non-XP patients.
It is well recognized that UV-irradiation induces immune suppression [25], and that cytokines are immuno-modulatory molecules that play crucial roles in immune regulation [26]. The cytokines are produced by a plethora of immune cells during inflammation and impact all the stages of tumorigenesis [27]. The most important Th1 cytokines are IL-2, IFN-γ and TNF-α. Th2 cells produce a large number of anti-inflammatory cytokines such as IL-4 and IL-10. It is well-known that the balance between pro- and anti-inflammatory cytokines determines the fate of immune responses.
We have noted that IL-2 was present in the serum of the studied individuals, albeit in different amounts (Figure 1A). In fact, IL-2 is integrated in natural immunity via its regulatory role in lymphocytic functions. For XP and non-XP patients, relatively high levels of serum IL-2, is evidence of the development of an anti-tumor immune response, limiting tumor propagation that takes place in the early stages of tumor development [28]. Paradoxically, it plays a major role in establishing immune tolerance [29], by limiting anti-tumor cytotoxic T cell responses, inducing them to undergo apoptosis, or enhancing the survival and expansion of regulatory T cells [30]. This contributes to the development of the tumors.
For IL-4, normal physiological levels ≤ 10 pg/mL. The observed levels of IL-4 in non-XP patients and in controls were under this value. In contrast, IL-4 was found to be at higher levels in the XP patient P1 (244 pg/mL). It is worth noting that for patient P1 who is XP, the tumor was in an advanced stage, and as it has been shown in previous studies [31], high levels of IL-4 favor tumor evasion from immunological control and its propagation (Figure 1B).
As for IL-4, IL-10 was present in the circulating serum of XP and non-XP cancer patients (Figure 1C). This might reflect lymph node metastasis, as has been demonstrated elsewhere [32, 33].
We didn’t find any detectable levels of IFN-γ in the sera of all the studied individuals. This could be due to the failure of the assay or to another underlying cause for the possible alteration in the production of this cytokine. For the controls, it is possible that the absence of IFN-γ in the serum is simply due to the fact that they were not developing an immune response against a pathogen at the time of sampling.
On the other hand, the absence or the low concentration of IFN-γ in the early phase of an immune response and concomitant production of IL-4 by cells of the mast cell/basophil lineage or T cells themselves is known to favor the development of Th2 cells [34]. It is also possible that IFN-γ levels were under the sensitivity threshold of the used test (2 pg/mL).
In fact, serum circulating IFN-γ is an indicator of NK cell activity in cancer patients [35]. Nevertheless, its absence could result in a reduction in the capacity of some cancer patients to produce this cytokine [36].
Alternatively, it has been evidenced that IFN-γ serum half-life is very short because of enzymes and complement proteins [37], and also because of the possible presence of auto-antibodies anti-IFN-γ in the blood [38]. Other studies have shown that IFN-γ is suppressed by IL-4 and in the presence of Th2 cytokines in general. Moreover, IFN-γ has been shown to be antagonized by TGF-β. The latter is produced at substantial levels by tumors [39].
Our results did not show significant differences between the three studied groups of XP/non-XP patients, and the control groups in the production of IL-2, IL-4, and IL-10 cytokines. This is probably due to the small sample size.
Other studies have shown that serum levels of TNF-α were high in various cancer patients [40]. For instance, in chronic lymphocytic leukemia, the serum level of TNF-α was 16.4 pg/mL. However, in a number of chromosomal abnormalities, its value could reach 27.5 pg/mL. The latter value remains below our results.
It has been reported, in an advanced breast cancer case, mean values of TNF-α before and after neo-adjuvant chemotherapy, to be reduced (15.9 pg/mL) and if proper chemotherapy was applied, the values of TNF-α would be even lower [41]. In other words, without chemotherapy these values are much lower than those of XP patients (Figure 1D), which are in favor of our hypothesis, about the contribution of DNA damage in the alteration of immune response in the course of skin cancer.
In fact, it is already known that TNF-α enhances the cytotoxic effects of chemotherapeutic agents on tumor cells [42]. Furthermore, it was shown that the increase in production of TNF-α is associated with tumor development.
It has been shown that RT (radiotherapy), which is considered to be an anti-tumor treatment, by producing double-strand DNA damage and subsequently tumor cell death, also induces inflammation [43]. Reciprocally, inflammation has the capacity to alter tumor cell response to RT. This factor is worthy to be considered in the interpretation of our results. Nevertheless, if RT induces an increase in the rates of inflammatory cytokines, such as TNF-α, in the two groups of patients (XP and non-XP), and all of them, underwent RT, the difference between them remains significant. This suggests a supplementary role for DNA damage, in the alteration of immune responses.
A growing body of evidence indicates that TNF-α can induce, apoptotic and necrotic cell death. Reactive oxygen species (ROS) have been shown to play a critical role in mediating necrosis [15]. Moreover, UV-induced DNA damage has been demonstrated to induce ROS formation [44, 45].
Taken together, these results indicate a connection between the two pathways.
TNF-α has also been reported to be elevated in the serum of patients with advanced stages of tumor development and correlates with an increased number and size of metastatic sites [46, 47].
In conclusion, the current findings suggest that XP patients with skin cancer had an altered cytokine expression level, compared with non-XP and healthy controls, whose baseline serum levels were lower than those of XP patients. This provides some support to the hypothesis that cancer symptoms are related, at least in part, to a cytokine-immunologic activation state. The balance between TNF-α induced cell survival and cell death signaling is crucial for TNF-α target cells. Controlling TNF-α would help prevent cancer development and harness the therapeutic properties of this cytokine. Therefore, it’s worth considering treating the inflammation with TNF-α inhibitors such as Etanercept, with the study of multiple polymorphisms in order to enhance the patient’s response to these TNF-α inhibitors and to lower side effects [48].
To sum up, it’s important to recognize that our analysis was limited by the following facts:
The information concerning some patients’ files may lack details (only one XP patient P3 had melanoma in addition to carcinoma).
The premature deterioration of the skin of XP patients led us to compare it with that of aged non-XP ones. As inflammation increases with age, younger non-XP patients would have less inflammation. Besides, if XP patients have significantly higher levels of inflammatory cytokines than older non-XP individuals, it’s conceivable that the level of inflammatory cytokines is also significantly higher in XP than in non-XP age-matched patients.
We could not investigate the patients, before and after, radio- and chemotherapeutic treatments. In contrast, they were investigated in the chemotherapy department, after they underwent RT.
In fact, determining cytokines profile before and after treatments may form a basis for future investigation, to explore the role of cytokines in tumor progression and their values as predictive parameters, to improve cancer treatment.
As this study is based on a very small sample size, it is paving the way for a larger investigation, in which we will set a goal to explore the effects of DNA damage, at a cellular scale, on the alteration of the immune response, in the course of carcinogenesis in XP patients.
5-FU: 5-Fluorouracil
RT: radiotherapy
XP: xeroderma pigmentosum
This work was supported by the Immunology, Dermatology and Oncology Department at INSERM Research Center 841, Henri Mondor Hospital, Créteil, France. We are grateful to Professor Armand Bensussan, Director of the Department, for having hosted us in his laboratory. We also wish to thank Professor Dragan Jovanovic of Mac Gill University, Canada, for helpful discussions. We also would like to thank Professor Scott King Johnson for language editing.
KA: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Writing—original draft. JB: Conceptualization, Formal analysis, Methodology, Validation, Writing—review & editing. FEM: Conceptualization, Investigation, Methodology, Validation, Writing—review & editing. HB: Conceptualization, Investigation, Methodology, Resources, Validation, Writing—review & editing. All the co-authors gave their final approval of the version to be published.
The authors have no conflicts of interest to declare.
Our study was approved by the Ethics Committee of A. Mami Hospital, Lariana, Tunisia. The study is in accordance with the Helsinki Declaration.
Informed consent to participate in the study was obtained from all the participants.
Not applicable.
All supporting data are available from the corresponding author upon request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2350
Download: 28
Times Cited: 0
Ryuta Muromoto ... Tadashi Matsuda
Masanori Fujii ... Susumu Ohya
Aparna Mukhopadhyay ... Debprasad Chattopadhyay
