Affiliation:
1Independent researcher, Biochem123 Education, NW7 4AU London, UK
Email: abrownbscmsc@gmail.com
ORCID: http://orcid.org/0000-0001-5238-6943
Affiliation:
2Independent researcher, 2G Keswick Road, SW15 2JF London, UK
†These authors contributed equally to this work.
Email: rogbe2005@outlook.com
ORCID: http://orcid.org/0000-0002-8200-0885
Affiliation:
3Independent researcher, 31195 Lamspringe, Germany
†These authors contributed equally to this work.
Email: ingo.fricke@protonmail.com
ORCID: http://orcid.org/0000-0001-7638-3181
Explor Immunol. 2024;4:691–721 DOI: https://doi.org/10.37349/ei.2024.00167
Received: April 13, 2024 Accepted: September 24, 2024 Published: November 01, 2024
Academic Editor: Calogero Caruso, University of Palermo, Italy
Measles virus (Morbillivirus abbreviated as MV, but more recently MeV) is the causal agent of measles disease, thought to have existed at least 4,000 years ago, affecting predominantly infants, but also immunocompromised individuals remaining a public health issue today globally. In this review, we discuss the historical background about MeV infection to modern-day research on measles disease, current epidemiology, but also what is known about immunisation against it. We report what is known about the viral structure and the function of the viral proteins. This additionally covers the cellular structure of MeV, mechanisms, and clinical aspects of infection. Including a review of topics like cellular receptor-associated entry factors, to the immunology of MeV infection. In this review, the current knowledge of innate immune responses during infection is explained, which involves changes to chemokine and cytokine expression, finalised by the present understanding of adaptive immune responses to MeV. The genomic stability of the MeV proteins is explained and suggestive that it could be the third pathogen with eradication potential (after the variola and rinderpest viruses). Further biological and immunological clarification as to how this could occur is explained below.
Comparatively less is known of the underlying cellular immunological mechanisms of natural measles virus (MeV) infection’s innate and adaptive immune responses in humans; initially, because the original wild-type (wt) MeV isolation occurred in 1954 alongside concurrent use in immunisation, which continued thereafter. This first isolation was known as the “Edmonston strain” derived from David Edmonston, a student at Fay School, which led to the first measles vaccine developed shortly after. Other relevant biological discoveries occurred prior and simultaneously. As early as 1869, Friedrich Miescher documented DNA isolates, then named “nuclein”. Synthesis of nuclein by white blood cells (WBCs), known as leukocytes, was noted to contain the elements carbon, nitrogen, and hydrogen with phosphorous in abundance, but not sulphur [1]. Miescher laid the foundations for future research.
Measles disease is defined as caused by the species Measles Morbillivirus infecting only humans. The causal virion is defined biologically within the taxonomical system by order Mononegavirales, family Paramyxoviridae, genus Morbillivirus and species Measles Morbillivirus; another Morbillivirus, the rinderpest virus (RPV), was the second virus reported to be eradicated globally prior to 2011, known as a cattle plague [2]. This was preceded by variola virus (VARV) eradication, the causal agent of smallpox disease affecting only humans, confirmed by the World Health Organization (WHO) in 1980 [3, 4]. Immunisation utilising MeV was tested in 1948 with the licensing of two vaccines in 1963 which were composed of a live-attenuated virus (LAV), derived from MeV, superseding in this instance simultaneous development of a withdrawn formalin-inactivated MeV vaccine strain [5–7]. Both were aimed at evoking a host immunogenic long-term response against the first Morbillivirus known to cause human disease [5–7].
In 1948, a pioneer Adams [8] examined how seven bacteria and/or viruses could be inactivated through gas/liquid exchange through bubbling nitrogen over Escherichia coli. It was then observed that a preventative chemical could restrict pathogen cellular replication, causal of pathological conditions [8]. Other pioneers such as Crick and Watson further clarified the nuclear structure of DNA in 1953; while various groups grappled and advanced this through experimental investigation. Debates occurred with arguably the key theoretical framework, in 1961, outlined by Jacob and Monod [9] of the role of messenger molecules, now known as mRNA, in gene regulation and protein production [10]. Therefore, during this period, it could be seen that primary chemical structures influence leukocytes traversing restrictive cellular barrier layers known as the glycocalyx and the endothelial surface layer. Secondly, viral antigens can be restricted during replication within the respiratory tract, vascular (endothelial), and epithelial cellular layers [11]. Thirdly, WBCs are the cells of the immune system that develop and evoke specific phenotypes permeating throughout the vascular system. This occurs through inhibitory and stimulatory proteins, as well as autocrine and paracrine hormonal cellular messengers [12]. Intercellular messengers include both cytokines and chemokines within the lymphoid tissue, organs, and cells. Most can be analysed through genetic and tissue expression effecting the host immune response which can predispose a person to different infections or cancer.
Measles disease was considered to be causal of more than 2 million deaths each year in 1980 (see Supplementary materials). Around 1981, as research evolved, Bellini et al. [13] published the first article discussing how this occurred through the host immune reactivity with the purified MeV haemagglutinin (H) protein stimulating immune cells to recognise a protein expressed by the MeV virion. Throughout the 20th century, guidelines were produced by the WHO, and many countries now utilise a routinely scheduled standalone MeV vaccine (MV), or measles-containing vaccine (MCV), also known to counter mumps virus (MuV) as well as the rubella virus (RuV), known as the trivalent MMR (measles, mumps, and rubella) vaccine in routine immunisation programmes to reduce the prospective rate of incidence and severity of MeV-evoked disease (see Supplementary materials) [12]. Host cell presentation of peptides called antigens through immunisation could thus stimulate the rate of immune system recognition to facilitate the rate of immune response to prevent multiple diseases.
Thirty-seven years later (2017), MeV disease mortality estimates remained around 140,000 individuals per year with variable infection/mortality rates globally, and in resource-limited countries. It is considered that environmental factors contributed to the decrease in the severity of MeV infection besides immunisation [14]. The rate of severe measles disease is affected by a myriad of factors. The same family of Paramyxoviridae also encompasses the Nipah virus (NiV), which, similarly to MeV, can cause severe neurological disease, as well as blindness, brain damage and encephalitis in a minority of infections [5, 15–18]. Potential explanations to elucidate this further during past, present, and future research are discussed.
The lack of antigenic variation of MeV is suggestive that eradication potential is possible. The significance of this review is that it provides key insights into MeV infection in both natural infection as well as studies of immunised individuals since MeV isolation. Furthermore, the effects of MeV proteins within cellular transduction are examined here based on many years of research. Therefore, the wider public health community will be able to explain further the importance of immunisation in prophylaxis against Measles disease.
There is no description of measles disease in the works of Hippocrates and Galen, although the disease may have been reported in Indian texts several centuries before. Most likely measles disease was misdiagnosed with other exanthematic diseases [19]. The first detailed description distinguishing smallpox disease from measles disease was by Rhazes (865–925 AD), a chief physician at a hospital in Baghdad [4]. Measles disease was considered to be widespread in Europe, Asia, India, and China in the Middle Ages. With the discovery of America and European colonial expansion from the 17th century onwards, measles spread from the Renaissance period to the 20th century becoming a global public health issue [4, 19].
Monovalent measles immunisation began in 1963 and was shown as prophylactic, with indications MeV immunisation could reduce infection and disease severity [20]. A team at Boston Children’s Hospital comprised of John Franklin Enders, together with Dr. Thomas C Peebles, isolated MeV. The individual infected patient blood serum sample was obtained from an 11-year-old boy during an outbreak in Boston, Massachusetts. Alongside Samuel Katz, and notably a pioneer Maurice Hilleman, who worked at Merck and Co., this led to the development of the first LAV [15]. A further MCV was developed in 1968, with the combined MMR vaccine following in 1971 which is utilised to counter antigens expressed by MeV, MuV, and RuV [15].
In 1974, the WHO introduced MCV into its expanded program of immunisation (see Supplementary materials) [5]. Even though measles is one of the most contagious infectious diseases ever (R0 range 12–16), population immunisation coverage of 95% is considered to be prophylactic through reducing viral infection transmission rate triggering epidemics [5]. The first vaccine developed by Enders’ team was derived from the MeV Edmonston-B strain, with this LAV having high antigenic stability, explaining remarkable efficacy, regardless of the MeV genotype [21]. A large decrease in disease incidence was observed since, and during cell culture, less virulence was observed. Retention of the ability of immunisation to induce a strong immune response with neutralising antibodies (nAbs) against MeV was also observed [21].
Subsequently, serial MeV passage in chick embryo fibroblasts (CEFs) yielded other attenuated vaccine strains denoted as “Moraten and Schwarz” inducing fever and rash in 10% of those immunised [22]. Comparison of protein sequences of the H protein, together with fusion (F) and nucleocapsid coding genes of MeV vaccine strains occurred thereafter through the Edmonston-Zagreb (EZ) strains of slightly different lineages. Human fibroblasts derived from lung tissue diploid cell lines (WI-38), were also used for cell passage, which is the vaccine strain most widely used in resource-limited countries [5]. Vaccine strains used were also derived from the chorioallantoic membrane-70 cell line (CAM-70), whilst others were named after the place of research including Leningrad-16, Shanghai-191 as well as AIK-C (A—America, I—Iran, K—Kitasato Institute, and C—virus adapted to chick embryo cells), each of which are closely related to MeV genotype A viruses with few sequence differences [5, 23]. Below is shown the chronology since initial MeV isolation (see Figure 1).
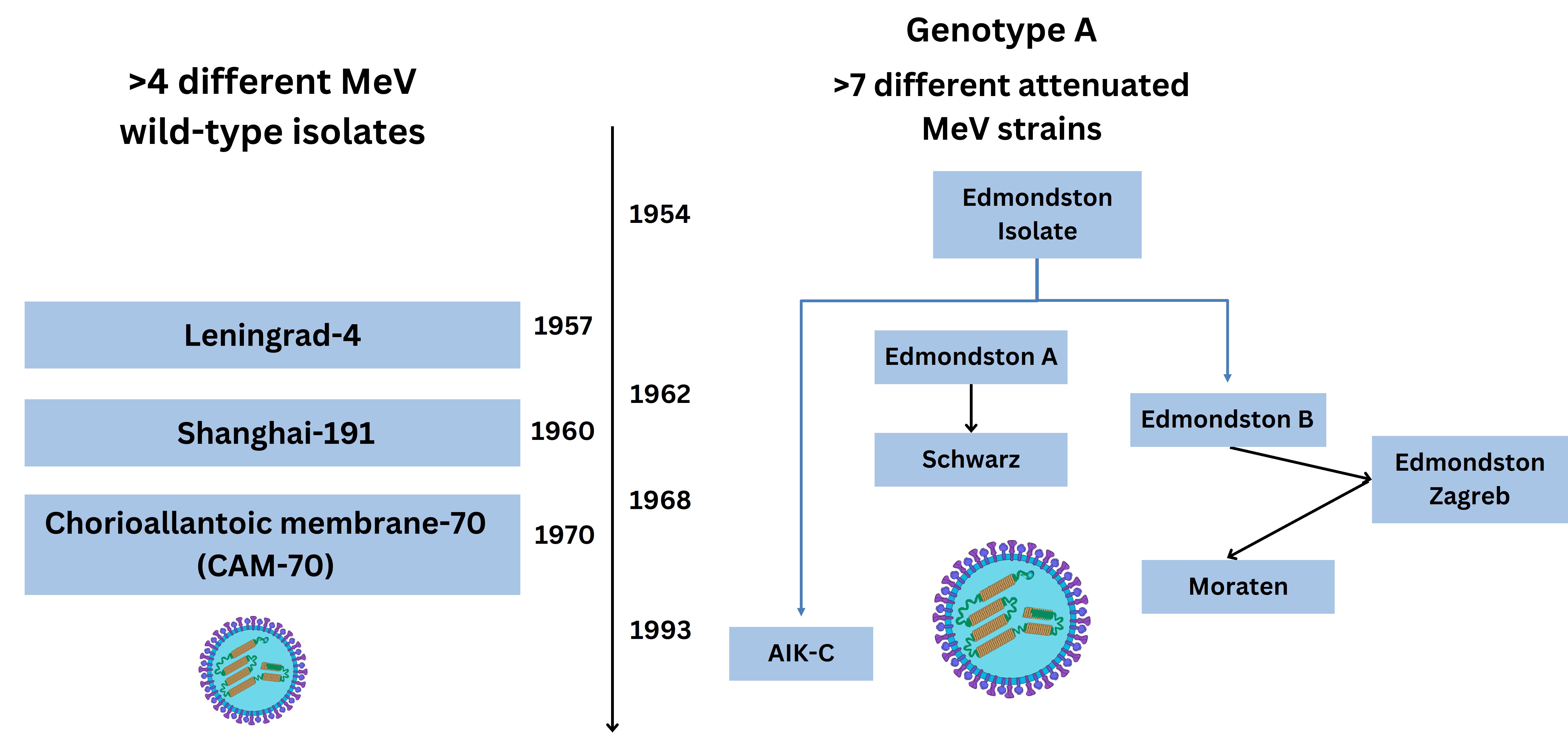
Evolution of 19th and 20th century MeV vaccine utilised strains. The virus schematic was adapted from ViralZone, SIB Swiss Institute of Bioinformatics (https://viralzone.expasy.org/86), licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. MeV: measles virus; AIK-C: A—America, I—Iran, K—Kitasato Institute, and C—virus adapted to chick embryo cells
The first MCV dose is administered as a single dose at 9–12 months of age followed by a second in routine schedules varying globally, but immunisation with two dose regimens is usual, although not in pregnancy or immunocompromised individuals (see Supplementary materials) [5]. The trivalent MMR vaccine is licensed for use in the United States of America (USA) and many other countries in the 21st century [15]. Universal immunisation, in the case of MeV, led to an overall decline in global incidence quantified as a 66% reduction (145 to 49 cases per million population between 2000 to 2018), concurrently accompanied by reduced measles disease mortality reduction of 73% (535,600 to 142,300 individuals) [24]. These figures are notable. Currently, immunisation against MeV can be administered as MCV, MMR, or a recent formulation including a chickenpox component (known as MMRV, with V abbreviating the varicella zoster virus, VZV) [25]. The COVID-19 pandemic led to setbacks in surveillance and immunisation efforts. The interruption/disruption of routine immunisation services has left millions of children vulnerable to preventable diseases like measles. Around 22 million infants globally missed at least one dose of an MCV throughout routine immunisation schedules in 2022 whilst other outbreaks around the globe did occur [4, 16, 26–28].
Immunisation against MeV is considered to induce long-term cellular immunity; however, less is explained about the underlying biological mechanisms of how this occurs [5, 29]. Since isolation, the attenuated MeV through cell-expressed antigens is utilised as a LAV through its ability to infect cells and evoke the required cellular immunogenic response without measles disease manifestation [5]. Attenuated MeV is being evaluated to target other viral antigens expressed by human immunodeficiency virus (HIV), Dengue fever virus (DENV), and chikungunya virus (CHIKV), discussed elsewhere [30, 31]. Furthermore, potential applications could probably include MeV as an oncolytic viral (OV) vector therapeutic (around 2022), with evaluation occurring for future potential treatment for cancers like glioblastomas [32–35]. The original LAV strain of MeV infects host cells using receptors. Characterised are at least three receptors that MeV employs. These are the cluster of differentiation (CD) molecule CD46, as well as CD150, but also nectin-4 [36]. The latter is a member of the immunoglobulin (Ig) superfamily and is known as a type I membrane adhesion molecule, only recently becoming recognised. This could be a cellular checkpoint that can shed its extracellular domain promoting angiogenesis by regulating the C-X-C chemokine receptor 4 (CXCR4) and C-X-C motif chemokine ligand 12 (CXCL12) axis [37–39]. The attenuated MeV strain could also have similarities to both vaccinia virus (VACV) and modified vector versions utilised to counter VARV leading to smallpox disease eradication prior [4].
Measles disease affliction generally can affect different ages, but particularly vulnerable infant populations and immunocompromised individuals [40]. Throughout 21st century vaccine development, efficacy was indicated in 2002 of more than 95.4% studied in individuals (n = 471) seroconverting and producing nAbs against MeV [41]. The efficacy and safety of MMR immunisation were the subjects of debate in the 21st century [42]; however, 2021 reports summarising population real-world data suggested efficacy of more than 90% to either the trivalent (MMR) or quadrivalent (MMRV) immunisation options [5, 43]. More recently, it has been indicated that MeV immunisation achieves nearly 98% seroconversion, generating antibodies predominantly neutralising the conserved H protein of the attenuated MeV vaccine strain [44–49].
The terms of vaccination and immunisation are derived from VARV research together with the VACV [4]. Research now only occurs with the latter evoking active prophylactic immunological responses in a host animal or human [4]. Active immunity is commonly used to describe the process of exposing a host to an antigen and can be natural or acquired; similarly, passive immunity can be either natural or acquired. The two terms are historically used to differentiate between types of host immune responses with the first utilised that may be long-lasting following infection or immunisation [50]. The second passive type of immunity refers to the transfer of antibody types in hosts, for example, IgG, or similar other licensed preparations like rabies Ig, as well as monoclonal antibody (mAb) preparations [51]. Different proteins utilised in research and viral vector vaccine development can be a beneficial factor in priming the innate and adaptive immune system cells to effect an immunogenic response reducing the severity of pathogenic infection [35]. This occurs through many immune cell phenotypes now known [28]. Longevity and kinetics of antibody production by B cells are factors, alongside T cells adequately stimulating a recall memory immune response. Below is presented the detail so far about immunological phenotypes of a host human response to MeV infection throughout the illness.
The MeV virion particle size is 15,894 kilobases (kb) from the 3’ end of the negative (–ve) sense single-stranded RNA genome [44, 52]. This encodes the nucleoprotein (N), followed by a conserved tetrameric H protein, F protein, matrix (M) protein followed by a trimer of phosphoproteins (P) combined with two non-structural proteins (C/V) with a larger polymerase (L) enzyme towards the 5’ end of the RNA genome [44]. The L protein polymerase sequentially transcribes through binding to MeV RNA at the 3’ leader sequence with polyadenylation occurring during synthesis with V protein produced through RNA editing and a P protein synthesised from the C protein. This process utilises host intracellular machinery for the RNA-dependent RNA polymerase (RdRp) to transcribe and produce proteins to produce the infectious virion. Viral attachment of the virion occurs through the MeV H protein attaching to cell receptors, with the F protein facilitating entry via the cellular phospholipid-rich plasma membrane (PM) where viral mRNA is capped and polyadenylated within cellular cytoplasm [53]. Therefore, H protein nAbs evoked by attenuated MeV is the first key mechanism of restricting viral entry. MeV virions traverse cell membranes and replicate intracellularly within cell cytoplasm, followed by cell egression [54]. Below is depicted the timeline history of the discovery of selected Paramyxoviridae, although two of these [respiratory syncytial virus (RSV) and human metapneumovirus (hMPV)] were later classified within the family Pneumoviridae in 2016 with later updates determined by the International Committee on Taxonomy of Viruses (ICTV) (see Figure 2) [55].
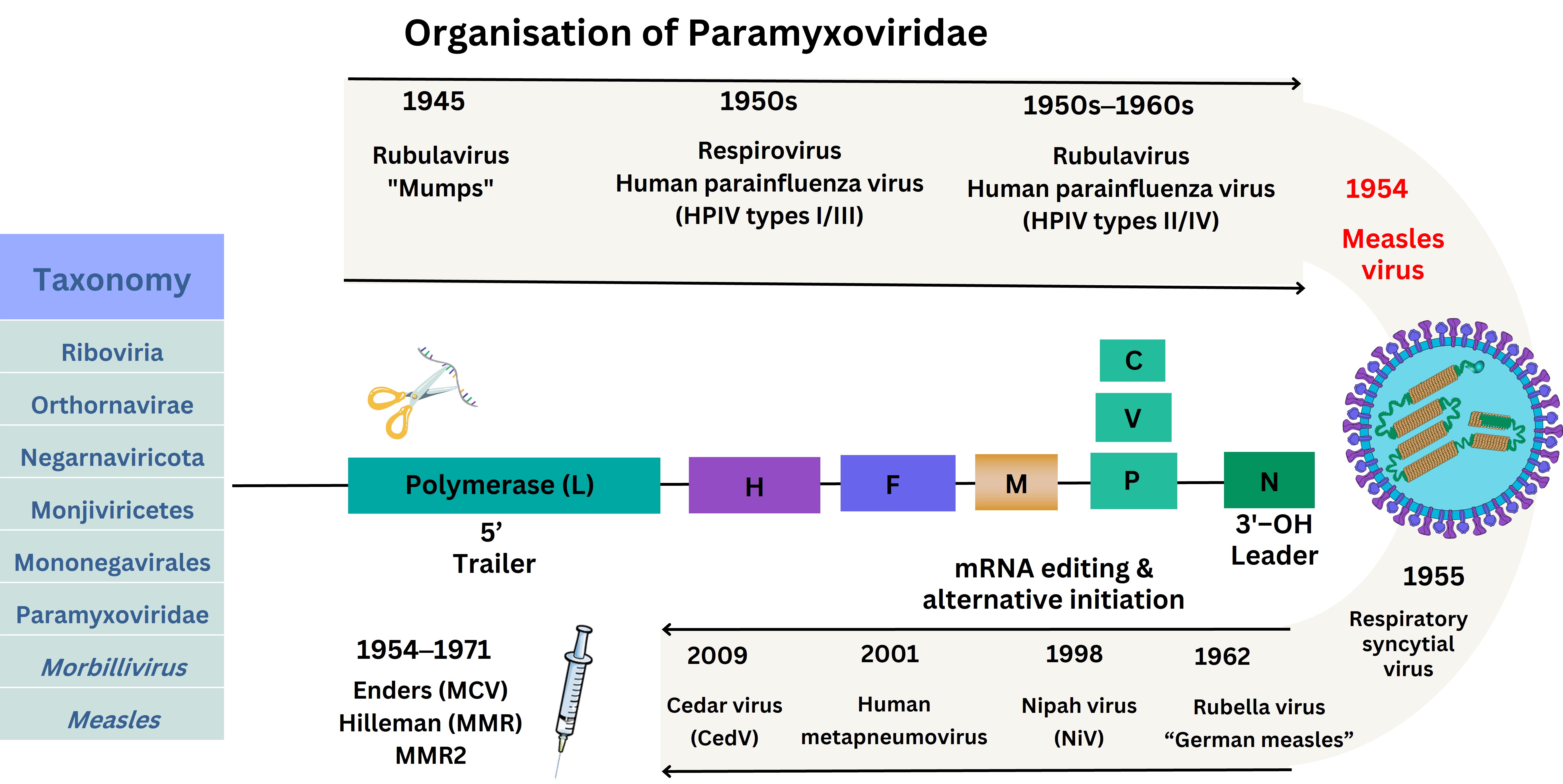
Structure of MeV and historical Paramyxoviridae isolation. The virus schematic was adapted from ViralZone, SIB Swiss Institute of Bioinformatics (https://viralzone.expasy.org/86), licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. HPIV: human parainfluenza virus; hMPV: human metapneumovirus; OH: hydroxyl group; MCV: measles-containing vaccine; MMR: measles, mumps, and rubella vaccine; H: haemagglutinin protein; F: fusion protein; N: nucleocapsid protein; M: matrix protein: P: phosphoproteins; C/V: non-structural proteins
In 2019, it was indicated that the MeV virion forms inclusion bodies (IBs), without a membrane, with three MeV-abundant N, P, and L proteins [56]. The MeV P protein was demonstrated to act as a chaperon and cofactor for the L protein, with a third multimerization domain (MD) affecting gene expression of MeV [47]; whilst the M protein of Paramyxoviridae is known to direct virion assembly interacting with cell membrane phospholipids like phosphatidylserine (PS) and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] that could be potential therapeutic inhibition targets, facilitating the spherical or filamentous protrusions formed during viral egress [45]. However, in 2020, fluorescence studies highlighted the N- and C-terminal P protein domains through liquid-liquid phase separation (LLPS) structures without a membrane before fusion, forming nucleocapsid-like particles that RNA molecules can localise with regard to MeV [57].
Genetic characterisation of the MeV virion indicates ancestry before 1915, with the H protein conserved, explaining why current diagnostics and therapeutics remain relevant for MeV infection prophylaxis and reducing potential pathophysiology [58]. Mutation rates of MeV were estimated in 1999 at 9 × 10–5 per base/replication with a genomic mutation rate of 1.43 per replication cycle indicating point mutations comparable between other –ssRNA viruses, including poliovirus as well as vesicular stomatitis virus [59]. Genomic sequencing classifies viruses based on nucleotides. To this effect, MeV clades were originally classified before 2011 designated by letter (e.g., A to H), known as clades, with 24 genotype sequences designated by a number (e.g., B3, H8) [60]. It was recommended by the WHO that 450 nucleotides encoding the carboxyl (-COOH) amino-acids (AA) of the N protein would be used to assign the genotype [61].
Between 2007 and 2015, protein epitope prediction and molecular mapping have remained in ongoing development for the immune system to be trained as more responsive [62–64]. During a host immune response, fragments (epitope peptides) are presented and processed through two classes of major histocompatibility complex (MHC type I/II), encoded by the human leukocyte antigen (HLA) utilised by antigen-presenting cells (APCs). The APCs include dendritic cells (DCs), monocytes, and macrophages (Mϕ) amongst a network of other characterised immune system cells [16, 28, 65].
A summary of genomic sequencing reports spanning 2005 to 2014 denoted that the predominant detected MeV strains in surveillance were B3, D8, and D4, with predominantly H1 whilst two others were monitored (G3/D9) (see Supplementary materials). Subsequently, in 2015, MeV antigenic stability was further attributed to inflexible F and H proteins indicating that MeV generates a host polyclonal antibody response against both F and H proteins [46]. At the same time in 2015, sporadic outbreaks in Canada occurred of MeV H1 and D8 genotypes [66]. Shortly thereafter in 2018, circulation of predominant MeV genotypes was confirmed as decreasing to four [67]. These were denoted as B3/D8, together with two others (D4/H1) during 2020 [67]. Out of these, two (B3/D8) are known to be endemic across six of the WHO regions. To this effect, continuing surveillance in Italy between 2015 to 2019 documented MeV genotypes (n = 1,273) submitted to the Genbank database [68, 69]. These reports utilise H protein as the genotype to identify MeV in line with WHO guidelines [68]. Comparisons with prior MeV genotypes during this MeV sporadic outbreak found unique details of one MeV genotype, B3, where alanine was substituted by valine (denoted as B3 A400V) [68]. It was crucially uniquely indicated that within this MeV B3 clade, 62% of individuals affected by MeV had been immunised prior [68]. The significance remains unknown at the date of writing. Furthermore, the other MeV D3 clade had an AA substitution to threonine, seemingly within the MeV H protein noose epitope (HNE) [62, 68]. The authors described epitopes in common that are targeted by the immune system including a receptor-binding epitope (RBE), a sugar-shield epitope (SSE), a loop epitope (LE), as well as a neutralising epitope (NE) [62, 68]. The HNE conformation (379–400 AA) within MeV forms an epitope region characterised by three cysteine residues with a surface-exposed loop where the epitope can be recognised by antibodies produced by B cells [62, 68]. In 2023, further genotyping indicated that D8 was a current MeV strain in circulation in a small sample (n = 2,682) analysed where 3 described mutations were noted to occur out of 16.8% of samples analysed according to the N-450 WHO sequence guidelines [70]. Many protein mutations can affect immunologically programmed responses to pathogens discussed below.
Measles disease is now rarer than in the 20th century, with immunisation programmes implemented in many countries together with ongoing genotype surveillance (see Supplementary materials). It remains a preventable contagious infection, with one vaccine dose usually given at around 12 months of age, followed by a second between 18 months to around 4 years of age (see Supplementary materials) [71]. The prodromal stage can be flu-like, accompanied by rising fever, coryza, cough, conjunctivitis, and fatigue [71]. Often there is a visible epithelial cell rash, although occasionally not, however examination of the buccal mucosa can show white “Koplick” spots. The classic blotchy, slightly raised red rash (non-itchy) can appear (day 3 to day 7) with symptoms lasting for a further 7 days by becoming flat with drier skin accompanied by skin colour change as the rash sheds [71]. Management is mostly symptomatic, with fever and fluid management as the main targets, plus rest and avoidance of strong light. This reduces the risks of complications occurring like pneumonia, acute encephalitis, and the devastating longer-term outcome of sclerosing panencephalitis, and is almost fatal [48]. Confirmation of the diagnosis is usually clinical, alongside testing sera for IgM or IgG, with the former appearing first, whilst the latter levels rise after symptom onset. Measles disease during pregnancy can increase the risk of miscarriage or preterm labour, largely due to the high fevers seen with this infection and the LAV is also not given during pregnancy [71]. Complications of MeV infection may be pronounced in immunocompromised and poorly nourished individuals. Such complications include otitis media (ear infection), pneumonia (lung infection), encephalitis (inflammation of the brain), as well as meningitis (inflammation of the lining of the brain) [48]. Cells that are infected by MeV include endothelial cells (ECs), neurons, and astrocytes, which cause delayed persistent inflammation through MeV infection instigating central nervous system (CNS) symptoms [48]. Such complications in 2015 were considered defined by four categories: namely MeV encephalitis, acute post-MeV encephalitis, MeV IB encephalitis, and subacute sclerosing panencephalitis [48]. The latter is a lesser observed phenomenon, but each is a serious and potentially fatal clinical phenomenon and is specified by incidence within the range of 6.5 to 11 individual cases per 100,000 MeV cases following most commonly infant MeV infection [48].
Measles cellular infection was investigated after immunisation with the attenuated MeV to occur through CD46, known as a membrane cofactor protein (MCP) discovered in 1986 [20]. Canadian and French research in 1993 by groups led by Dörig et al. [72] and Naniche et al. [73] showed MeV required CD46 for binding, fusion, and replication, but could be inhibited by two types of antibodies [74]. The two types of antibodies are mAbs as well as polyclonal antibodies defined by protein specificity. Therefore, CD46 is considered the initial adhesive entry receptor MeV employs as a ligand for cellular entry across the PM with MeV H and F proteins required for syncytia formation [73, 74]. It is considered that CD46 is expressed by many nucleated cells [74]. During 2010, clarification of CD46 extracellular structure domains elucidated interaction with complement proteins as depicted below, through the crystal structure complexed with human adenovirus type 11 [75]. This is depicted below (see Figure 3).
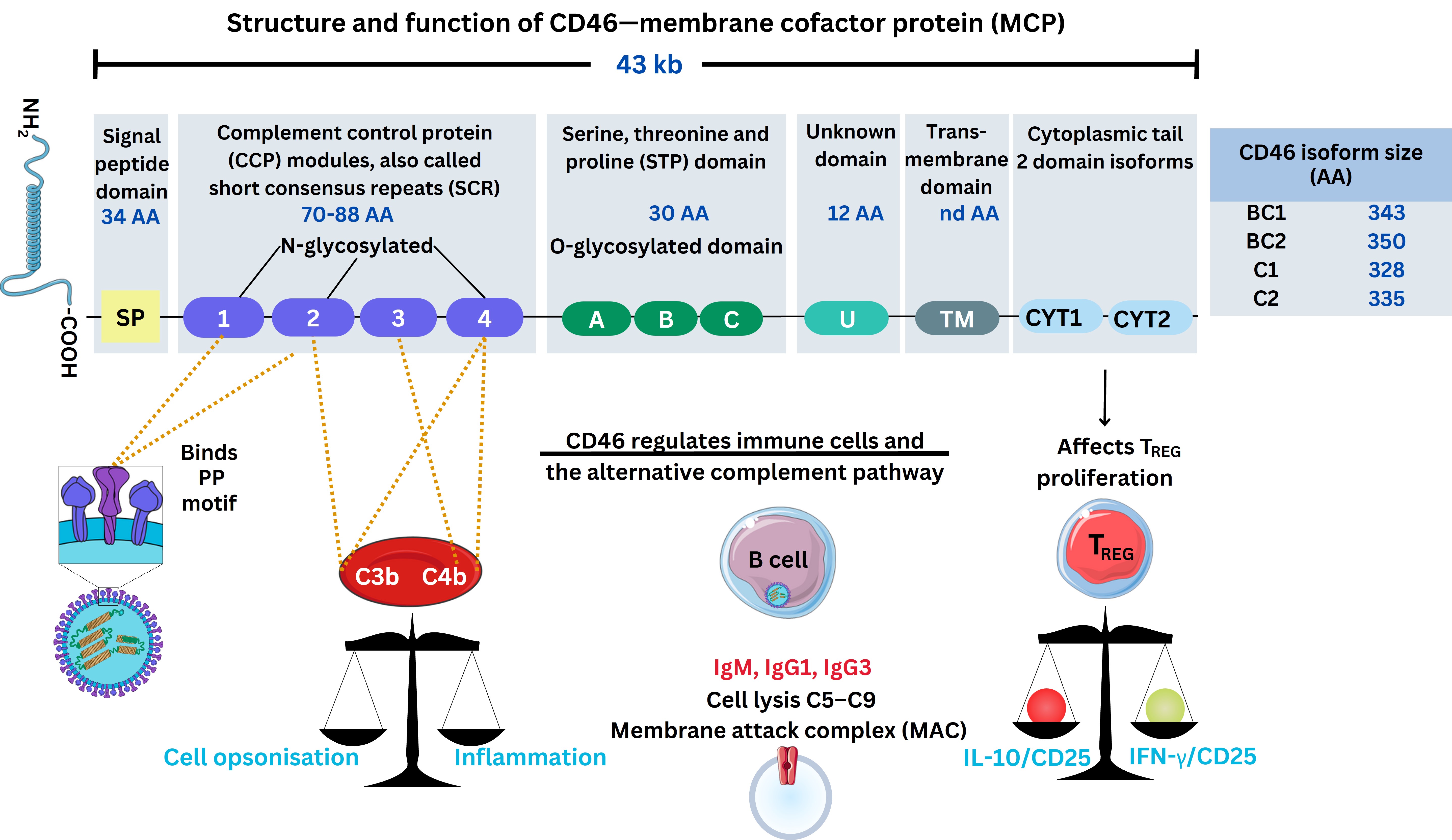
A depiction of CD46 structure and role during MeV infection. The virus schematic was adapted from ViralZone, SIB Swiss Institute of Bioinformatics (https://viralzone.expasy.org/86), licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. The Figure was partly created with Servier Medical Art (https://smart.servier.com/), licensed under a Creative Commons Attribution 4.0 Unported license. C3b: complement factor 3b; IL-10: interleukin 10; CD25: cluster of differentiation 25; TREG: regulatory T cell; AA: amino-acids; IgM: immunoglobulin M; IFN-γ: interferon-γ; kb: kilobases; MeV: measles virus; PP: proline-proline motif
CD46 can be activated and is expressed within the myeloid cellular lineages binding to complement proteins [complement factor 3b (C3b)/C4b)], a crucial part of coagulation system pathways. Antibodies synthesised by B cells in response possess more than two domains with an antigen-binding receptor [fragment-antigen-binding (Fab)] domain recognising pathogenic epitopes, together with a crystallizable fragment (Fc) protein domain structure. The latter effector Fc receptors [FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16)] are crucial in effector cell function [76]. These affect antibody opsonization (binding) through cellular membrane receptors effecting an immune response through signalling, and homeostatic complement regulation synthesizing fibroblast growth factors (FGF), as well as angiogenic factors regulating vascular growth. Knowledge of this was less well known then, however, the CD46 receptor usage is demonstrated to be preferentially expressed during oncogenic disorders and is described as a “pathogen magnet” in various infections [74, 77]. Therefore, CD46 is localised with many proteins that enhance FGF necessary for angiogenesis during common skin and systemic viral infections affecting the vasculature through different organ systems [78]. During 2002, other research in vitro did indicate that the MeV H protein uses other receptors to determine cell specificity [78].
Many MeV sporadic outbreaks have occurred since isolation. It is now known that MeV infects WBCs called lymphocytes, expressing the second receptor known as signalling lymphocytic activation molecule 1 (SLAMF1, CD150) [79]. The SLAMF1 receptor is expressed by activated B cells, T cells, DCs, and monocytes (see Supplementary materials). This second receptor, SLAMF1, is considered to be expressed throughout the primary immune system organs (bone marrow/thymus), secondary (spleen, tonsils, lymph nodes), as well as tertiary lymph systems (e.g., bronchus-associated lymphoid tissue, BALT), but also by platelets and haematopoietic stem cells (HPSCs) [79].
Nectin-4 (poliovirus-receptor-like 4, PVRL4) is a third receptor of relevance during MeV infection, overexpressed in specific tumour carcinomas (breast, lung, colorectal, pancreatic, ovarian cancer), and is usually expressed at lower levels during infancy when MeV infection frequently occurs [80]. Nectin-4 clarification came as recently as 2012, similar to poliovirus receptors (PVR) like CD155 [81–83]. The others are individually considered as nectin-1 (CD111), an entry factor receptor for herpes simplex virus (HSV, HSV-1/HSV-2), with nectin-2 (CD112) an entry factor of human herpes viruses (HHV), whilst nectin-3 (CD113) was also characterised [84]. Nectins are classified as an Ig superfamily glycoprotein similar to antibodies mediating cell-cell adhesion. As recently as 2014, Mateo et al. [38] did indicate that specific loops of nectin-4 govern MeV H protein attachment. Crucially, nectin-1 was then implicated to form a part of this adhesive mechanism, but also forming a heterodimer with nectin-4 with the MeV H protein competing at this interface [38]. However, MeV also infects airway epithelial cells lacking SLAMF1 [85]. Nectin-4 regulation could be controlled during the cell cycle usually expressed at low cell membrane levels but could be inhibited. Furthermore, DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) can cross-link with host antibodies at the cell PM surface [85]. Nectin-4 protein is also used as a counterpart specifically expressed on certain subtypes of cells including DCs [80, 81, 86]. Each of these was characterised using X-ray diffraction between 2007 to 2013 with 25 known structures relevant to understanding MeV pathogenesis (see Supplementary materials). In 2022, an initial report yet to be peer-reviewed potentially clarified that immune cells are affected directly through draining lymph nodes (dLNs) within the tonsils [87]. Nectin-4 is concurrently considered a ligand for the inhibitory lymphocyte receptor (T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory motif domains, TIGIT) expressed by both T cells and NK cells [88, 89].
In 2011, the first report appeared describing a fourth receptor of relevance in the context of other non-pathogenic or pathogenic Phleboviridae (Uukenimei virus/Rift Valley fever virus). This is known as the DC-SIGN (CD209) receptor. Recent data indicated this receptor RNA is predominantly located within specifically two types of APC, namely classical and intermediate monocytes (see Supplementary materials). Its corresponding ligand (CD209L) is indicated to have comparatively high RNA expression within fibroblasts and ECs according to data on the protein atlas (see Supplementary materials). Just prior in 2007, DC-SIGN was shown as a relevant trigger on DCs that could be induced involving Toll-like receptors (TLRs) via acetylation of the nuclear transcription factor p65 leading to activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [90]. Reports showed that this serves as an adhesive receptor facilitating virion internalisation, as well as uptake via endocytic pathways for MeV entry [85].
It was shown in the years following 2016 that mAbs inhibit MeV cellular entry and resulting disease, through binding to CD46, SLAMF1, and nectin-4 [62]. More recently, delineating the unknown causes of how MeV may cause neurological complications like encephalitis is only just emerging. It was concluded that CD46 may be dispensable during MeV infection of neurons. Latency after MeV infection remains largely unknown within the neuronal/astrocyte synaptic cleft. However, Poelaert et al. [91] did show that astrocytes could be dependent on glutamate excitatory AA transporters leading to potential MeV-induced syncytia formation.
Measles disease is frequently characterised by skin rashes employing the nectin-4 receptor [5, 39, 92, 93]. Reduction in lymphocyte counts can occur (lymphopenia) through excessive apoptosis (cell death/proliferation) in many disorders, where the regulatory homeostatic immune system is imbalanced through host cell receptor viral entry and cytokine regulation [93]. Chemokines affect this cellular checkpoint by balancing the immune cell signalling system, in an autocrine/paracrine fashion similar to hormones [28]. Measles virions disturb this homeostatic cellular function during natural infection. In 2013, Richetta et al. [94] illustrated this utilising knockout of the non-structural MeV C protein in vitro to show that CD46 and Cyt-1 were required together with the Golgi-associated protein. MeV proteins were therefore found to be able to escape from autophagic degradation during MeV cellular infection, in effect sustaining host cell replication in an early and late wave during autophagosome cellular egress [94]. Below is a depiction of some of the intracellular proteins and pathways known to date (see Figure 4).
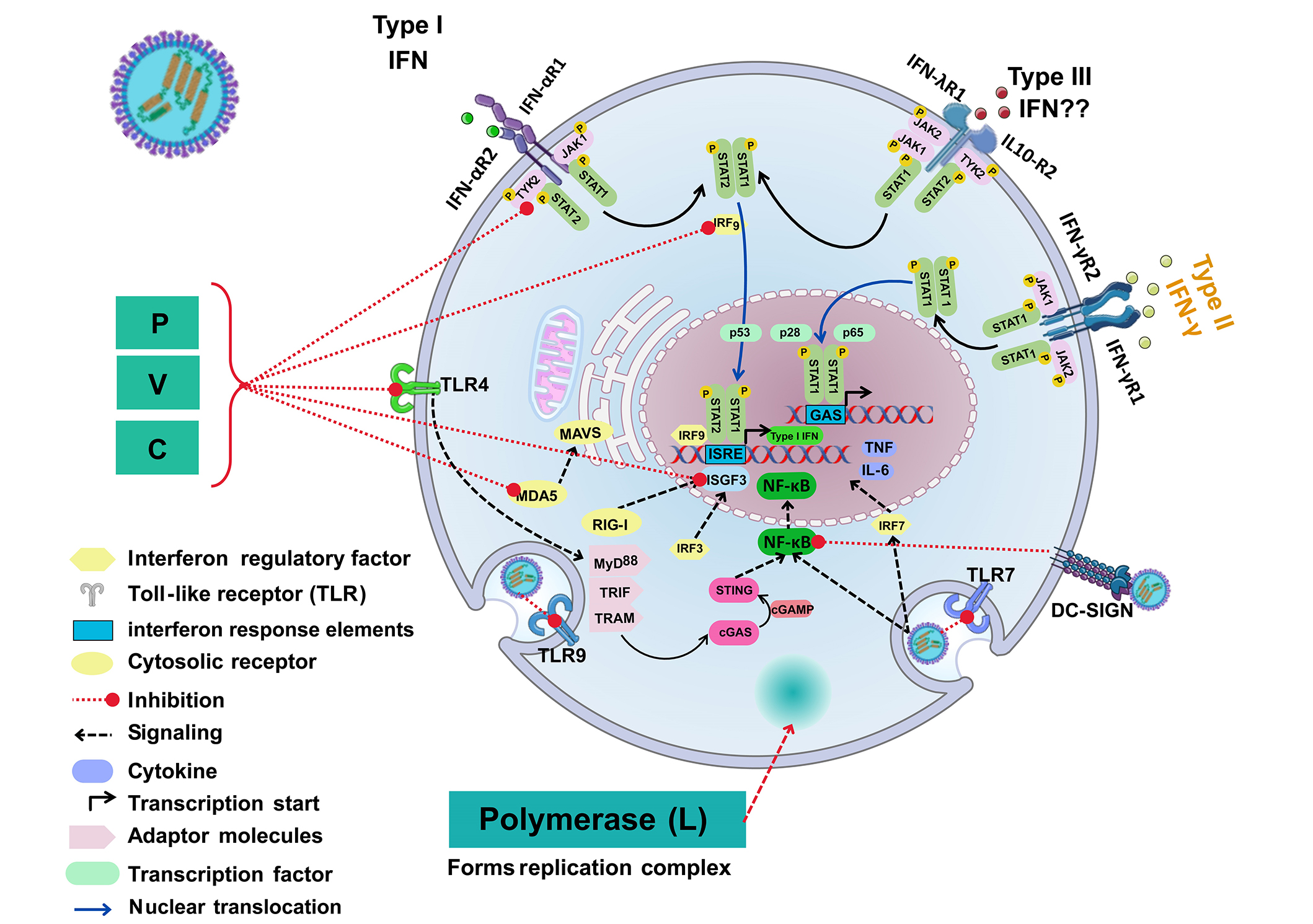
A graphical summary of the literature until 2023: interaction of MeV or attenuated MeV with extra and intracellular proteins. The virus schematic was adapted from ViralZone, SIB Swiss Institute of Bioinformatics (https://viralzone.expasy.org/86), licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. IFN-αR: type I interferon receptor; IFN-γR: type II interferon receptor; IFN-λR: type III interferon receptor; TNF: tumour necrosis factor; IL-6: interleukin 6; MyD88: myeloid differentiation primary response 88; TRIF: Toll/interleukin 1 receptor domain-containing adaptor inducing interferon-β; TRAM: TRIF-related adaptor molecule; STAT: signal transducer and activator of transcription; TYK: tyrosine kinase; JAK: Janus kinase; IRF: interferon regulatory factor; RIG-I: retinoic acid-inducible gene I; MDA5: melanoma differentiation-associated protein 5; MAVS: mitochondrial antiviral signaling protein: cGAS: cyclic guanosine monophosphate-adenosine monophosphate synthase; STING: stimulator of interferon response gene; cGAMP: cyclic guanosine monophosphate-adenosine monophosphate; ISRE: interferon stimulating response element; GAS: gamma activated sequence; ISGF3: interferon stimulating growth factor; Type III IFN??: no data available; DC-SIGN: dendritic cells-specific intercellular adhesion molecule-3-grabbing non-integrin; TLR7: Toll-like receptor 7; P: phosphoproteins; C/V: non-structural proteins; MeV: measles virus
The wild-type measles virus (wtMeV) cellular mechanisms involved in the causation of characteristic exanthema are comparatively unknown, although it is considered that this occurs during the recovery phase with both lymphoid and myeloid cells infected with MeV [95]. This is then followed by epithelial cells which express nectin-4 as well as SLAMF1 (CD150) within the vasculature potentially explaining why the skin rash appears systemic and is instigated by immune system cells [95].
The phenomenon of vaccine failure has been known for 50 years since Cherry et al. [96] described MeV outbreaks between 1971 to 1973, but the reasons remain elusive [97]. Some years later in the late 20th century, it became evident that the wtMeV strain infected DCs, replicated and caused loss of DC allogeneic stimulation of innate and adaptive immune system T cells [98]. This effectively suppresses viral antigen presentation whilst spreading throughout secondary lymphoid organs and restricting the repertoire of natural antibodies produced [99]. Reports from Isa et al. [100] in 2001 documented wtMeV infection, as the duration and kinetics of the immune response before and since MeV discovery remains of interest in ensuring longer-term health in vivo. The prominent role of other cellular receptors during MeV infection appeared in 2012 when those cells expressing DC-SIGN from both bronchoalveolar fluids (BALF), as well as LNs could transmit MeV to B cells that usually can produce antigen-specific antibodies [85, 101].
Kinetics of the immune response indicates that during natural MeV infection, two antibody types, IgM and IgG, are synthesised around 11 days after infection, peaking at 17–24 days for IgG in non-human primates (NHP) in vivo [102]. However, there are at least four relevant subtypes of IgG (IgG1, IgG2, IgG3, IgG4), as well as two subtypes of IgA (IgA1, IgA2), alongside IgE and IgD, with others like IgY in avian species [28]. Nevertheless, it was shown using immunofluorescence assays that one type of IgG (IgG1) is predominant in blood sera in individuals (n = 154) after a rash appearance. In addition, with IgG1 present, IgG2/IgG3 appears to spike at day 2–3; moreover, both IgG1 and IgG4 remain present 10–30 years after infection or immunisation (seropositivity 100% and 86%) [100]. This cellular development of antibody response, in this case, attributed the relevance of IgG2/IgG3 95.5% seropositivity to convalescence rather than memory responses [100]. Population serology studies in 2020 (n = 1,092) examined nAbs present between 10 to 12 years after either infection or immunisation [103, 104]. Decreases in measles disease mortality occurred more than 30 years prior when much of this remained unknown and still does. nAbs are considered to negate the biological and infectious effects of a pathogen. It was indicated in 2020 that IgM-measured was crucial in reducing host viral propagation and effecting a host immune cell response, as the second key antibody type parallel to IgG for diagnostic assays [100]. Other research investigated antibody production, to either infection or MMR immunisation (n = 88), by age range to show that IgG3 is the dominant IgG produced (63.3%) in response to MeV infection/immunisation in children age 3 or under without synthesising IgG2; additionally, IgG2 was 42.6% of the total IgG response in children over 4 rising to 62% in convalescent adults [105]. During natural MeV infection, IgG1 and IgG3, are considered to be the dominant earlier humoral antibodies produced [105]. These remain key observations because, in vivo, in mice rather than humans, three subtypes of IgG2 exist [106–108]. Indeed in 2019, monomeric human IgG2 was described as having less effector function, but still therapeutically relevant through FcγRII (CD32) and FcγRIII (CD16), effecting microbial pathogen clearance through antibody-dependent cell cytotoxicity (ADCC) which utilises both Mϕ and neutrophil function [109].
During a 10-year study following MeV, as well as MuV antigens evoking nAbs after immunisation with MMR (n = 98), comparisons were made between 7 to 17 years post-immunisation of individuals. This data did not indicate a statistical difference between the production of either nAbs to MeV or MuV; but did indicate that 42% of individuals experienced more than 20% waning of MeV antibody titres with an established antibody correlate (120 milli-international units per millilitre, mIU/mL) [110]. Furthermore, the waning of IgG antibodies occurred specifically against MuV rather than nAbs against MeV [110]. Further to this in 2019, scientists from Boston in a crucial study during natural MeV infection of un-immunised individuals (n = 77), further clarity came through serological analysis [111]. It was found that the host antibody repertoire produced could be quantified with up to 73% reduction during natural MeV infection in infants [111]. During this study, parents graded disease severity as 44% in acute and 56% in severe MeV infection [111]. This change instigated by MeV infection may alter the human host’s immune response to other pathogens including HHV as well as papillomaviruses amongst other bacterial infections (e.g., Streptococci) for up to 5 months after natural MeV infection with much still unknown [111].
Recently between 2017 to 2021, circulating B3/D8 MeV genotypes were examined during an outbreak in Italy by Bianchi et al. [49] who confirmed B3/D8 MeV genotypes to show that breakthrough infections could also occur in immunised individuals (n = 864). Specifically, they estimated < 2.6% of individuals were non-responsive to MMR immunisation as measured by antibody production [49]. The significance of this remains unknown to now.
During MeV infection, it was similarly observed that B memory (BMEM) cells were reduced, which would usually develop and stimulate other cells to form antibody-secreting cells (ASCs). Together with BMEM cell count reduction, an accompanying reduction in antibody secretion of two predominant types within serum and mucosal compartments (IgG/IgA) was observed, although increases in other B cells, transitional B cells, occurred being bone marrow resident B cells [112]. MeV therefore has been confirmed to selectively deplete and affect naive B cell development with signalling pathways largely unknown, but potentially affecting the adaptive immune response during pathology [112]. During the acute phase of MeV infection, circulating B cells as well as T cells are infected through MeV differential affinity to CD46 and other cell receptors. CD46 receptors are present throughout the lymphoid tissues, germinal centres (GCs), and dLNs. On another note, MeV infection is associated with a robust immune response through the attenuated MMR immunisation, but infection points to a temporal lack of memory of B and/or T cell response but the level of this remains obscure. Many factors affect the rate of antibody generation and persistence, but TMEM cell responses play a crucial role.
Since MeV immunisation began, technological evolution and genetic sequencing have discovered other protein factors in the immune system. These include type I interferon (IFN), type II IFN or type III IFN discovered between 1957 to 2003, besides a host of pattern recognition receptors (PRRs), for example, TLRs, as well as interleukin (IL) cytokines. These can modulate the immune cell phenotype in responding to infection.
To this effect in 2011, the reasons for differential antibody production were observed in MMR-immunised subjects (n = 454), with variations observed in TLR2 associated with increases in antibody production, whilst in contrast TLR4 which was associated with less antibody production within this study [113]. Authors attributed this to an innate immune regulatory gene (mitogen-activated protein 3 kinase 7, MAP3K7), which can mediate cell signal transduction through a transforming growth factor-β (TGF-β), evenly expressed throughout the immune system leukocytes essential for normal cell function [113–115]. In 2012, further studies examined CD46 receptor single nucleotide polymorphisms (SNPs) genotyped in children (n = 137) to show significant correlation could occur with MeV-specific IgG concentrations with a specific CD46 genotype (rs7144), seemingly affecting both B cells and T cells [116]. In the aforementioned study, MeV antibody titers below 324 mIU/mL were considered seronegative of which 10.2% of individuals did not produce antibodies [116]. Whilst during 2020, an Australian retrospective report investigating MeV infection (n = 297) spanned 2008 to 2017, it was outlined that sometimes primary and secondary MeV vaccine failure could potentially be observed [117]. Antibody responses could still be present and were classified as nonimmune (IgM+/–/IgG–), indeterminate (IgM+/IgG+), but also waning immunity (IgM–/IgG+), further elucidating potential usefulness as indicators [117].
Little was known of antibody seroprevalence to MeV in individuals with cancer, until two studies of individuals (n = 959) with solid malignancies and haematologic malignant neoplasms were published in 2021 [118]. It was shown that 25% of individuals in these groups lacked antibodies for MeV, whilst 38% lacked antibodies against MuV. Variable seroprevalence was noted with age groups characterised by higher seroprevalence in increasing age [118]. Concurrently recipients of haematopoietic stem and progenitor cell (HSPC) transplants also possess significantly fewer nAbs against both MeV and MuV. Whilst in other paediatric cancer cohorts, it was also noted that protective antibody titers were also reduced more significantly in acute lymphoblastic leukaemia (ALL) patients, but similarly, MuV antibody waning was further noted [119].
Regarding the cellular mechanisms MeV utilises to disrupt cellular homeostasis, as early as 2005, observations noted that MeV was causal in inhibiting the production of a required homeostatic type I IFN-α/β cytokine [120]. Furthermore, in 2011 in 1-year-old infants, DC-SIGN and SLAM SNPs were compared through genotyping to find type II IFN-γ responses required also varied in conjunction with antibody IgG levels [121]. More recently, MeV was also shown to inhibit two PRRs, TLR7 and TLR9, usually expressed within the plasmacytoid DC (pDC) lineages commonly producing type I IFN whilst presenting viral antigens required for B cell development in GCs [122]. This was emphasised in NHP, where cellular stimulation using combined TLR3/TLR9 agonists with an MCV was seen to induce high concentrations of IFN synthesis in vivo as well as cytokines like IL-10 [123]. TLR9 particularly is known to be expressed by B cells and pDCs and is a factor in other skin disorders like systemic lupus erythematosus (SLE) [115]. With regards to the MeV-induced downregulation of type I IFN production by DCs. In 2014, Mesman et al. [124] did show in vitro DC-SIGN could inhibit cellular phosphatase activity regulating RIG-I as well MDA-5. In effect, DC-SIGN is required by MeV for cellular infection, as well as early MeV transcription and replication and suppresses DC type I IFN production affecting the adaptive immune system response as discussed below [124].
Chemokine research evolved since 2011, with investigations into the role of CXCL12 beginning, and is considered to be affected during MeV infection that may potentially affect APCs. It was postulated that the Runt-related transcription factor 3 (RUNX3) gene was a regulatory transcription factor that could regulate and maintain both T cell and monocyte receptor (CD4/CD14) expression affecting monocyte differentiation with individual angiogenic and immunosuppressive activity [28, 125, 126]. CXCL12 is known as a B cell developmental growth factor (GF) also called stromal-derived factor 1α (SDF-1α). This homeostatic chemokine is ubiquitously expressed throughout the human body.
Further reports from 2016, using unbiased mRNA-sequencing technology, confirmed that immunisation against MeV elicited the production through cellular mRNA of CXCL12, together with the expression of one cell receptor, CD93, and one cytokine, IL-6 [127]. Chemokine further characterisation has largely occurred in the 21st century. As mentioned, CXCL12 protein synthesis was observed to be downregulated during MeV infection [128]. Therefore, it is plausible that this represents a key pathway with which MeV infection can alter both monocyte lineages as well as T cell phenotypes during disease. Interestingly, CD93 is a C-type lectin transmembrane receptor affecting cell adhesion and phagocytosis by APCs. In addition, CD93 appears to have a central checkpoint function discovered, with a negative correlation to type I helper T cell (TH1), NK cells, but also myeloid-derived suppressor cells (MDSC) in cancer as well as follicular helper T (TFH) cells [129]. It was furthermore considered that blockade of CD93 could sensitise tumours to immune-checkpoint therapy [129]. Whereas, IL-6 in immune responses is a well-characterised cytokine, performing a role as a chemoattractant for neutrophils during pro-inflammatory immune responses; while CD93 is found expressed by cell lineages including myeloid cells, HSPCs, NK cells, and platelets concurrently with neuronal, microglial, and ECs [130]. It was further clarified that IL-2 along with TNF-α, and type II IFN (IFN-γ) are required for effective innate host responses during MeV infection [129]. Previous articles indicated that increases in levels of the soluble IL-2 receptor (IL2R also known as CD25), a membrane—shed marker of regulatory T (TREG) cells discovered in the 21st century can occur [129]. Furthermore, this was accompanied by cyclical IL-17 changes produced by TH17 cells and other cells [129]. This is unsurprising, and the cytokine TNF-α is not only expressed within epithelial cellular layers during infection, but also during premalignant oncological conditions, where epithelial layer differentiation can be affected during inflammatory responses [131, 132].
Development in 2020 indicated a second chemokine, CXCL10, was observed in serum concentrations and could be a correlate of severity during MeV infection [133]. These were interesting observations because the receptor for CXCL10 is CXCR3 expressed on many immune cells, including DCs, required for antigen presentation. More recently it was observed that MeV infects cytokeratin-positive epithelial cells in bronchial and appendix epithelia, accompanied by disruption of alveolar and bronchial epithelial cells as well as multinucleated cells expressing CD11c, characteristic of the DC or Mϕ cell phenotypes expressing CD68 [134]. Below is depicted the cytokine and chemokine roles in the immune system as discussed below (see Figure 5).
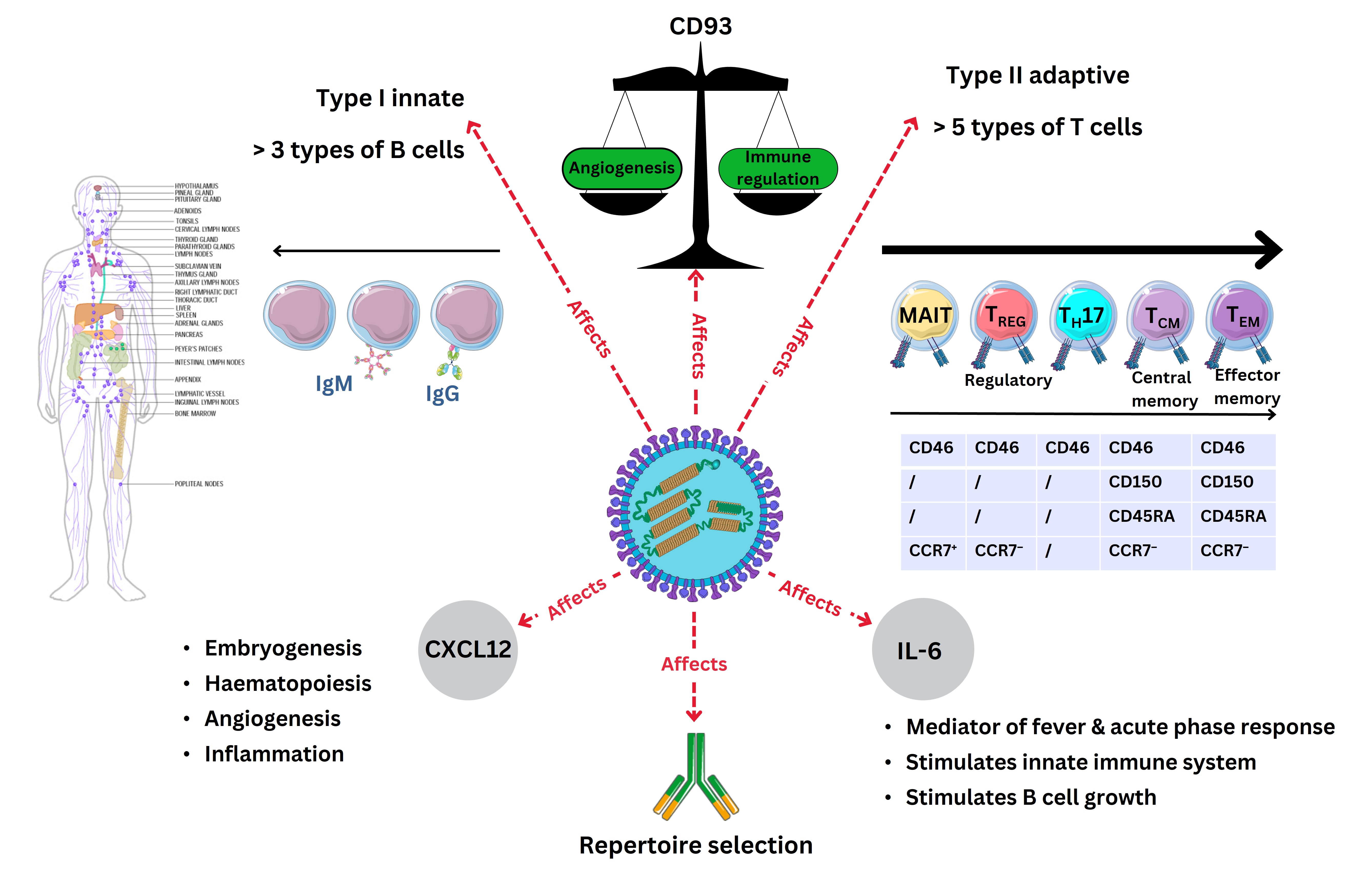
A depiction of the role of CXCL12 and IL-6 in MeV infection with adaptive immune cells defined by CD molecules during MeV infection. The virus schematic was adapted from ViralZone, SIB Swiss Institute of Bioinformatics (https://viralzone.expasy.org/86), licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) License. The Figure was partly created with Servier Medical Art (https://smart.servier.com/), licensed under a Creative Commons Attribution 4.0 Unported license. MAIT: mucosal-associated invariant T cells; TEM: effector memory T cells; TCM: central memory T cell; IgM: immunoglobulin M; IL-6: interleukin 6; CXCL12: C-X-C motif chemokine ligand 12; MeV: measles virus; TREG: regulatory T cell
Further details remain to be explored in conjunction with the role of TLRs. Since 2006, p38 mitogen-activated protein kinases (MAPK) and the role of TLR2 were determined to affect host cell responses and proliferation [115, 135]. During 2019, it was however indicated that a highly conserved nuclear protein like WD (tryptophan-aspartic acid) repeat-containing protein 5 (WDR5) could regulate MeV N and P proteins instigating viral IB growth [136]. Subsequently, in 2021, TLR2 SNPs during MeV infection in individuals (n = 100) suggest certain host genetic mutations (rs3804100) may affect cell signal transduction and susceptibility within the respiratory tract upon MeV infection [137].
In 2003, when type III IFN was discovered, it was implicated that the MeV C protein may suppress type I IFN (IFN-α or IFN-β) [138]. The resultant inhibition by MeV infection of the JAK1 enzyme crucial to nuclear IFN signal transduction, in effect may temporarily modulate the type I IFN response, altering type I IFN synthesis with research continuing [139]. More recently, since type III IFN discovery, in 2015, it could be observed in vivo that this lack of IFN response was also accompanied by a lack of type III IFN response and measured by lack of specific mRNA gene transcripts (MX/ISG56) usually leading to lack of translation of type I/III IFN protein expression, a known epithelial layer expressed IFN [140]. More recent discoveries from 2021 show MeV can modulate mitochondrial DNA (mtDNA) in common with both +ssRNA and –ssRNA viruses by affecting the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) pathway affecting each of the type I/II/III IFN secretion pathways required for immune responses [26, 65, 141]. Therefore, MeV could modulate the homeostatic IFN systemic response essential to antiviral innate/adaptive cellular reactions. Investigations by Clifford et al. [142] examined the TLR role within individual infants (n = 238) who received MMR, but also then contracted MeV. It was then shown that TLR7 SNPs did not affect functional responses to MeV immunisation, however, CD46 and TLR8 variants potentially could affect a host immune response to infection and immunisation.
Atabani et al. [98], in 2001, confirmed that natural MeV infection could dampen IL-12 cytokine production in DCs, whilst other researchers reported in 2012 additional suppressive effects on both innate B and T cells to conclude that three crucial immune cell phenotypes could be infected by MeV [98, 102]. The effector host cell response to MeV infection requires these three. The adhesive nature of MeV to DC-SIGN on DCs in epithelial cellular layers is implicated as one route of affecting MeV cell infection, with other lymphocytes expressing CD150 present mainly in lymphoid tissues [79]. Furthermore, MeV transmission between T cells from cells expressing CD150 indicated that virological synapses could be formed where viral proteins accumulated. This could occur through activation of DC-SIGN and was investigated together with leukocyte functional antigen 1 (LFA-1) as well as a non-glycosylated tetraspanin (CD81) [143]. These were notable findings as LFA-1 is abundantly expressed by leukocytes and required by T cells as a motility factor utilising intercellular adhesion molecules (ICAM) within epithelial and EC layers [143]. For example, effector memory T (TEM) cells, but also recall of other TH cells, as well as cytotoxic T (TC) cell responses across membrane barriers are required to provide longer-term adaptive immunity. Other T cells include and are defined phenotypically as above naive T (TN) cells, together with TREG cells, whilst other TH17 cells secrete chemical cytokines like IL-17 amongst other T cell phenotypes [28].
T cells can be infected through MeV F proteins binding to the PM surrounded by receptors described above. The T cell phenotypes affected include memory T (TMEM) lymphocytes lacking expression of receptor proteins, like the leukocyte common antigen, CD45 (denoted as CD45RA−), or expressing others usually by TMEM cells (denoted as CD45RO+) [102]. These specific T cells traverse and diffuse through EC layers, as well as within lymphoid tissues (bone marrow/thymus) and dLNs, utilising leukocyte-specific adhesion molecules like CD62 ligands (CD62L). It was noted that two classes of T cells were preferentially infected namely TEM cells, as well as TCM cells, leading to the hypothesis that natural MeV infection provokes immune cell temporal amnesia [28, 102]. However, other innate immune cells developing into B cells were observed as proliferating within LNs (follicular B cells), measured by antigen Kiel 67 (Ki67), a cellular proliferation marker. Suggestions were that apoptosis did not occur as measured by caspase-3 expression within T cells, but rather that MeV-infected cells were preferentially depleted by TC cells usually producing an array of effector enzymes like perforins and granzymes [28, 102].
Immunisation against MeV traditionally occurs in two doses in infants providing a prophylactic benefit by training the immune system to recognise attenuated MeV epitopes presented to T cells. The rationale of this is as described with attenuated MeV through immunisation resulting in cell-derived processed epitopes being presented to the immune cell phenotypes expressing CD46 and therefore metabolised efficiently upon stimulation [111]. Recent diagnostics commonly used up to 5 days after infection are real-time polymerase chain reactions (rtPCR); whilst serology assays have been reviewed elsewhere for MeV indicative of the sensitivity of 90.6% but also 100% specificity to date that are screened for viral variations [144].
More recent research on MeV infection (n = 26) is connotative of other T cell phenotypes affected. These comprise of TFH cells alongside at least four other key T cell phenotypes, TH1 and TH2, as well as TREGs, with TH17 cell reduction occurring [145]. However, this involves the APCs processing antigens requiring GFs like IL-4 and IL-13, to effect functional T cell responses. To this effect, scientists in 2017 researched these potential factors including SNPs in the IL-4 cytokine pathway in individuals (n = 137) [146]. Specifically, one polymorphism (S503P) was documented within the corresponding IL-4 receptor subunit (IL-4Ra) that could affect immunisation responses [146]. How polymorphisms affect response to infection or immunisation remains unknown, but it is known that APCs utilise IL-4 signalling to effect APC growth, thereby facilitating the presentation of viral antigens and effecting host production of IgG antibody subtypes [146]. However, in 2020, the role of TFH cells was further clarified in acute MeV infection. It was then seen that signalling through the expression of the inducible T cell costimulator (ICOS, CD278) was activated together with the expression of CXCR5 with two cytokines (IL-6 and IL-21) observed in individuals (n = 42) with MeV-specific serum IgM antibodies [147].
Comparatively less is known about the role of NK cells during MeV infection or other immune cell phenotypes. However, since 1954 MeV isolation, many of the T cell phenotypes are now defined by membrane expression of both chemokine receptors and respective ligands accompanied by either membrane or soluble CD protein expression by T cells. These are commonly denoted by the leukocyte common antigen (CD45), together with CCR7, frequently expressed by migratory TN cells. The phenotypes specifically observed to be infected in NHP during MeV infection were TCM cells (CD45RA−CCR7+), or TEM cells (CD45RA−CCR7−) with both expressing SLAMF1 [102, 148]. Similarly, MeV is known to infect naive B cells (IgD+CD27−), as well as BMEM cells (IgD−CD27+), as well as other B cells that all express a B-lymphocyte antigen (CD20+), but also the dominant antigen-presenting receptor, the type MHC class II receptor (HLA-DR) usually presenting 9–30 AA of pathogen degraded cellular processed peptides as a ligand for TCR recognition [102, 148]. In 2017, the T cell response was further analysed indicative of CD4+ T cells producing type II IFN-γ during the MeV infection rash period along with cytokines required for Mϕ maturation into either M1ϕ/M2ϕ phenotypes (e.g., IL-4, IL-10, and IL-13) [149]; just as antibody production occurring in a TH1 type response is considered to be beneficial. However, other cytokines like IL-17 were synthesised and secreted up to 126 days after infection, although the other 2 key types of T cells (TREG and TH17 cells) roles have not as yet been measured [149].
Both of the two cell types expressed a retinoic acid nuclear receptor [retinoid-related orphan receptor γt (RORγt)]; furthermore, both were shortly after described to be specific for the MeV H and N proteins [150]. As recently as 2021, other emerging reports further confirm that MeV infects lately characterised mucosal-associated invariant T (MAIT) cells expressing CD3+ with MHC class I-related gene protein (MR1) [151, 152]. These were crucial because the MR1 protein can bind to vitamin metabolites such as those produced during riboflavin synthesis (e.g., vitamin B2) or during bacterial infection with others obscure [153–156]. Other T cell phenotypes are defined that include γδ T cells that are also a factor which include the Vγ9Vδ2 T cell phenotypes in the developmental immune response [157]. To this effect, However, 2024 reports are only just clarifying (n = 38) some. It is now clarified that no significant difference occurred in cytokine production by monocytes after MMR immunisation; however, a metabolic shift may occur in γδ T cells. Specifically, the dominant peripheral blood Vδ2 T cells are increased, whilst being able to produce both TNF as well as type II IFN-γ necessary for T cell activation and proliferation to infection [158]. Subsequent re-stimulation of CD3/CD28 Vδ2 T cells was further seen to be able to induce mitochondrial metabolic changes [158]. While infectious MeV can be cleared, in 2017 it was evidenced that MeV RNA persists in peripheral blood mononuclear cells (PBMCs), together with secretions for months after [149, 159]. This was observed in NHP between 84 to 140 days after infection through type II IFN-γ release required to clear viral infections [149, 159]. TH cells expressing CD4 were observed as crucial early in infection. The TH cells expressing CD4 were active earlier in infection and appeared polyspecific later during infection against MeV H- and N-expressed proteins [149, 159]. This was accompanied by an increase of CD4+ T cells secreting IL-17 (1.35–2.27%) that were MeV H protein-specific [149, 159]. However, TC cells were still active at 113 days after infection indicative that immune responses are still sensing MeV-presented epitopes [149, 159]. The TC cell response therefore does continue to occur. In 2018, Arbore et al. [160] examined CD46 deficiency to note the optimal type II IFN-γ response and resulting cytotoxicity was dependent on both CD46 and TC cells. Notably CD46 stimulation was indicated to be a stronger checkpoint than CD28 on T cell phenotypes (expressing CD4/CD8). This could occur with the upregulation of CD107a, and increased activity of the serine protease granzyme B effecting the apoptotic function in a perforin-dependent pro-apoptotic manner. It was also shown then that the inflammasome NLRP3 (nucleotide oligomerization domain, leucine-rich repeat, and pyrin domain-containing protein 3) sensing of microbial pathogens could be independent of MeV infection [160]. Notably, it was then observed that this CD46 receptor could be co-stimulatory and reflect divergence through metabolic pathways whilst directing an optimal TH1 response. However, stimulating complement (C5a) production could regulate optimal CD8+ T cell responses through receptors (C5AR1 and C5AR2 expressed by T cells) [160].
Above some of the research will have included in vivo/in vitro studies subject to guidelines. Immunisation is subject to both regulatory as well as local authority jurisdiction for further guidance and is dependent on supply chains as well as ongoing diagnostic tool development discussed elsewhere (see Supplementary materials). Safety monitoring of immunisation occurs and is of consideration but discussed elsewhere, whilst similarly, vaccine efficacy remains difficult to quantify during MeV-caused disease [161, 162]. New vaccines remain in development [163]. LAVs are subject to clinical guidance; specifically for individuals with diagnosed immunodeficiency (e.g., severe combined immunodeficiency disease, SCID), or immunosuppressed (e.g., during acute or chronic leukaemia/lymphoma treatment) (see Supplementary materials).
In recent years, the European Centre for Disease Control and Prevention (ECDC) surveillance reports up to 2023 indicated that the incidence of MeV detected cases between 2018 and 2019 (34.4 and 27.2 per million population) has decreased in 2023 to 5.2 cases per million population, without attributable fatality caused by MeV (see Supplementary materials). The most recent mortality data in 2018 characteristic of overall MeV-caused disease fatality globally illustrates around 140,000 individuals remain affected predominantly under the age of 5 and also immunocompromised individuals [5]. This is affected by vaccine hesitancy, but also by implementing immunisation programmes and schedules through cooperation globally [164, 165]. It is considered that immunisation coverage exceeding 90% or 95% could potentially lead to the near eradication of MeV, similar to other viruses like VARV long since extinct [165]. Recent reports indicate that 86% of MeV-diagnosed cases occurred in un-immunised individuals, with 66% of cases in un-immunised adults. This occurred in countries where the range of two doses of MCV uptake varied between 71% to 99% (see Supplementary materials). Such figures are notable. Sporadic MeV cases can occur as the immune response and resulting rates of measles disease prevalence could be affected by a myriad of factors, as well as immunisation evoking immune system responses. It is currently indicated that serious complications of measles disease can be acute encephalitis and sclerosing panencephalitis occurring 7–10 years after initial MeV infection [5]. Nevertheless, the longevity of immunological responses to the attenuated MeV, since MCV or MMR immunisation inception remains unknown. Yet there has been a reduction in overall MeV disease case counts and disease burden since the progressive introduction of immunisation [166, 167]. Given the high seroconversion rates observed after MMR immunisation, it could be considered that vaccines targeting MeV may yet lead to eradication, although unknown genetic factors can affect the immune response [165].
In 2024, differential MeV-induced antibody profiles were examined in China (n = 2,629) recently [168]. These were denotative of a potential antibody threshold at around 14.3 years of age with antibody concentrations around 200 mIU/mL suggesting waning immunity contrary to previous indicators [168]. However, T cell responses vary during development adding to the complexities [169]. The arbitrary scale of antibody responses is being compared globally, with reagents used determined by the specificity and sensitivity of the mAb [27]. Timing of immunisation is indicated and could affect the nAb response against MeV usually occurring in infants [170].
Whilst CD150 was confirmed as a key MeV cellular entry receptor before 2018, it was noted that MeV infects TN cells and BMEM cells, as well as both DCs, M1ϕ/M2ϕ, but not the other key APCs that are monocytes in vivo. Research opinions vary on whether MeV infects monocytes, however, historically this was observed in 1975 research [171, 172]. The wtMeV may appear causal in the cytotoxic activity of lymphocytes entering B cell follicles between acute to severe MeV infection [144]. Seemingly, MeV immunosuppression has utility beyond what was originally known, with the role of TREG cells and NK cells remaining mostly in the dark. However, Griffin et al. [173] in 1990 examined NK cell responses which did appear unresponsive but could be rescued in vitro by the DC maturation/stimulation cytokine IL-12. Cytolytic activity of Paramyxoviridae is known in similar viruses of this family like NiV [16].
Other factors largely unknown that MeV affects during disease were noted in 2011, when a systematic review examined synthetic vitamin A supplementation in infants aged 6 months to 5 years as reducing overall mortality by up to 30% [174]. Vitamin A (retinoids) effects on the immune system phenotypes remain comparatively unknown, as the discovery of the relevant stimulated by retinoic acid 6 (STRA6) protein receptor occurred in 2013, remaining central to vitamin A metabolism [155]. Furthermore, in France during 2017, a trace element, selenium (n = 94), was found to be reduced in the sera of individuals with acute MeV-caused disease [175, 176]. These were interesting findings because selenium is considered to be essential to human health [176, 177]. Other recent studies before and since the recent COVID-19 pandemic are indicative that CD150 has a role in DC maturation. Since other DC phenotypes were observed between 2006 to 2018 and specifically in 2017, further developments will be interesting to see [135, 178, 179]. Current knowledge indicates that the SNPs within the predominant host CD46 receptor were only observed from 2012, at least with attenuated MeV strains, where CD46 was highly expressed on monocytes; but also a specific CD46 genotype (7144CC) may affect CD46 cellular function and resultant activation as well as the host response to MeV immunisation [116, 180]. Furthermore, many unknowns remain regarding SNPs of the TLRs also affecting a host immune response. The role of type III IFN in MeV infection remains elusive as other gene or protein deficiencies may occur that affect host viral and bacterial immune responses during development and throughout life [181].
However, guidelines illustrated that there remain many unknowns. For example, the usage of LAVs, as in MMR, in specific individuals with diagnosed primary or acquired immunodeficiency disorders can be contraindicated in certain populations of immunosuppressed individuals despite the documented decline in overall infections (see Supplementary materials). To this effect, the details above provide further detail. Reports remain scarce on MeV and immunodeficiency in 2024. However, it was 1952 when Bruton discovered X-linked agammaglobulinemia (XLA) that could plausibly result in humoral immune response deficiency in humans [182]. Some years later in 1996, Griffin [183] to this effect continued this line of research by developing an in vivo research model to further examine strains of the attenuated MeV requiring further clarity. Future research should therefore consider the other T cell phenotypes and transcriptome studies. It can be considered through the outline above that there are similarities with the usage of VACV which similarly induces immune responses that have led to the eradication of smallpox disease caused by VARV through immunisation development with research ongoing [4, 183–187].
The longevity of humoral/adaptive correlates to MeV infection or vaccine correlates of protection remain unknown, although longitudinal studies suggest natural infection and/or immunisation against MeV does induce high concentrations of nAbs preventative of pathogenic disease. The relevance of MeV as an infectious disease is that through nAb production to the attenuated MeV, stimulation occurs of the rate of recalled BMEM and TMEM cell responses, and a combined duration of potentially 10 years or more is likely. However different viral infections have individually different physiological and immunological responses. Measles immunisation seemingly induces a beneficial host repertoire of antibody types that stimulate immune cells to produce chemokines and cytokines reducing host chronic disease severity and/or reducing MeV cellular replication through training the immune system. This is likely to occur because of a conserved MeV H protein. Above the role of both innate and adaptive immune cells is outlined in response to MeV infection underpinning how immunisation evokes a host immune response.
At this time, two MeV accessory proteins (C/V) are known that could affect the type I IFN receptor transduction STAT1/2 proteins. Innate immune responses to MeV infection may be independent of type I/III IFN synthesis with much remaining uncharted and the topic of ongoing research. Currently, 253 clinical trials investigating measles have been completed with 32 awaiting (see Supplementary materials). Beyond the outline above, comparatively much remains unknown concerning the MeV replication mechanisms employed within cells, but is indicated by the formation of IBs [53, 160, 188]. Further clarity will be required as to how other T cell phenotypes are affected by MeV infection. Overall longer-term autoregressive models conducted by Pezzotti et al. [128] of immunisation against 10 vaccine-preventable diseases over 115 years (spanning 1900 to 2015) indicated that immunisation can effectively reduce disease [128]. To this effect, it is now indicated in long-term studies that vaccine reduction of infections causing disease occurs in the order of diphtheria, MuV, VZV, and then MeV [128].
Despite the comparative success of immunisation against MeV to date, with lack of MeV antigenic variation, much remains obscure on a pathogen that has high transmission rates affecting predominantly infants under the age of 5. Alternatives to traditional vaccines are only now emerging, with microneedle patches registered in phase 1/2 by the Pan African Clinical Trials Registry (PACTR202008836432905) developed potentially soon to enter phase 3 clinical trials designed to counter MeV and RuV as alternatives to the initial MMR [189]. MeV was initially declared eradicated in the USA (2000), but also in the United Kingdom (2016) with outbreaks occurring after. More recently in 2020, a further five countries (Bhutan, the Democratic Republic of Korea, Maldives, Sri Lanka, Timor-Leste) are now considered to be leading the way in limiting MeV transmission for more than one year with B3, D4, D8, and H1 as the reported circulating MeV genotypes prior (see Supplementary materials). Given these similarities, the longer-term considerations of the benefit of immunisation are outlined above in scientific terms, much of which was unknown in 1954 upon the isolation of the MeV pathogen. However further research is required in future years.
AA: amino-acids
APC: antigen-presenting cell
BMEM: B memory cell
CD: cluster of differentiation
CXCL12: chemokine ligand 12
CXCR4: C-X-C chemokine receptor 4
DC: dendritic cell
DC-SIGN: dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
dLNs: draining lymph nodes
EC: endothelial cell
ECs: endothelial cells
F: fusion
Fc: crystallizable fragment
FGF: fibroblast growth factors
GCs: germinal centres
GF: growth factor
H: haemagglutinin
HHV: human herpes viruses
HLA: human leukocyte antigen
HNE: haemagglutinin protein noose epitope
HPSCs: haematopoietic stem cells
HSV: herpes simplex virus
IBs: inclusion bodies
IFN: interferon
Ig: immunoglobulin
IL: interleukin
LAV: live-attenuated virus
LFA-1: leukocyte functional antigen 1
LN: lymph nodes
M: matrix
mAb: monoclonal antibody
MCV: measles-containing vaccine
MeV: measles virus
MHC: major histocompatibility complex
MMR: measles, mumps, and rubella
MuV: mumps virus
Mϕ: macrophage cell
N: nucleocapsid
nAbs: neutralising antibodies
NHP: non-human primates
NiV: Nipah virus
NK: natural killer
pDC: plasmacytoid dendritic cell
PM: plasma membrane
PRRs: pattern recognition receptors
RuV: rubella virus
SLAMF1: signalling lymphocytic activation molecule 1
SNP: single nucleotide polymorphism
TC: cytotoxic T
TCM: central memory T
TFH: follicular helper T
TH: helper T
TLR: Toll-like receptor
TMEM: T memory
TN: naive T
TNF: tumour necrosis factor
TREG: regulatory T
VACV: vaccinia virus
VARV: variola virus
VZV: varicella zoster virus
WBCs: white blood cells
WHO: World Health Organization
wtMeV: wild-type measles virus
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1003167_sup_1.pdf.
The authors would like to thank: Jan Sheringham for editing and Enrique Chacon Cruz for expert advice, as well as the Queios community for valuable critique and input.
BB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing—original draft, Writing—review & editing, Validation, Visualization. CI: Data curation, Formal analysis, Methodology, Investigation, Writing—review & editing, Software. IF: Data curation, Formal analysis, Investigation, Methodology, Writing–review & editing, Resources, Software, Visualization, Supervision.
The authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
