Affiliation:
Hepatogastroenterology Division, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples 80138, Italy
Email: raffaele.pellegrino@unicampania.it
ORCID: https://orcid.org/0000-0001-5074-230X
Affiliation:
Hepatogastroenterology Division, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples 80138, Italy
ORCID: https://orcid.org/0000-0002-7367-4175
Affiliation:
Hepatogastroenterology Division, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples 80138, Italy
ORCID: https://orcid.org/0000-0002-4182-2858
Affiliation:
Hepatogastroenterology Division, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples 80138, Italy
ORCID: https://orcid.org/0000-0001-8049-0115
Explor Immunol. 2024;4:770–779 DOI: https://doi.org/10.37349/ei.2024.00171
Received: August 28, 2024 Accepted: November 12, 2024 Published: November 21, 2024
Academic Editor: Cunte Chen, South China University of Technology, China
The article belongs to the special issue The Role of Immune Checkpoint Molecules in Cancer and Hematological Malignancies
Immunotherapy, a primary anti-neoplastic treatment, exploits the patient’s immune system to kill neoplastic cells by modulating immune checkpoints such as cytotoxic T-lymphocyte antigen 4 and programmed cell death 1. Despite an apparent anti-neoplastic efficacy, immunotherapeutic agents are often accompanied by multiorgan toxicity, including gastrointestinal ones. This particular class of immunotherapy-related adverse events, mainly represented by diarrhea and colitis, necessitates a nuanced treatment strategy. Current treatments are primarily based on standardized severity grading systems to guide and proportion therapeutic interventions, ranging from simple behavioral modifications or conventional molecules (such as anti-diarrheal) to advanced biological treatments. Tofacitinib, a pan-Janus kinase inhibitor, emerged as a potential option for managing immune-related (IR) colitis by targeting hyperactivated T cells within the colic microenvironment. However, evidence supporting the use of tofacitinib in IR colitis is primarily derived from case reports and small case series, lacking robust randomized clinical trial data. While preliminary findings demonstrate encouraging clinical control of IR colitis with tofacitinib, further research is warranted to elucidate its efficacy, safety, optimal dosage, and treatment duration. Although there are some worries about its effects on cancer response and safety, current evidence indicates that tofacitinib could be seen as a possible treatment choice if other therapies with more robust evidence profiles have not been successful.
Immunotherapy is a form of anti-neoplastic therapy that stimulates the patient’s immune system, encouraging it to target and eliminate neoplastic cells by modulating two key immune checkpoints, namely cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) [1]. The immunotherapeutic agents currently employed include anti-CTLA-4 (i.e., ipilimumab), anti-PD-1 (i.e., nivolumab and pembrolizumab), and anti-PD-L1 (i.e., atezolizumab, avelumab, and durvalumab). These agents are indicated for various malignancies, including but not limited to melanoma, non-small-cell lung cancer, and tumors of the stomach and colon [1].
Nevertheless, despite a significant enhancement in the oncological prognosis of patients, these pharmacological agents are burdened by a multiorgan toxicity that also affects the gastrointestinal (GI) tract [2].
The role of tofacitinib, a Janus kinase (JAK) inhibitor, is increasingly emerging as a potential therapeutic agent of interest for managing complex cases of lower GI toxicity, particularly colitis, induced by immunotherapy [3].
This review aims to discuss and gather available evidence on using tofacitinib to manage lower GI toxicity (i.e., colitis) in patients undergoing immunotherapy to treat pre-existing neoplasia.
GI toxicity represents one of the most frequent forms of immune-related adverse events (irAEs), and it usually manifests with colitis or diarrhea, with the last one being the most frequent by far [4]. A recent systematic review weighted that diarrhea occurs in 35–40% of patients receiving an anti-CTLA-4 agent, 11–17% of those treated with anti-PD-1, and 32% treated with both agents. Colitis, on the other hand, occurs in 8–11% of patients treated with anti-CTLA-4, 0.3–3% of those treated with anti-PD-1, and 14% of those treated with combination therapy [5]. Diarrhea and colitis usually appear after the second or the third administration of the immunotherapy agent but can also occur as early as the first administration or after the end of therapy [2].
The severity of immune-related (IR) diarrhea and colitis is divided into four grades according to the Common Terminology Criteria for Adverse Events (CTCAE) [6], and according to the European Society for Medical Oncology (ESMO) guidelines [6]. This severity grading system guides the prognostic impact of immunotherapy-related damage. It determines the therapeutic approach’s significance, ranging from simple behavioral modifications in milder grades to systemic steroid therapies and advanced biological treatments in more severe and resistant forms.
Concerning diarrhea, the grading system is based on increased frequency of bowel movements, with grade 1 corresponding to less than four evacuations per day above baseline, grade 2 to an increase in bowel movement frequency beyond four but less than six above baseline, grade 3 to an increase beyond six above baseline, and grade 4 to life-threatening complications such as the onset of hematochezia, abdominal pain, significant dehydration, and fever. For colitis, the grading system relies on abdominal pain instead, with grade 1 corresponding to asymptomatic colitis (diagnosed by imaging, endoscopy or laboratory tests), grade 2 corresponding to mild-to-moderate abdominal pain, grade 3 to severe abdominal pain, and grade 4 to life-threatening complications [6].
Diagnosis of IR colitis can be difficult due to the lack of specific laboratory or instrumental hallmarks. The primary differential diagnoses of IR colitis are infection, diverticulitis, and tumor-related symptoms, e.g., intestinal metastases, peritoneal carcinomatosis, and radiation proctitis [7].
Endoscopic features of IR colitis include erythema, erosion, ulceration, and luminal bleeding. The inflammatory pattern has been observed to manifest as continuous inflammation in most patients treated with anti-CTLA-4 while displaying a patchy endoscopic distribution in patients undergoing treatment with anti-PD-1 agents [5].
Histologic specimens generally show acute inflammation characteristics, including lamina propria expansion, neutrophil infiltration, and crypt abscesses. Chronic inflammation features can often be seen, e.g., mononuclear cell infiltrate, basal plasmacytosis, granulomas, and distortion of crypt morphology [5, 8].
According to the ESMO guidelines [6], the management of IR colitis and diarrhea depends on the severity of the disease (according to the grading mentioned above) and ranges from symptom management to immunosuppressive agents. Management of grade 1 toxicity consists of symptomatic management (e.g., low-fiber diet, loperamide, psyllium) without discontinuing immune checkpoint inhibitors (ICI). For grade 2 colitis, ESMO guidelines recommend withdrawal of immunotherapy agents and administration of oral prednisolone at a dose of 40–60 mg per day. In grades 3 and 4, administration of intravenous corticosteroids (methylprednisolone 1 mg/kg/day) is recommended in addition to discontinuation of the ICI. In case of steroid therapy failure or recurrence of symptoms after corticosteroid withdrawal, additional immunosuppressant therapy can be considered. Infliximab (5 mg/kg, in a single dose or repeated on days 14 and 42) is by now considered the first choice after steroid failure. Vedolizumab (300 mg on days 1, 14, and 42) can be an alternative option; since it has a relatively slow response, it may not be the ideal treatment in most severe forms.
In cases where resistance is observed even to such advanced therapies, other interventions have been described, including fecal microbiota transplantation (FMT) and, in extreme cases, surgery [6]. However, no clear recommendation currently integrates the use of other advanced pharmacological agents, such as novel small molecules (e.g., tofacitinib).
Another critical point for deep discussion is the relationship between restarting ICI after a case of IR colitis, especially in cases graded two or higher, and the risk of recurrence after halting colitis treatment and resuming immunotherapy. Tran et al. [9] examined a large patient cohort in a meta-analysis (over twelve thousand patients), identifying a permanent discontinuation rate of ICI following lower GI toxicity in 48.1% of patients. In 48.6% of cases, ICI were reintroduced after only temporary suspension, and among this plethora of patients, 17% experienced an IR diarrhea/colitis recurrence. Moreover, in another large pharmacovigilance observational study, Dolladille et al. [10] also highlighted that among various IR adverse events, IR colitis (OR 1.77) is one of the forms of immunotherapy toxicity associated with an increased risk of recurrence following ICI rechallenge. This underscores the need to carefully assess the necessity of introducing additional immunosuppressive agents early on when dealing with recurrent hard-to-prevent cases of IR colitis [11]. Another significant area of research where we require more evidence is a comparison of recurrence rates of IR colitis following treatment with advanced therapeutic agents (i.e., biologics and small molecules) to identify which of the available options provides the most significant benefit and preventive power in this context.
Tofacitinib (Xeljanz®, Pfizer Inc.) is a small molecule that acts pharmacodynamically by non-specifically inhibiting molecules belonging to the JAK family, although it demonstrates a specific preference for JAK1 and JAK3 [12].
In in vitro enzymatic assays, tofacitinib can inhibit JAK1, JAK2, and JAK3 with half-maximal inhibitory concentrations ranging from 1.6 nmol/L to 3.2 nmol/L. However, it also exhibits inhibitory activity against non-receptor tyrosine-protein kinase 2 (TYK2, 34 nmol/L) [13]. By inhibiting these kinases, tofacitinib can thus impede downstream signal transduction mechanisms that rely on signal transducer and activator of transcription (STAT), which, through phosphorylation events, promote the synthesis of pro-inflammatory interleukins (ILs) and other cytokines (i.e., IL-2, IL-4, IL-6, IL-7, IL-15, IL-21, interferon-α, and interferon-γ) [12].
Tofacitinib is approved for treating ulcerative colitis, rheumatoid arthritis, psoriatic arthritis, polyarticular juvenile idiopathic arthritis, and ankylosing spondylitis [14].
Although the exact pathogenetic mechanism underlying ICI-induced colitis has not yet been fully elucidated, a key role seems to be represented by the hyperactivation of T cells (most of all tissue-resident memory T cells), which invade the lamina propria of the colic mucosa [15–18]. Furthermore, the immunologic profile of ICI-induced colitis appears to differ depending on the immunotherapy agent used, with an increased number of CD4+ T cells found in the lamina propria in anti-CTLA-4-induced colitis and an increase in CD8+ T cells in the lamina propria and epithelium in anti-PD-1 colitis [16]. In this setting of enhanced T cell activation, tofacitinib has been shown to reduce the number of activated CD8+ tissue-resident memory T cells in the context of colic mucosa, thus emerging as a valuable therapeutic option in the management of IR colitis [17].
The blockade of the JAK-STAT and JAK-TYK2-STAT pathways induced by tofacitinib, which, being a pan-JAK inhibitor, exerts a broad-spectrum inhibition of these systems, and can potentially counteract the hyperactivation of the T-cell compartment (especially CD4+ and CD8+ cells) in the pathogenic microenvironment of IR colitis by downregulating the pro-inflammatory cytokines responsible for the damage, which had previously been upregulated by immunotherapy stimulation [3]. Notably, in-depth flow cytometry data show that in patients with IR colitis and activation of T cells (predominantly CD8+ CD103+ T resident memory cells), six weeks of tofacitinib treatment results in a significant reduction in T-cell activation and response [17]. This occurs through the downregulation of mediators of the pathways as mentioned above (e.g., JAK1, 3, and STAT1–5) [17].
A possible consideration arising from using tofacitinib and other JAK inhibitors is their possible interference with the ICI since they enhance immune system activity while tofacitinib opposes it. It has been shown that mutations in genes coding for JAK1 or JAK2 can cause resistance to PD-1 inhibitor therapy [19]. On the other hand, excessive JAK-STAT signaling and, specifically, hyperactivation of JAK1 by IL-6 is responsible for an increased expression of PD-L1, thus promoting cancer immune evasion [20]. The latter mechanism seems to predominate over the first one, explaining the results obtained in the published case reports and series.
Moreover, independent of the already described populations, it is well known that natural killer (NK) cells play a significant role in tumorigenesis and the anti-neoplastic response, contributing to the necroptosis of cancerous cells [21]. NK cells typically suppress tumors through CD-16-dependent antibody-dependent cellular cytotoxicity by releasing damaging enzymes such as granzyme and perforin. They also secrete pro-inflammatory cytokines like tumor necrosis factor (TNF) and interferon-γ or initiate tumor cell death through the Fas-Fas ligand pathway and the TNF-related apoptosis-inducing ligand pathway and its receptor [22]. Moreover, Figueroa-Romero et al. [23] demonstrated that, in vitro models of NK cells, as well as NK cells derived from healthy subjects and patients with amyotrophic lateral sclerosis, tofacitinib is capable of suppressing IL-15 and STAT1 in these cells, thereby protecting neurons from the cytotoxic damage induced by NK cells. It is, however, known that when cancer cells are targeted by NK cells, they can use interferon-γ-induced PD-L1 as a form of immune evasion [24]. This also occurs because interferon-γ induces upregulation of class I major histocompatibility molecules [25] and downregulates cell receptors for NK cells (such as NK group 2 member D) [26]. In this regard, Okita et al. [24] demonstrated that tofacitinib, in the context of non-small cell lung cancer cell lines, can block the interferon-γ-induced transition from a sensitive NK cell phenotype to a resistant one. This could be a potential mechanism to overcome tumor immune escape from interferon-γ-mediated immune response.
Lastly, the pathogenic implications related to the role of myeloid-derived suppressor cells (MDSCs) cannot be overlooked. These immature cells have immunosuppressive functions and are closely linked to cancer progression by facilitating escape from the anti-tumor immune response [27, 28]. Several pre-clinical studies on the relationship between MDSCs and tofacitinib are available. Nishimura et al. [29] demonstrated in murine models of inflammatory arthritis that tofacitinib reduced disease severity by increasing the population of these cells in the bone marrow of the mice. Similar results were reported by Sendo et al. [30] in a murine model of rheumatoid arthritis-associated interstitial lung disease, where tofacitinib improved this clinical condition by promoting the expansion of MDSCs in the lungs. Figure 1 summarizes these molecular considerations regarding the use of tofacitinib in the setting of IR colitis.
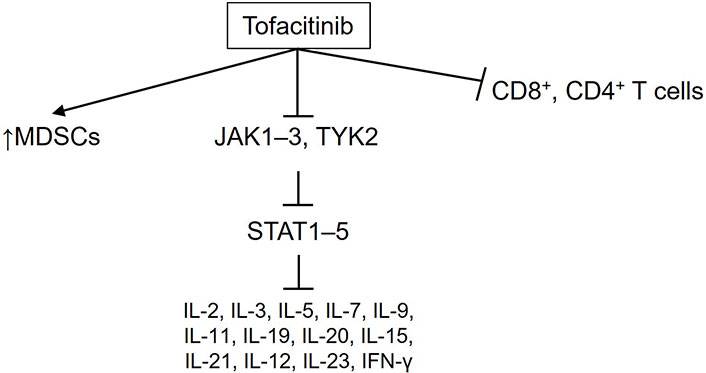
Key relevant molecular pathways modulated by tofacitinib, as discussed in this review, in the pathogenesis and progression of immune-related colitis. MDSCs: myeloid-derived suppressor cells; JAK: Janus kinase; TYK2: tyrosine-protein kinase 2; STAT: signal transducer and activator of transcription; IL: interleukin; IFN-γ: interferon-γ. ↑: increase in the production of these cells
Although the precise impact of interference between ICI and JAK inhibitors used concurrently has not been fully clarified, most of the available case reports [31–34] suggest that the use of tofacitinib has not altered the response to ICI while still being associated with the maintenance of cancer response to ICI.
Another central point in the clinical debate on using tofacitinib (and the whole JAK/TYK2 inhibitors family) is, so, its safety profile. It is indeed actually known that JAK inhibitors could increase cardiovascular and cancer risk in patients with pre-existing risk factors compared to TNF inhibitors, and warnings have been issued for infections and venous thromboembolism [35, 36]. This evidence has caught the attention of pharmacovigilance agencies; in fact, the European Medicines Agency (EMA) has recently recommended limiting the use of tofacitinib in patients older than 65 years of age, past or current smokers, or with other cardiovascular or cancer risk factors, to cases where no other treatment is fitting [37].
However, this datum has been reevaluated, considering that recent meta-analyses of real-world data have not reported major cardiovascular events or thromboembolic complications [38]. Additionally, subsequent analysis of tofacitinib’s registrational trials in ulcerative colitis has shown a thromboembolic events incidence rate lower than 0.05 [39].
These considerations have led to international consensus on managing thromboembolic risk in patients undergoing therapy with JAK inhibitors, and it has emerged that in patients with ulcerative colitis, tofacitinib does not increase the risk of major cardiovascular events. Furthermore, following current data, there does not appear to be an increased risk of venous thromboembolism in ulcerative colitis patients treated with tofacitinib (which, conversely, is dose-dependent in patients with rheumatoid arthritis) [40].
Another concept to remember is the neoplastic risk associated with chronic therapy with tofacitinib, which is relevant when this therapeutic agent is used in IR colitis (arising in patients already undergoing immunotherapy for a pre-existing neoplasm).
A recent meta-analysis evaluated the relative risk (RR) of cancer between tofacitinib and other control treatments, failing to achieve statistical significance (RR 1.06, P = 0.95), even when compared to placebo (RR 1.04, P = 0.95), although it demonstrated a slightly higher RR when conducting a head-to-head comparison with anti-TNF agents (RR 1.40, P = 0.02) [41].
These data suggest, albeit with the preliminary caution of awaiting further robust studies, a safety profile of tofacitinib regarding the risk of cancer that does not position it substantially differently from other available biological therapeutic agents.
There is a deficiency of high-quality evidence (e.g., derived from meta-analyses or placebo-controlled randomized clinical trials) regarding the use of tofacitinib (and generally for all JAK/TYK2 inhibitors) in IR colitis. Consequently, the available evidence is primarily drawn from small case series and case reports.
Esfahani et al. [31] reported in 2020 the case of a patient affected by gastric cancer (with mismatch repair deficiency subtype) treated and responsive to pembrolizumab, a PD-1 inhibitor. Although a good cancer response was obtained, recurrent IR colitis occurred during the treatment. The initial management with steroids, infliximab, and vedolizumab failed to reach the clinical remission of IR colitis. Therefore, tofacitinib 10 mg twice daily was administered, leading to clinical remission in 5 days. In addition, colitis remission was maintained four months after discontinuation and no recurrence of tumor activity was observed during or after therapy with tofacitinib.
Bishu et al. [32] published a case series including a total of four patients; three of them were treated with an ipilimumab/nivolumab combination for melanoma, while one received pembrolizumab and indoleamine 2,3-dioxygenase (IDO) inhibitor as adjuvant therapy for lung adenocarcinoma. Two of the three patients receiving ipilimumab/nivolumab and the one receiving IDO inhibitor developed steroid-dependent colitis during immunotherapy. All of them were initially treated with infliximab, with partial or any response, and then switched to tofacitinib (10 mg twice daily), reaching steroid-free remission in 4 to 6 weeks of treatment. Furthermore, all three patients had already achieved cancer remission before the start of tofacitinib therapy. The fourth case relies on a slightly different scenario since the patient was previously diagnosed with Crohn’s disease. After steroids, infliximab, and vedolizumab failure, the patient received a first treatment with tofacitinib 10 mg three times per day, which gained a complete remission by week six and then interrupted for cancer progression. When ICI were reintroduced, IR colitis recurrence occurred, and a new treatment with tofacitinib 10 mg twice per day was begun, obtaining remission in one month. In conclusion, all four patients attained clinical remission of IR colitis with tofacitinib administration; the three patients who achieved tumor remission at the initiation of tofacitinib therapy have sustained remission at subsequent follow-ups, whereas only the fourth patient experienced tumor progression while on tofacitinib.
In other instances, combination therapy involving FMT and tofacitinib has been reported. In Sasson et al. [17] experience, a patient developed IR colitis during treatment with pembrolizumab (in addition to carboplatin and pemetrexed) for non-small cell lung cancer. After steroid and infliximab failure, an attempt was made with the FMT. Despite an initial response to transplantation, colitis’s macroscopic and histologic features were maintained at endoscopic controls. Tofacitinib (10 mg twice daily) was then started, and remission was achieved after five weeks.
Similarly, in Holmstroem et al. [42], in a patient with IR colitis that occurred during ipilimumab/nivolumab combined therapy for metastatic melanoma, an initial approach with FMT was tried after “conventional therapy” failure. Again, there was an initial improvement in the clinical outcome followed by a re-increase in the number of bowel movements. Hence, tofacitinib (10 mg twice daily) was started with prompt clinical remission.
Sleiman et al. [34] presented the case of a patient with metastatic colon adenocarcinoma, microsatellite instability-high molecular subtype, treated with ipilimumab/nivolumab combination, who developed a recurrent IR colitis. Specifically, the first episode of colitis was successfully treated with steroids, but recurrence appeared during steroid tapering. During the second episode, the patient underwent therapy with prednisone and infliximab; remission was achieved, and no symptoms occurred during steroid tapering. Due to cancer progression, ICI therapy had to be resumed, but this led to a new IR colitis episode. This time, no response was obtained with steroids and infliximab, nor was remission observed by introducing vedolizumab. Thus, tofacitinib 10 mg twice daily was started, leading to symptom remission in 4 months.
Finally, in Sweep et al. [33], a patient treated for metastatic melanoma with ipilimumab and nivolumab received tofacitinib 10 mg twice per day for ICI-induced colitis after steroids, infliximab, and vedolizumab failure. Similarly to previous cases, symptom control was rapidly achieved, and complete clinical remission was maintained even after tofacitinib tapering.
In conclusion, IR colitis constitutes a significant proportion of immunotherapy-associated irAEs. A considerable number of IR colitis cases require advanced management with biological therapies beyond steroid therapy.
Leading therapies include infliximab and vedolizumab; however, more data are emerging for other mechanisms of action already employed in other inflammatory bowel diseases (primarily among inflammatory bowel diseases).
JAK inhibitors, particularly the pan-JAK inhibitor tofacitinib, show promising results from preliminary evidence in achieving reasonable, even rapid, clinical control of IR colitis. Nonetheless, randomized trials evaluating its efficacy and safety are still lacking. It is essential to consider the impact of JAK inhibitor treatment on cancer response control (although preliminary data from published reports provide reassuring insights).
Furthermore, clarifying the ideal dosage, treatment duration, and suspension criteria for this drug is necessary. While there are many pieces to the puzzle, current evidence may warrant consideration of this therapeutic molecule as a viable option following failures of molecules (e.g., infliximab) with more established evidence [6].
CTLA-4: cytotoxic T-lymphocyte antigen 4
ESMO: European Society for Medical Oncology
FMT: fecal microbiota transplantation
GI: gastrointestinal
ICI: immune checkpoint inhibitors
ILs: interleukins
IR: immune-related
JAK: Janus kinase
MDSCs: myeloid-derived suppressor cells
NK: natural killer
PD-1: programmed cell death 1
RR: relative risk
STAT: signal transducer and activator of transcription
TNF: tumor necrosis factor
TYK2: tyrosine-protein kinase 2
RP and AGG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Validation, Supervision. GP and GI: Investigation, Writing—original draft, Writing—review & editing, Validation. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest pertaining this article.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Luis Cabezón-Gutiérrez ... Vilma Pacheco-Barcia
Lingli Zhao ... Gaoli Niu
Rawaa AlChalabi ... Ahmed AbdulJabbar Suleiman
Qing Bao ... Hailin Tang
Fakher Rahim ... Issenova Balday
Neha Kannan ... Giuseppe Minervini
