Abstract
The tripartite network, including the nervous, immune, and endocrine systems, plays a significant role in regulatory and effector processes in the male body. On the one hand, males perform their reproduction function by generating spermatozoa in conditions of self-tolerance maintenance because most spermatozoa antigens (“sequestrated antigens”) are unknown to the immune system. On the other hand, in everyday life, a male body encounters hostile external infections, some of which colonize the skin and barrier surfaces and present a cancer threat to male genital tracts. This is human papillomavirus (HPV), the “silent killer”. Therefore, the male immune system has to function in a contradictory situation using either active immune responses, self-tolerance mechanisms, or both simultaneously. This review focused on the functional organization of the male immune system, including its coordination with the nervous and endocrine systems, and immune processes at the level of the whole organism, as well as on obvious changes, which have currently happened. The male immune system should function in conditions of the strong influence of testosterone and biosocial impulses coming from the nervous system. In the last century, researchers obtained data showing a decrease in the male reproduction function because of a stable negative dynamic of spermatozoa count and quality. Nowadays, depressing statistical indicators of male fertility have been published. 15% of couples are unable to conceive a child, where 50% of the causes of infertility relate to males, and up to 15% of male infertility cases are due to immunological disorders. It can be assumed that the male immune system starts to function when self-tolerance is partially lost, and the previous balance has been destroyed. Furthermore, sperm allergy has become a new topic in male immunology.
Keywords
Male immune system, “sequestrated antigens”, self-tolerance, male infertility, sperm allergyIntroduction
Nowadays, the immune system as a whole and its parts are no longer considered in isolation but are perceived by researchers as a mutual network with tripartite communication and three compartments [1]: immune, endocrine, and nerve. Lymphocytes [2], innate immune cells, endocrinocytes, neurons, neuroglia, cytokines, hormones [3], neurotransmitters [4], and neuropeptides are present in all parts of this triune mega system, and its molecules are even produced in all three compartments. This position is particularly relevant to the organs of sexual activity and reproduction of the male body [5], combining what may initially seem to be completely different functions of sexual pleasure, protection against peripheral invaders, and an odyssey of persistent opportunistic microbes [6], ensuring the extension of gender and building the biosocial career of an individual. Prominent thinkers and researchers of different fields of knowledge, such as Freud [7], Kinsey et al. [8], and Selye [9] put forward, based on the male sexual system, their theories, and humanity as the whole the basics of a phallocentric picture of the world, male dominance and the centuries-old dispute about male circumcision were born. In Westernized countries, the social sciences, adult behavior, and the upbringing of the next generation have challenged phallocentrism to a historic tournament. However, this wide discussion falls outside of the scope of this paper and may serve merely as background information. The purpose of this review paper is to assess and interpret published data on the male immune system combined with the reproductive system, their influence on fertility rates, relatively new data on the role of the prepuce in the transmission of infections linked with sexual activity, new understanding of the composition of cells and molecules contributing to the maintenance of self-tolerance, and new disorders in the male immune system.
Male immune system at a glance
The omics revolution opened up new opportunities for studying the immune system of the male reproductive tract, making it possible to categorize in a new way the composition and functional activity of all cells of innate immunity, T and B lymphocytes, all effector and regulatory molecules in connection with interaction with the endocrine and nervous systems and describe in more detail the course of the main immunological processes: innate immunity, adaptive immune responses, and auto- or self-tolerance. Taking into account the purpose and peculiarities of embryonic development of male genitalia, they are distinguished by the presence of several physiological barriers, the absence of organized lymphoid follicles in the distal part, which is unique for all other components of the mucosal immune system of the body, the presence of various cells of an initially mutual developmental lineage, and the presence of “sequestrated antigens”, normally unknown to the immune system.
Cells, molecules, and related processes in the male immune system
Under the epithelial cover of the penis and prepuce, there are many immune system cells, including macrophages, NK cells, dendritic cells, neutrophils, CD4+ T cells (most subsets, such as Th1, Th17, pTreg, effector CD4+ T cells), CD8+ T cells, γδT cells, etc., which take part in immune processes (see Figure 1). A composition of lymphocytes allows them the development of almost all possible immune processes, including immune inflammation, and many forms of programmed cell death or tolerance. The cells function here and may migrate to the draining inguinal lymph nodes [10]. The penis is innervated by the dorsal nerve, pudendal nerve, and cavernous nerve, and as for females, neuritis of N. pudendis can become a serious problem for a male person [11].
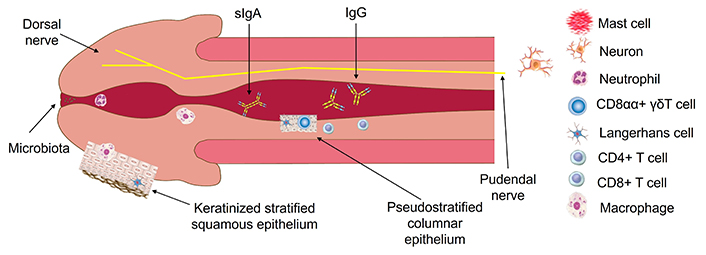
The penile immune system. The penis and prepuce (removed here) first meet the new microbiota, including hostile microbes, and other human bodies—sexual partners. Evolution provided male organs with strong protective properties to withstand danger whenever detected. The penile immune system consists of cells and molecules of: (1) innate immunity, such as neutrophils, type 1 macrophages, Langerhans cells, dermal dendritic cells, NK cells, γδT cells in the form of CD8αα subset, mast cells, etc.; (2) adaptive immune responses. Innate immunity protects the penis using the cells mentioned above and urgent defensive mechanisms, such as phagocytosis, pyroptosis, NK-cell-mediated apoptosis, “acute-phase” reaction, complement activation, etc. For one week, the immune system launches adaptive immune responses: (i) advanced B-cell-mediated pathway with the production of IgM, IgG, and IgA, which are directed against bacteria; (ii) CD8+ T cell and CD4+ T cell-mediated pathways, upregulated by Th1 and Th17, that protect against intracellularly located pathogens (preferably, viruses)
Note. Parts elements in Figure 1 are reprinted from [12], © 2019 Springer Nature Switzerland AG
Male testes serve as the spermatogenesis place [2, 3, 13], whereas epididymis is an intermediate point of remodeling [14, 15] on the path of spermatozoa from the testes to fertilization places in the female reproductive tract. All along this long path, spermatozoa must not be attacked by the defensive molecules of the male immune system, and after that, by the more aggressive factors of the female immune system. However, all the time both immune systems have to perform their main function to kill external microbial invaders, their own reactivated opportunistic germs, and newly appearing cancerous cells. Therefore, tests and epididymis are related to autotolerance zones where most autoantigens ontogenetically unfamiliar to the immune system (“sequestrated antigens”) should not be recognized and adaptive immune responses should not be directed against them with the formation of effector autoantibodies, autoreactive CD8+ and CD4+ T cells and lifelong memory cells [16]. This would be a pathway to persistent autoimmune inflammation and infertility. The structure of the epididymis is very complex and is characterized by the presence of at least two distinct sections, the caput and cauda [14, 17]. Such a gateway system is probably a kind of additional guarantee for maintaining self-tolerance. In general, the processes occurring in the epididymis correspond to the classical patterns of natural immunity, adaptive immune responses, and self-tolerance [18]. The end result, fertility or infertility, depends on a balance of all processes, which is most often disturbed by infection.
There is a difference in how the male and female immune systems function. In males, a weaker containment of external infections often falls short of the logical end result—the destruction of pathogens and complete clearance of the body from infection. In women, as a rule, the maximum containment of the pathogen by the forces of innate immunity and invader elimination by effectors of adaptive immune responses. However, women more often develop autoimmune disorders as another extreme of the functioning of the immune system. This transcriptional sexual dimorphism can be explained by many reasons: from genetic (for example, the presence of two X chromosomes) to the strongest influence of estrogens in contrast to androgens [19, 20].
Prepuce as the “Achilles’ heel” of the male immune system and whole male body
The foreskin and glans penis are not only a source of sexual pleasure but also the first to meet new microbiota, including hostile microbiota, and other human bodies as sexual partners. Evolution would have to endow them with strong protective properties to withstand danger whenever detected. Areas of the penis and the outer foreskin successfully cope with this task by being covered with a keratinized stratified squamous epithelium with its ancient protective keratinization mechanism. However, the inner leaf with the slowly keratinizing epithelium is essentially the “Achilles’ heel” of the male body, because it contains many receptors for such dangerous viruses as HIV and human papillomavirus (HPV), allowing them to enter deep into the tissue of the foreskin and transfer to another person. The presence of such receptors as CD4, CCR5, CXCR4, L1/L2 receptors [21], and EGFR [22, 23] promotes viral acquisition by penile tissues, which has lately been convincingly demonstrated [22, 23]. Male circumcision is a medical procedure, which is scientifically indisputably preventive in terms of reducing the spread of HIV and other sexually transmitted infections, as well as the penetration of pro-oncogenic types of HPV, which significantly diminishes the prevalence of cervical cancer in women, penis cancer in men, and oropharyngeal and colorectal cancer in both [24–31]. The WHO has shown in practice the success of a male circumcision strategy in terms of reducing the spread of HIV [32]. Furthermore, an expert assessment is known to consider that the prepuce is an evolutionary vestige [33–35] and tissue, which too often leads to medical problems.
Epithelial cells covering the male genitals make an essential contribution to innate immunity, as in other barrier organs. However, the epithelium of the male genitalia has another specific function—ensuring enjoyable sexual activity in contact with other people, and close exchange of microbiota, body fluids, and chemical substances of lubricants, creams, lotions, etc. At the evolutionary level, humanity is faced with an increased variety of such contacts, including homosexual ones; the emergence of new types of sexually transmitted infections; the proven influence of some viruses, such as HPV, on tumorigenesis in the male and female genital tracts; and concerned biosocial factors. From the dominant viewpoint of the medical community, an uncircumcised male should be aware of his responsibility for spreading the “silent killer” [36], which is HPV. Fortunately, another effective preventive method against HPV, vaccination has been developed and used for many years. There is no vaccine against HIV yet, however one 100% protection HIV antiretroviral medication is available now. Lenacapavir received breakthrough drug status because it uniquely inhibits several stages of the HIV-1 life cycle and shows the highest clinical efficacy [37].
Microbiota, or microbiome, exist in every barrier organ and spread even deeper than previously thought. For example, it is known that semen is not sterile. Based on the assessment of intestinal microbiota, all microbiotic components can be divided into two parts: (1) opportunistic, which is potentially dangerous to the body; (2) immunoregulatory or tolerogenic, which maintains a state of tolerance when there is no threat of external infection or reactivation of one’s own opportunistic microbes [38]. These useful bacteria such as Lactobacteria spp., Bifidobacteria spp., Clostridia spp., and Bacteroides spp. ferment dietary fibers to eight short-chain fatty acids such as propionate, acetate, butyrate, etc., which are maturation factors for two main cell lineages of the tolerance maintenance system, tolerogenic dendritic cells and peripheral pTreg cells [18]. Nowadays, with the advent of omics technologies, many species of penile microbiota, including bacteria, fungi, and viruses are differentially described in uncircumcised and circumcised males [6, 39–41]. From one point of view, since there are no own tolerogenic microbes in male microbiota, the required factors originate from the gut and, additionally, have to come from the female vagina rich in Döderlein’s flora like Lactobacilli [42]. This can mean that constant heterosexual activity is beneficial for male health.
In uncircumcised men, normal urethral microbiota contains Prevotella spp., Porphyromonas spp., Anaerococcus spp., Streptococcus spp., and Finegoldia spp., whereas in circumcised men, normal urethral microbiota includes Corynebacterium spp., Staphylococcus spp., and Gardnerella spp. [38, 41]. Regardless of circumcision, Prevotella spp., Streptococcus spp., and Corynebacterium spp. are prevalent in the male urethra [40]. An increase in penile microbiota Gardnerella spp. can influence the incidence of bacterial vaginosis in female sex partners [43]. Over 30 viruses are found in the male genital tract [6], and many of them persist in semen, fall into the urethra, and can be spread due to sexual activity.
Tolerance breakdown to sequestrated antigens in male infertility and evolutionary immune senescence in males
Nowadays, 12 months of regular unprotected sexual activity does not result in pregnancy in 15% of couples [44]. Up to 50% of infertility is related to men [45], and up to 15% of male infertility is caused by immunological origin [14], which promotes research in the male mutual immune and reproductive system. However, most studies on the pathogenesis of male infertility have been conducted in animal models [16]. These mechanisms, as shown by modern data, relate mainly to the breakdown of the immunological tolerance to those proteins of the male sexual system, which are so-called “sequestrated antigens” according to the classical research by the outstanding immunologist of all time Frank Macfarlane Burnet [46] and phenomenon clonal ignorance observed in several “immune-privileged” human organs [47, 48]. Such male pathologies as urethritis, prostatitis, varicocele, epididymitis, oligospermia, asthenospermia, and azoospermia are either secondary autoimmune or infection-caused disorders [45, 49], whereas any infection can contribute to immune tolerance breakdown, too. On the other hand, there is our understanding linking male infertility to the microbiota and data obtained by microbiomics and mass-spectrometry in the male reproductive system [44, 45].
The self-tolerance maintenance system includes many cellular and molecular components (see Table 1). The system works as a fine-tuned tool. Simultaneously, it has to downregulate adaptive responses to “sequestrated antigens”, and not affect the containment of obvious foes like pathogens.
Immune tolerance maintenance system [17]
| No. | Category | Members | References |
| 1 | Main cell lineages | Tolerogenic dendritic cells | [50, 51] |
| Peripheral (pTreg) Foxp3+ cells | [2, 45, 52] | ||
| 2 | pTreg subsets on mucosal surfaces (they do not express Foxp3) | Type 1 regulatory T cell (Tr1) | [53] |
| Type 3 helper T cell (Th3) | |||
| 3 | Other cell lineages | M2a macrophages | [54–56] |
| Myeloid-derived suppressor cells | [57] | ||
| Regulatory B cells | [58, 59] | ||
| 4 | Own body-derived molecules | Immunosuppressive cytokines (IL-10, TGF-β, IL-35) | [45, 60, 61] |
| Coinhibitory molecules | [62, 63] | ||
| Blocking antibodies | [64] | ||
| Protolerogenic neurotransmitters and neuropeptides | [4, 65–68] | ||
| 5 | Other | Tolerogenic microbiota | [38, 41] |
The main tolerogenic factors, including tolerogenic dendritic cells, peripheral regulatory Foxp3+ T cells, M2a macrophages, IL-10, TGF-β, IL-35, etc., [18] are present in the male reproductive system but undergo the weakening influence by the following mechanisms: (1) increasing the production of pro-inflammatory cytokines IL-6 and TNF-α, which act on sperm membranes, exposing their autoantigens, and, due to inflammation, activate fibrosis in the spermatogenesis zone; (2) decreasing immunosuppressive activity of regulatory T cells due to toxic tryptophan metabolites like kynurenines, which act on both effector and inhibitory T cells; (3) polarization of protolerogenic M2a macrophages in pro-immunogenic M1 macrophages [16].
Weakening of immune tolerance is closely connected with immune senescence, age thymic involution, and replacing naive T cells with memory T cells. In males, aging is also associated with erectile dysfunction, and a decrease in sperm count and quality, including viability, motility, and morphological criteria of spermatozoa [69]. Therefore, a low self-tolerance level does not allow the survival of a significant count of spermatozoa.
Many reports regarding the decreased sperm concentration and quality in Europe and worldwide have been published in the last 80 years [70–75]. They all confirmed the existing problem, which correlated with male infertility. However, there was no data on infertility history in all the research and the final conclusions. Generally, in the last 50 years, a 32.5% decrease in sperm concentration has been noted [70], and earlier, over another 50-year period, a significant decrease in the mean sperm count from 1.13 × 108/mL in 1940 to 6.6 × 107/mL in 1990 was reported [71].
Is 80 years too short a term for the evolution of a species? However, taking into account serious global changes on the planet, such as the loss of biosphere balance, climate change, a decrease in species biodiversity, and the appearance of new species like SARS-Cov-2 maybe it is time to think about the future of H. sapiens.
Tolerance breakdown to allergens, and allergic processes in the male immune system
Allergic disorders are found in the intimate tract in men quite often but are not a topic for wide research and discussion, although some of them have been published in serious immunological and andrological journals [76]. According to clinical signs, these disorders display most diverse, from local [77–80] to systemic manifestations [81–83], and their etiological nature may be unusual: semen, vaginal secretion, and chemical substances from hygienic products, latex particles from condoms, food products, household mites, textile dyes, and medications. In uncircumcised men, the prepuce can accumulate tiny pieces of these items and serve as a source of allergens because it is impossible to perfectly maintain the ideal cleanliness of the penis at the molecular level of hygiene. Also, a rare case of insect toxic-allergic reaction due to bites of Hymenoptera insects on the man’s penis has been described [84].
Allergic reaction to sperm
Allergy to sperm occurs not only in women but also in men either to their own or to the semen of other men. A case history of a patient who developed systemic reactions after anal intercourse has been published, although this patient never displayed sperm allergies after vaginal coitus. This case illustrated that the route of exposure to sperm may play a role in developing allergen tolerance or sperm allergies, including anaphylaxis [85].
In general, patients with intimate allergies are heterosexual men, men who have sex with men, and transgender people. However, an atopic constitution was identified in most of the total number of persons, and some of them already suffered from pre-existing atopic allergic pathology, along with an atopic hereditary burden.
Sperm allergy is an under-researched, poorly identified, and under-estimated disorder. Both mechanisms, IgE-mediated and type IV hypersensitivity, are possible either separately or in combination [86]. Nevertheless, type IV hypersensitivity has to be differentiated from mucosal manifestation of protein contact dermatitis [87]. Specification of proposed mechanisms is conducted using skin prick testing (SPT) [88], however, in most cases, the exact circumstances of suspect allergy in the male genital tract remain undetected [89]. The local allergic reaction to semen typically occurs within 30 min after exposure to sperm manifesting like the early-phase and rarer late-phase atopic response [90] by the following symptoms: vaginal or penile burning, itching, local urticaria, and redness. Unfortunately, there are no statistically reported rates on the prevalence, incidence, and prognosis of sperm allergy [91, 92]. Attempts of allergen-specific immunotherapy (AIT) with autological sperm in Gulf War veteran sexual couples with local reactions to sperm have been published [77]. There are also other publications [93, 94].
The sperm and spermatozoa contain a variety of glycoproteins, which participate in multiple processes in both male and female genital tracts. Additionally, sperm may be contaminated with a mixture of pro-inflammatory and anti-inflammatory factors, such as IL-6, IL-8, CCL-2 (MCP-1), CCL-5 (RANTES), TNF-β, CCL2 (MCP-1), TGF-β, and IL-10, and be a source of possible errors [95]. There are key criteria for the difficult diagnosis of sperm allergy, including the use of condoms, particularly made of lambskin, negative SPT with suspect culprit allergens, and a low value of specific serum IgE [81]. It took a while before Weidinger et al. [96] could isolate from seminal fluid a major allergen, which turned out to be a prostate-specific antigen (PSA) well-known to urologists as an essential diagnostic marker of prostate cancer.
Post-orgasmic illness syndrome
Currently, research continues according to one more sperm-associated disorder, which develops in males only, post-orgasmic illness syndrome (POIS), which was first described by Dutch researcher Waldinger [97].
To date, about sixty cases of POIS have been reported in the medical literature, but its prevalence, incidence, and treatment modality remain unknown [98–106]. The condition develops in both circumcised and uncircumcised men, at medium age, 34.07 ± 6.65 years, and with any exposure to sexual activity. POIS is closely connected to ejaculation and orgasm and develops right away after these events. The first symptoms are a flu-like feeling, nasal obstruction, conjunctivitis, perspiration, then extreme muscle weakness, incoherent speech, and headache. Most patients had a main symptom in common extreme fatigue [104]. The majority of symptoms last for about a week and disappear after that.
Sonkodi et al. [107] proposed a hypothesis of acute compression proprioceptive axonopathy in the muscle spindle and the influence of excitotoxic neurotransmitter L-glutamate [108]. It is possible that “neurogenic inflammation” has an influence here [109]. However, successful attempts of AIT have been described that may be considered in favor of the preferred atopic nature of the disorder [110, 111]. In addition, hormonal therapy, antihistamines, and Silodosin were used to result in full recovery. However, all these treatment modalities are experimental in nature and have not been assessed in placebo-controlled trials [105, 106].
Probably, POIS presents a combination of two phases of allergic inflammation, the early-phase atopy reaction and late-phase atopy inflammation-dependent condition, whereas the chronic phase is aborted [18].
Conclusions
The individual lifespan of a man depends on many factors but is regulated by the triune mega system, including the nervous, endocrine, and immune systems. The immune system of the genital tract belongs to the lower floor of the general mucosae-associated lymphoid network (MALT) with close communication with gut-associated lymphoid tissue (GALT). The immune system faces complex exclusive tasks: to repel external and internal enemies, and to ensure the inviolability of spermatozoa, whose antigenic composition is unknown to it. Spermatozoa are the key to the future life and survival of the species in the complicated conditions of the planet. However, as studies showed, over the past century, the male immune system no longer works in balance, develops undesirable processes such as sperm allergies, and steadily reduces the count of spermatozoa and their quality. As a result, male infertility rates are increasing, and human-wide indecision about the male circumcision problem and slow vaccination distribution leads to an increase in cancer and the spread of sexually transmitted infections. If the existing trends continue, then this will bring the species H. sapiens to the brink of survival.
Abbreviations
| HPV: | human papillomavirus |
| POIS: | post-orgasmic illness syndrome |
Declarations
Acknowledgments
The authors dedicate this article to thank Professor Alexander Gudkov, a prominent Russian urologist. Professor Gudkov did much to attract doctors of different medical communities to the immune system of men, particularly to the dependence of the favorable course of the postoperative period on a healthy immune system, and the need to be attentive to secondary immune-compromised and allergic conditions in the male reproductive system. In addition, he provided important insights into the analysis of this study.
Author contributions
VK: Conceptualization, Writing—original draft, Writing—review & editing. AK: Conceptualization, Writing—original draft, Writing—review & editing, Visualization.
Conflicts of interest
Both authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.