Affiliation:
1Anesthesia and Pain Management, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
Email: mcascella@unisa.it
ORCID: https://orcid.org/0000-0002-5236-3132
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0009-0004-5305-451X
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0000-0003-2806-2521
Affiliation:
1Anesthesia and Pain Management, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0000-0002-9349-7970
Affiliation:
3Unit of Anesthesia, ASL Napoli 1 Centro, 80145 Naples, Italy
ORCID: https://orcid.org/0000-0002-5986-3594
Affiliation:
4Anesthesia and Intensive Care, U.O.C. Fondazione Policlinico Campus Bio-Medico, 00128 Roma, Italy
ORCID: https://orcid.org/0000-0002-8962-5685
Affiliation:
5Medical and Experimental Head and Neck Oncology Unit, Istituto Nazionale Tumori Di Napoli, IRCCS “G. Pascale”, 80131 Naples, Italy
ORCID: https://orcid.org/0000-0001-8158-7509
Affiliation:
6Unit of Innovative Therapies for Abdominal Metastases, Istituto Nazionale Tumori Di Napoli, IRCCS “G. Pascale”, 80131 Naples, Italy
ORCID: https://orcid.org/0000-0002-2901-3855
Affiliation:
7Pharmaceutical Department, ASL Napoli 3 Sud, 80056 Naples, Italy
ORCID: https://orcid.org/0000-0001-9488-6057
Affiliation:
1Anesthesia and Pain Management, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0000-0002-0316-5930
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0000-0001-6280-8142
Affiliation:
8Epidemiology and Biostatistics Unit, Istituto Nazionale Tumori Di Napoli, IRCCS “G. Pascale”, 80131 Naples, Italy
ORCID: https://orcid.org/0000-0002-8455-3328
Affiliation:
9U.O.C. Odontostomatologia, A.O.R.N. A. Cardarelli, 80131 Naples, Italy
ORCID: https://orcid.org/0000-0003-0177-1301
Affiliation:
10Maxillofacial Surgery Unit, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples Federico II, 80138 Naples, Italy
ORCID: https://orcid.org/0000-0003-3947-8733
Affiliation:
11Department of Research, Fondazione Paolo Procacci, 00193 Rome, Italy
ORCID: https://orcid.org/0000-0002-3822-2923
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0000-0002-0232-1452
Affiliation:
2Oncology Unit, Department of Medicine, Surgery and Dentistry, University of Salerno, 84081 Baronissi, Italy
ORCID: https://orcid.org/0000-0001-6431-8278
Affiliation:
12The TRIAL (Try to Research and to Improve the Anticancer Links) Group, 82100 Benevento, Italy
Explor Immunol. 2024;4:802–821 DOI: https://doi.org/10.37349/ei.2024.00174
Received: April 28, 2024 Accepted: November 01, 2024 Published: December 04, 2024
Academic Editor: Cunte Chen, South China University of Technology, China
The article belongs to the special issue Immunology and Pain
Cancer-related pain represents one of the most common complaints of cancer patients especially for those with advanced-stage of disease and/or bone metastases. More effective therapeutic strategies are needed not only to improve the survival of cancer patients but also to relieve cancer-related pain. In the last decade, immune checkpoint inhibitor (ICI)-based immunotherapy targeting programmed cell death-1 (PD-1) and its ligand 1 (PD-L1) has revolutionized cancer care. Beyond its anticancer role, PD-1/PD-L1 axis pathway is involved in many other physiological processes. PD-L1 expression is found in both malignant tissues and normal tissues including the dorsal root ganglion, and spinal cord. Through its interaction with PD-1, PD-L1 can modulate neuron excitability, leading to the suppression of inflammatory, neuropathic, and bone cancer pain. Therefore, since the intricate relationship between immunotherapy and pain should be largely dissected, this comprehensive review explores the complex relationship between PD-1/PD-L1-based immunotherapy and cancer-related pain. It delves into the potential mechanisms through which PD-1/PD-L1 immunotherapy might modulate pain pathways, including neuroinflammation, neuromodulation, opioid mechanisms, and bone processes. Understanding these mechanisms is crucial for developing future research directions in order to optimize pain management strategies in cancer patients. Finally, this article discusses the role of artificial intelligence (AI) in advancing research and clinical practice in this context. AI-based strategies, such as analyzing large datasets and creating predictive models, can identify patterns and correlations between PD-1/PD-L1 immunotherapy and pain. These tools can assist healthcare providers in tailoring treatment plans and pain management strategies to individual patients, ultimately improving outcomes and quality of life for those undergoing PD-1/PD-L1-based immunotherapy.
Cancer arises from a complex interplay of genetic and molecular alterations that disrupt normal cell regulation, leading to uncontrolled growth and proliferation. These changes enable the immune system to recognize danger signals and organize an immune response [1]. Although T-cells are a key part of this response, the immune system’s full capability involves multiple cells and pathways. The term immunotherapy encompasses a wide variety of methods and therapies, including cytokines, vaccines, oncolytic viruses, adoptive cell transfer, and antibody-based immunotherapy [2]. Notably, immunotherapy-based strategies have been a game changer in anticancer treatment. Among the many types of immunotherapies, antibody-based immunotherapy targeting immune checkpoint pathways has become the most utilized in clinical practice for the treatment of cancer patients for its efficacy and safety. Researchers have identified and studied several pathways involved in the immune response that are targeted by immunotherapy. The programmed cell death ligand-1/programmed cell death-1 (PD-L1/PD-1) pathway is one of the most studied of them. PD-1 is a cell-surface receptor encoded by the PDCD1 gene. It acts as a checkpoint that regulates the immune response, preventing excessive and dangerous reactions [3]. Tumor cells can express PD-L1/PD-L2, which are ligands of PD-1. Briefly, the PD-1: PD-L1/L2 pathway inhibits the function of T-cells promoting the survival and proliferation of the tumor. Consequently, the disruption or blockade of these pathways leads to a stronger immune response. Since immunotherapy works by blocking the inhibitory signal from PD-1, T-cells can recognize and attack tumor cells (Figure 1).
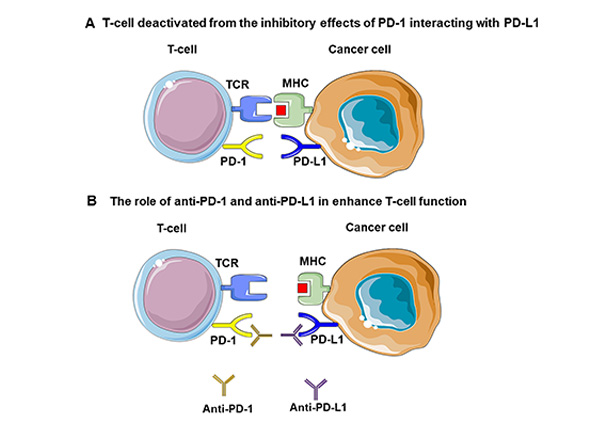
PD-1/PD-L1 interaction. (A) Tumor cells may express PD-L1 or PD-L2, which bind to PD-1 receptors on T-cells. This PD-1/PD-L1 pathway suppresses T-cell function, aiding tumor survival and growth. (B) Blocking this pathway through immunotherapy (anti-PD-1 and anti-PD-L1) unleashes T-cell activity, enabling the immune system to target and attack tumor cells more effectively. PD-L1: programmed cell death ligand-1; PD-1: programmed cell death-1; TCR: T-cell receptor; MHC: major histocompatibility complex. Parts of the figure were adapted from pictures provided by Servier Medical Art, licensed under CC BY 3.0
One of the most common complaints of cancer patients is pain. According to the revised definition of the International Association for the Study of Pain (IASP), pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage [4]. Evidence-based analyses suggest that the overall prevalence of pain in cancer patients is 64%. Yet, pain prevalence is higher in advanced-stage cancer patients (74%) and in those affected by bone metastases (83%) [5].
While offering significant advancements in cancer treatment, immunotherapy can be linked to a range of complications. These immune-related adverse events (irAEs) include inflammation and damage of vital organs like the lungs, liver, or colon, as well as autoimmune reactions where the immune system mistakenly attacks healthy tissues. Additionally, immunotherapy may lead to systemic side effects like fatigue, nausea, and flu-like symptoms, which can significantly impact patients’ quality of life [6]. On the other hand, PD-1/PD-L1 blockades have clearly been demonstrated to improve the quality of life of treated cancer patients as compared to standard of care [7, 8]. Among these challenges, the intricate relationship between immunotherapy and pain should be largely dissected. Immune cells, glial cells, and cancer cells can influence nociceptors to regulate pain [9]. The result is the potential occurrence of a wide range of painful clinical manifestations such as arthritis-type pain, as well as neuropathic and visceral pain [10]. A recent evidence-based analysis revealed that the use of targeted inhibitors might increase the risk of back pain while potentially lowering the occurrence of myalgia. Conversely, immune checkpoint inhibitors (ICIs) that target PD-1 in lung cancer patients may not lead to arthralgia or muscular pain [11]. Therefore, links between inflammation, peripheral neuropathy, cytokine release, direct tumor-immune system interaction, tissue damage, drug-related toxicity, and painful clinical manifestations require in-depth investigations. Moreover, the study of the PD-1 axis offers important research prospects in pain medicine. For example, emerging research suggests that PD-L1/PD-1 could be a promising neuroimmune target for pain relief [12].
The primary objective of this review is to thoroughly examine the relationship between PD-1-based immunotherapy and cancer-related pain. The aim is to understand in detail the underlying mechanisms through which PD-1 immunotherapy might influence pain pathways. Furthermore, we will discuss the clinical implications of these findings to optimize pain management strategies in cancer patients receiving immunotherapy. Ultimately, we aim to provide guidance on future research directions and improve the quality of patient care.
The PD-1 pathway is a crucial component of the immune system. It plays a vital role in inhibiting immune responses and promoting self-tolerance. PD-1 (also known as CD279) is a 55-kDa transmembrane protein that contains 288 amino acids with an extracellular N-terminal domain, a membrane-permeating domain, and a cytoplasmic tail located at the N and C ends, respectively, with two tyrosine bases [13]. PD-1 interacts with its ligands PD-L1 (also known as CD274) and PD-L2 (also known as CD273) [14, 15].
PD-1 can be expressed on the surface of many types of immune cells including B cells, monocytes, natural killer (NK) cells, macrophages, dendritic cells (DCs), monocytes, and activated tumor-specific T-cells. In addition, it is also expressed across inflamed tissues and in microglia and neurons [16, 17]. The functional PD-1 expression in peripheral sensory neurons influences neuronal excitability (Figure 2). Conversely, PD-L1 is expressed in immune cells and non-hematopoietic cells as well as in non-lymphoid tissues [18, 19]. In these cells, the expression of PD-L1 can be also induced by specific cytokines such as interferon-gamma (IFN-γ) [18]. Notably, PD-L1 is also found on the surface of many types of cancers and correlated with worse prognosis [20]. Noteworthy, under physiological conditions, PD-L1 mRNA is abundant in many tissues, but PD-L1 protein is scarcely expressed on the cell surface, indicating stringent post-transcriptional control of PD-L1 expression [6]. In contrast, PD-L2 is mainly expressed on professional antigen-presenting cells (APCs) such as DCs, and macrophages but can be also expressed on other types of cells based on the characteristics of the tumor microenvironment [15]. The different binding mechanisms of PD-L1 and PD-L2 to PD-1 may explain the differential contributions of these ligands to immune response [21]. The interaction between PD-1 and their ligands mediates a co-inhibitory signal which promotes the exhaustion of activated cytotoxic T-cells [22]. Because both PD-L1 and PD-L2 are mainly expressed in peripheral tissues, this co-inhibitory signal mainly modulates the immune tolerance within locally infiltrated tissues. As a result, the overexpression of PD-1 ligands by cancer and immune cells in the infiltrated tumor tissue promotes the exhaustion of activated cytotoxic T-cells. Using this mechanism, cancer cells can escape from activated T-cell-mediated destruction [23]. Disruption of PD-1/PD-L1 axis by restoring the exhaustion of activated cytotoxic T-cells promotes the T-cell-mediated killing of cancer cells [24]. In addition, PD-1/PD-L1 axis plays an important role in the regulation of development and activation of other types of immune cells such as Treg cells [25]. More in detail, this process is mediated by the downregulation of phospho-Akt, mTOR, S6, and extracellular signal-regulated kinase 2 (ERK2) and concomitant with the up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) [25]. Nevertheless, further studies are needed to clarify all the functions mediated by PD-1/PD-L1 axis.
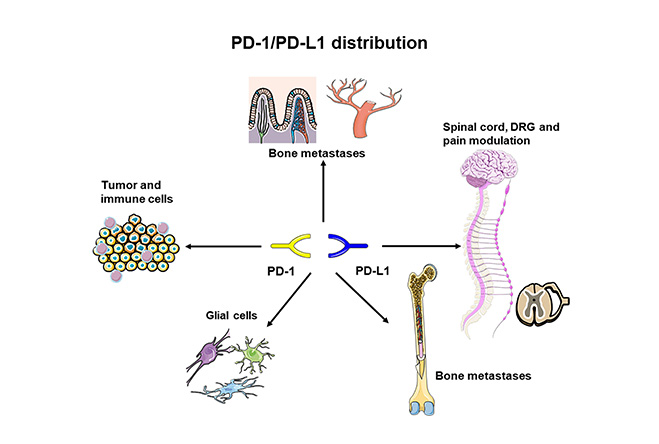
PD-1/PD-L1 tissue distribution. PD-1/PD-L1: programmed cell death-1/programmed cell death ligand-1; DRG: dorsal root ganglia. Parts of the figure were adapted from pictures provided by Servier Medical Art, licensed under CC BY 3.0
Based on this biological rationale, novel drugs also called “Immune Checkpoint Inhibitors (ICIs)” targeting PD-1/PD-L1 axis have been developed for the treatment of cancer patients [26]. This innovative immunotherapeutic approach has radically changed the management of many types of solid tumors. Indeed, ICIs have demonstrated to improve the survival outcomes as well as the quality of life of cancer patients as compared to standard of care. Many clinical trials have led to the approval for the clinical use of ten anti-PD-1 antibodies (nivolumab, pembrolizumab, cemiplimab, sintilimab, camrelizumab, toripalimab, tislelizumab, zimberelimab, prolgolimab, and dostarlimab) and three anti-PD-L1 antibodies (atezolizumab, durvalumab, and avelumab) [27].
However, not all patients achieved a long-term clinical benefit from this novel immunotherapeutic approach. Many factors may influence the efficacy of anti-PD-1/PD-L1 immunotherapy. Emerging evidence suggests that environmental, dietary, and lifestyle factors as well as genetic differences influence the response to immunotherapy and the occurrence of the irAEs [28]. For instance, better clinical outcomes were reported in cancer patients with a fiber-rich diet. The latter increases the levels in the gut of the short-chain fatty acids (SCFAs) like butyrate. SCFAs in turn decrease the levels of proinflammatory cytokines and promote the immune cell differentiation and function by improving the efficacy of anti-PD-1/PD-L1 immunotherapy [29]. On the same line, some studies suggested that a ketogenic diet (the majority of energy was obtained from fats and proteins rather than carbohydrates) may improve the efficacy of ICIs [28–30]. More in detail, ketogenic diet promotes pro-immunomodulatory effects including the increase of CD8+ T-cells and decrease of the number Treg cells, the levels of interleukin (IL)-10, and the expression of CTLA-4 and PD-L1 [28–30]. Despite the role of diet, different studies have shown that obesity or overweight status improves the efficacy of ICIs in many types of cancers [31]. In contrast, other lifestyle factors such as alcohol consumption and smoking seem to negatively influence the efficacy of ICI-based immunotherapy. However, the results in this field are contrasting [32]. Beyond environmental factors, individual genetic differences can also impact ICI efficacy as well as the occurrence of irAEs [33, 34]. For instance, in our previous study we have demonstrated that single nucleotide polymorphism (SNP) in PD-L1 effectively predicts which advanced NSCLC patients achieved a clinical benefit from anti-PD-1/PD-L1 immunotherapy [35].
However, further comprehensive studies are needed to understand how the environmental, dietary, and lifestyle factors as well as genetic differences influence the efficacy of ICIs and the occurrence of irAEs.
The mechanisms underlying pain modulation by PD-1 immunotherapy remain a subject of ongoing research and exploration. Several potential mechanisms and interactions may contribute to this phenomenon. Preclinical findings from pain models suggest that the PD-1/PD-L1 pathway is implicated in pain mechanisms in the dorsal root ganglia (DRG), sciatic nerve, and spinal cord dorsal horn (SDH) [36, 37]. In addition to inflammatory and neuroinflammatory processes, there may also be a neuromodulation process, direct interaction with the opioid system as well as the impact on bone resorption and bone pain.
Inflammation is a set of complex changing responses to tissue injury primarily caused by toxic chemicals, environmental agents, trauma, overuse, or infection [38]. Neuroinflammation is a type of localized inflammation occurring in the peripheral nervous system (PNS) and central nervous system (CNS). Examples include multiple sclerosis, Guillain-Barré syndrome, Alzheimer’s disease, meningitis, peripheral neuropathy, and Parkinson’s disease [39]. Moreover, neuroinflammation underlies cognitive impairments related to chemotherapy (known as “chemobrain”) [40] as well as cognitive alterations following surgery (postoperative cognitive dysfunction) [41]. Collectively, in these disorders, inflammation plays a key role in disease progression and symptom development.
This complex phenomenon is associated with leukocyte infiltration, activation of glial cells, and production of inflammatory mediators such as chemokines [CXCL1, chemokine C-C motif ligand 2 (CCL2), and CX3CL1], proteases [matrix metalloproteinase-9 (MMP-9), cathepsin S, and caspase-6] [42], and the canonical (β-catenin) and non-canonical (e.g., the calcium and the planar cell polarity) Wnt signaling pathways [43]. In this complex scenario, neuroinflammation plays an important role in chronic pain maintenance via neuron-glial and neuron-immune cell interactions [44]. Under physiological conditions, glial cells maintain the homeostasis of electrolytes such as potassium, and glutamate, in CNS and PNS, protecting against excessive nociceptor stimulation due to the release of inhibitory mediators. When glial cells can no longer maintain homeostasis, gliopathy is established. Therefore, dysregulated activation of glial cells serves as an amplifier of pain, by producing proinflammatory and pronociceptive mediators upon activation, presumably initiated by neuronal signals. They enhance pain states via activation of key signaling pathways, such as the mitogen-activated protein (MAP) kinase pathways. Astrocytes release ATP and glutamate in response to the activation of hemichannels involved in cell-to-cell communication and signaling such as connexin 43 (Cx43) and pannexin 1 (PNX1), as well as the purinergic receptor P2X7 which plays a critical role in the immune response, cytokine release (e.g., IL-1β), and apoptosis processes [45]. Moreover, different glial mediators such as TNF-α, IL-1β, IL-6, CCL2, and the brain-derived neurotrophic factor (BDNF), modulate excitatory and inhibitory synaptic transmission even at low concentrations. Additionally, other glial mediators such as ATP, CCL2, IFN-γ, basic fibroblast growth factor (bFGF), and MMP-2 can further activate glial cells [46]. On the contrary, the production of anti-inflammatory and antinociceptive mediators by glial cells leads to the resolution of acute pain; the failure of this mechanism is part of the complex transition from acute to chronic pain [47]. The PD-1/PD-L1 axis is involved in this convolute process. For example, in murine models of experimental autoimmune encephalomyelitis, PD-L1 is upregulated on microglial cells, astrocytes, and infiltrating mononuclear cells near the meninges, particularly in regions with the strongest inflammatory response [48]. Significantly, the M2 polarization refers to a specific activation state of microglial cells, which shifts them towards an anti-inflammatory and tissue-reparative phenotype. When microglial cells undergo M2 polarization (anti-inflammatory or regenerative phenotype), they produce anti-inflammatory cytokines, promote tissue repair, and support neuronal health. This is in contrast to the M1 state where microglia exhibit a pro-inflammatory response (pro-inflammatory phenotype). Consequently, M2 polarization is associated with reduced inflammation and is beneficial for maintaining a balanced immune response and protecting neural tissue. The interactions between PD-1 and PD-L1 suppress the expression of pro-inflammatory cytokines by activating microglia and inducing the immunoregulatory M2 polarization of microglial cells [49].
Primary nociceptors share some characteristics with immune cells and interact with them. These nociceptors express key immunomodulators, facilitating communication between the nervous and immune systems. In the context of cancer pain mechanisms, a cascade of events leads to increased hypersensitivity and excitability of nociceptive neurons, resulting in heightened pain perception. These events can be triggered by factors associated with tumor growth and its interaction with the surrounding tissue. Tumor-induced inflammation via inflammatory mediators such as cytokines (e.g., IL-1, IL-6) and chemokines can sensitize nociceptive neurons, making them more responsive to stimuli [50]. Moreover, cancer cells can release growth factors like nerve growth factor (NGF) and vascular endothelial growth factor (VEGF), which can directly activate and sensitize nociceptive neurons. NGF, in particular, is known to enhance pain signaling by binding to its receptor (TrkA) on sensory neurons. Additionally, tumor growth can create an acidic microenvironment due to anaerobic metabolism. The lower pH can activate acid-sensing ion channels (ASICs) on nociceptive neurons, leading to increased excitability and pain. Other processes such as the upregulation of voltage-gated sodium channels, the sprouting of sensory neurons with increased density of nociceptive fibers in the tumor region, and the amplification of pain signals, facilitate the development of chronic pain [51, 52]. Even the PD-1/PD-L1 pathway affects neuron excitability and suppresses it in DRG through the modulation of sodium and potassium channels and other mechanisms [2]. In this regard, the Chen et al. [36] study on wild-type (WT) (unaltered genome) and Pdcd1-deficient mice, also known as Pd1−/− mice, demonstrated that PD-L1 is an endogenous inhibitor of pain, inducing analgesia through PD-1. They also showed that altered pain sensitivity in Pd1−/− mice is not a result of developmental defects in sensory neurons. As a matter of fact, in naive Pd1−/− mice baseline pain sensitivity is increased, suggesting that PD-1 has an essential role in regulating basal pain sensitivity. Other findings are of paramount importance. For example, PD-1/PD-L1 expression affected nociceptive neuron excitability, producing significant analgesic effects in both mouse and human studies. Zhao et al. [53] noted that PD-1 agonists, including PD-L1 and H-20, trigger the phosphorylation of Src homology 2 domain-containing tyrosine phosphatase-1 (SHP-1). This process can modulate neuronal excitability and alleviate both acute and chronic pain. They also suggested PD-1 as a new candidate target for designing analgesic peptides. This effect can be beneficial in controlling excessive immune activity and pain processing, but it may also potentially suppress anti-tumor immunity. Conversely, blocking of the PD-1/PD-L1 pathway activation can induce spontaneous pain and hypersensitivity. Furthermore, PD-1/PD-L1 signaling potentiates the TREK2 potassium channel. These modifications of sodium and potassium channels are regulated by the tyrosine phosphatase SHP-1, which is activated by PD-L1 in DRG neurons via phosphorylation [54]. Interactions with the PD-1 axis have also been explored in the context of chemotherapy-induced neuropathy. In the development of paclitaxel-induced neuropathy, there is an observed increase in PD-L1 expression in macrophages in the DRG [55]. This increase is dependent on the activation of Toll-like receptor 4 (TLR4) by paclitaxel [56]. TLR4 activation can lead to the activation of the NF-κB signaling pathway, which results in the expression of genes involved in inflammation and immune response. Moreover, TLR4 is also involved in pain modulation. Activation of TLR4 can lead to neuroinflammation and sensitization of nociceptive (pain-sensing) neurons, contributing to pain perception. Taken together, these findings demonstrated that the PD-1/PD-L1 pathway can produce antinociception through neuromodulation.
Nivolumab is an FDA-approved fully humanized IgG4 monoclonal antibody. It selectively targets PD-1 and has shown great success in treating melanoma, lymphoma, and lung cancer. Of note, nivolumab but not control human IgG, induced marked mechanical allodynia for 5 hours. The PD-L1-induced analgesic effects were blocked by both the mouse anti-PD-1 antibody RMP1-14 and nivolumab, suggesting that PD-L1 inhibits pain via PD-1 [36].
PD-1 mRNA and PD-1 protein are extensively found in neurons within the spinal cord and brain [36]. In SDH, lamina II interneurons play a crucial role in processing nociceptive (pain-related) signals. These interneurons are primarily excitatory and establish connections with primary C-afferent fibers and lamina I projection neurons [57]. The interactions within this nociceptive circuit enable the transmission and modulation of pain signals from peripheral sources to higher centers in the brain. This complex circuitry is essential for the detection, interpretation, and response to pain, making. Notably, blocking PD-1 with nivolumab may reduce GABA-induced currents mediated by the inotropic GABAA receptor in this neuronal circuit via ERK pathways [16]. This pathway is a key protein in the MAP kinase (MAPK) signaling circuit and plays an essential role in cell growth, differentiation, and survival. There is a strong link with neuroinflammation as ERK proteins, particularly ERK1 and ERK2, are activated by phosphorylation in response to various stimuli, such as growth factors, hormones, and cytokines [40–42]. Once activated, ERK proteins can move from the cytoplasm to the nucleus, where they regulate gene expression by phosphorylating transcription factors and other target proteins. In the context of neuroscience, ERK activation has been linked to neuronal plasticity, learning, and memory [58]. Additionally, ERK plays a role in regulating pain perception, as it is involved in the modulation of synaptic transmission and nociceptive signaling [59]. For instance, ERK activation in peripheral sensory neurons can enhance neuronal excitability and contribute to heightened pain sensitivity [36, 52]. Regarding the PD-1 axis, Jiang et al. [16] demonstrated that nivolumab binds to neuronal PD-1, leading to ERK activation and subsequent GABAergic inhibition. This mechanism probably also involves the complex neurokinin pathways as the neurokinin-1 receptor is expressed in a large percentage of projection neurons in the spinal lamina I which simultaneously expresses the Pd1 transcript. Interestingly, neurokinin is a family of neuropeptides including substance P, neurokinin A, and neurokinin B that play a key role in transmitting signals in the nervous system. These peptides, and the related pathway, are involved in various physiological processes, including pain perception, and inflammation [57].
Functionally, at the behavioral level, mice lacking Pd1 exhibited compromised GABA-mediated analgesia and anesthesia. This indicates that the absence of Pd1 can affect the normal pain-modulating effects of GABA in the nervous system, potentially leading to altered pain perception and response to anesthetics [16]. These findings suggest that the PD-1 axis may play a critical role in maintaining proper pain control and could have implications for pain management in clinical settings.
Opioids act by engagement of specific opiate receptors including mu, delta, kappa, and the not naloxone-sensitive nociceptin/orphanin receptor, respectively mu opioid receptor (MOP), delta opioid receptor (DOP), kappa opioid receptor (KOP), and nociceptin/orphanin receptor (NOP) [60]. These receptors are found predominantly in the CNS, and PNS, but are also present in vascular, cardiac, lung, gastrointestinal, and peripheral blood mononuclear cells [61]. Opioid agonists bind to G-protein coupled receptors, leading to changes in the conformation of the G-protein intracellular part, thus resulting in a reduction of cAMP by inhibiting adenylate cyclase. The net effect of these changes is cell hyperpolarisation and a reduction in neurotransmitter and mediator release [62].
Within the CNS, opioid-induced analgesia is produced by activation of MOP receptors in the midbrain. Here, MOP agonists can activate descending inhibitory neurons leading to greater neuronal traffic, or can directly inhibit nociceptive afferents in the periphery. In addition, they can indirectly inhibit spinal pain transmission and reduce spinal nociception [63].
It has been demonstrated that also PD-1/PD-L1 inhibition has effects on MOP channels. For example, Wang et al. [64] proved that anti-PD-1 treatment with nivolumab diminishes morphine antinociception in WT mice. They also demonstrated that administering nivolumab intrathecally blocked the antinociceptive effects of intrathecal morphine in non-human primates. Their investigations highlighted that PD-1 and MOR are co-localized in DRG neurons, spinal nerve axons, and spinal cord axonal terminals. Moreover, morphine-induced antinociception is diminished in Pd1−/− mice. Interestingly, different antinociceptive cellular actions such as suppression of calcium currents in DRG neurons, inhibition of spontaneous excitatory postsynaptic currents (sEPSCs) in spinal DRG neurons, and induction of outward currents in SDH neurons, were all compromised in the Pd1−/− preclinical murine model. Finally, nivolumab treatment in adult WT mice treated with morphine showed the same effect as morphine instillation in Pd1−/− mice [64]. The interaction extends beyond just receptor dynamics. Another preclinical investigation, conducted on a humanized NSG (NOD scid gamma) mouse model, revealed that chronic morphine administration elevates the number of circulating CD8+ T-cells expressing the inhibitory receptor PD-1. It suggests that chronic administration of MOR agonists might affect the immune system’s function, potentially leading to changes in responses to infections or other challenges [65].
The study of interactions between immunotherapy and opioids requires more in-depth investigation. Probably, the effects extend beyond molecular interactions. For example, in a study cohort of patients with recurrent/metastatic head and neck squamous cell carcinoma undergoing anti-PD-1 treatment, Scheff et al. [66] found that higher opioid use was linked to significantly shorter progression-free survival and overall survival, as well as a lower presence of CD8+ T-cells in the tumor microenvironment. A recent systematic review and meta-analysis confirmed these findings [67]. Botticelli et al. [68] proposed the possibility of a negative drug opioid-ICI interaction that could weaken the immune response to anti-PD-1/PD-L1 treatments.
The potential involvements of the PD-1 axis on pain processes are shown in Figure 3.
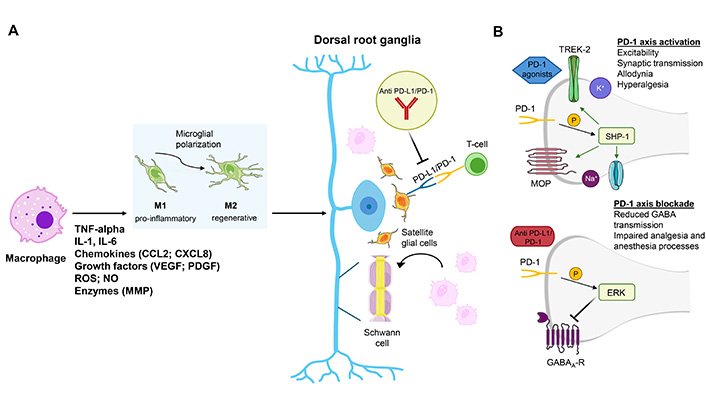
Links between the PD-1 axis and pain processes. (A) PD-1 and PD-L1 interactions influence neuron and glial cell function, potentially impacting chronic pain maintenance and pain perception. The PD-1/PD-L1 pathway plays a significant role in neuroinflammation. The interactions between PD-1 and PD-L1 suppress the expression of pro-inflammatory cytokines by activating microglia and inducing the immunoregulatory M2 polarization of microglial cells with pain modulation. (B) PD-1 axis involvement in DRG (and SDH, although not shown in the picture) excitability (via GABA processes or voltage-dependent channels), immune response, and its connection with opioid mechanisms (via opioid receptors) suggest complex interactions that can affect pain management and analgesia, including responses to immunotherapy. Specifically, the activation of PD-1 axis triggers the phosphorylation of SHP-1. Phosphorylated SHP-1 by modulating Na+ and K+ channels as well as MOPs reduce allodynia and hyperalgesia. In contrast, the blockade of PD-1 axis promotes the phosphorylation of ERK. Phosphorylated ERKs by reducing the GABA-mediated transmission induce an impairment of analgesia and anesthesia processes. IL: interleukin; CCL2: chemokine C-C motif ligand 2; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor; ROS: reactive oxygen species; NO: nitric oxide; MMP: matrix metalloproteinase; PD-L1/PD-1: programmed cell death ligand-1/programmed cell death-1; SHP-1: Src homology 2 domain-containing tyrosine phosphatase-1; MOP: mu opioid receptor; DRG: dorsal root ganglia; ERK: extracellular signal-regulated kinase; SDH: spinal cord dorsal horn. Parts of the figure were adapted from pictures provided by Servier Medical Art, licensed under CC BY 3.0
The majority of cancer pain is caused by bone metastases [69]. Bone cancer pain is mostly a consequence of osteolytic bone lesions caused by metastases from many forms of cancer. Different pathways and processes are involved in the genesis of bone cancer pain, for example, the over-activation of osteoclasts [70]. The receptor activator of nuclear factor-kappa B (RANK) is a receptor found on the surface of osteoclast precursors. When RANK is activated, it triggers a signaling cascade that leads to the differentiation, activation, and survival of osteoclasts. The ligand RANK-L is a protein that binds to the RANK receptor on osteoclast precursors, activating the signaling pathway. It is primarily produced by osteoblasts and other cell types such as T-cells. Furthermore, osteoprotegerin (OPG) is a decoy receptor that binds to RANK-L, preventing it from interacting with RANK. It inhibits the activation of osteoclast precursors and reduces bone resorption. Therefore, the RANK/RANK-L/OPG pathway is the main actor in osteoclastogenesis [71]. Nivolumab is a fully-humanized monoclonal antibody interfering with the PD-1/PD-L1 link by blocking PD-1 on immune cells. However, it remains unclear whether PD-1 blockade affects primary or metastatic bone cancer [72]. In a preclinical study, Pd1−/− mice, WT mice, and nivolumab-injected WT mice were inoculated into the intramedullary canal of femurs with Lewis lung cancer cells (LLC). From this study, Pd1−/− mice and nivolumab-injected WT mice perceived less pain than WT mice, so demonstrating that nivolumab has a protective effect on bone pain and destruction. Probably, PD-L1 promotes low-dose RANKL-induced osteoclastogenesis through c-Jun N-terminal kinase (JNK) activation and CCL2 secretion [71]. Therefore, in bone cancer, nivolumab, acting as an anti-PD-1 agent can interfere with osteoclast formation, attenuating bone pain.
Table 1 outlines the potential pain modulation mechanisms associated with the PD-1 axis.
PD-1 axis and pain modulation
| Mechanism | Finding | Effect | Reference |
|---|---|---|---|
| Neuroinflammation | Leukocyte infiltration, glial cell activation, and production of inflammatory mediators | Pain mechanisms in DRG, sciatic nerve, and SDH | [38–47] |
| PD-L1 upregulation on microglial cells, astrocytes, and mononuclear cells near meninges in high inflammation areas | Glial cells involvement | [48] | |
| PD-1/PD-L1 suppresses the expression of pro-inflammatory cytokines and induces M2 polarization. | Microglia activation | [49] | |
| Tumor-induced inflammation via cytokines and growth factors sensitizes nociceptive neurons. | Increasing hypersensitivity and excitability of nociceptive neurons | [50] | |
| Cancer cells create an acidic microenvironment that activates ASICs on nociceptive neurons. | Pain chronification | [51] | |
| Upregulation of sodium channels, sensory neuron sprouting, and pain signal amplification | Pain chronification | [36, 52] | |
| PD-1 agonists, (e.g., H-20) can modulate neuronal excitability and alleviate both acute and chronic pain. | PD-1 can be a target for designing analgesic peptides, but it may also potentially suppress anti-tumor immunity. | [53] | |
| PD-L1 expression in macrophages in the DRG and TLR4 activation | Potential effects on CIPN | [56] | |
| Neuromodulation | Nivolumab reduces GABA-induced currents in CNS neurons. | Impact of the PD-1 axis on pain mechanisms | [16, 36] |
| Effects on opioid mechanisms | PD-1 and MOR co-localization in DRG neurons, spinal nerve axons, and spinal cord axonal terminals | Anti-PD-1 treatment can affect opioid effects. | [60] |
| Chronic morphine administration elevates circulating CD8+ T-cells expressing PD-1. | Opioid-induced PD-1 expression | [61] | |
| Higher opioid use is associated with shorter PF and OS, and lower CD8+ T-cells in the tumor microenvironment. | Potential drug-drug interaction | [66–68] | |
| Effects on bone metastases | Nivolumab and Pdcd1-deficient mice experience less pain and destruction in bone cancer. | PD-1/PD-L1 pathway involvement in bone metastases and osteoclasts | [71] |
| PD-L1 promotes osteoclastogenesis through JNK activation and CCL2 secretion. | Nivolumab can block osteoclast formation. | [71] |
PD-1: programmed cell death-1; PD-L1: programmed cell death ligand-1; DRG: dorsal root ganglia; SDH: spinal cord dorsal horn; ASICs: acid-sensing ion channels; TLR4: Toll-like receptor 4; CIPN: chemotherapy-induced peripheral neuropathy; CNS: central nervous system; MOR: mu-opioid receptor; PF: progression-free; OS: overall survival; JNK: c-Jun N-terminal kinase; CCL2: chemokine C-C motif ligand 2
The relationship between PD-1/PD-L1-based immunotherapy and cancer-related pain presents several clinical implications and challenges that healthcare providers must address to optimize patient care. To this end, preclinical and clinical research must be developed, and the potential of artificial intelligence (AI) must be harnessed.
Effective pain management in patients undergoing PD-1/PD-L1-based immunotherapy requires a thorough understanding of the underlying mechanisms through which these treatments may influence pain perception, either alleviating or exacerbating pain. Therefore, there is a need for further research to elucidate the specific mechanisms by which PD-1/PD-L1 immunotherapy modulates pain, as well as to identify biomarkers that could predict patients at higher risk for pain-related complications. For this aim, preclinical research plays a key role, and ad hoc pain models should be developed. A captivating area of research examines how analgesic pathways intersect with other processes that contribute to the chronicity of pain. For instance, studies have shown that the PD-L1/PD-1 checkpoint pathway influences hippocampal neuronal excitability, as well as learning and memory behavior [73].
Clinically, vigilant monitoring and prompt intervention are crucial to manage pain and irAEs. Comprehensive pain assessment through validated pain assessment tools and questionnaires is mandatory. Furthermore, clinical research must be focused on identifying patterns and potential correlations between treatment and pain as well as therapy-induced complications such as chemotherapy-induced peripheral neuropathy (CIPN) and other manifestations of neuropathy. Gathering large datasets is essential to examine the interactions between ICIs, opioids, and other analgesics. To achieve this, AI-based data-driven approaches are necessary. High-quality clinical studies are also required to perform evidence-based medicine (EBM) analyses [74].
Patient monitoring and feedback regarding pain levels and potential side effects can help adjust treatment plans and pain management strategies in real-time. More importantly, the collection of prospective data and quality of life assessments, known as patient-reported outcome measures (PROMs), patient-reported outcomes (PROs), and patient-reported experiences measures (PREMs) is crucial to evaluate the effectiveness of treatments in pain control and in increasing the overall well-being of patients [75]. PROs provide patient reports of their health, quality of life, or functional status associated with the healthcare or treatment they received. PROMs are the tools used to measure PROs and are often self-completed (by the patient) questionnaires. PREMs are tools that report patient satisfaction scores with a healthcare service. They are generic tools that are often used to capture the overall patient experience in healthcare. PREMs are often used in a larger population and non-specific settings such as in the outpatient clinic [76]. However, the implementation of PROMs and PREMs in routine healthcare faces numerous obstacles. These include the inherent complexity of the healthcare system where if not well guided, many groups and individuals can prevent or inhibit adoption; problems of noise, data sharing (interoperability), and poor planning [77]. Addressing these challenges requires a multidisciplinary approach involving oncologists, pain specialists, researchers, and patients themselves.
AI can be very useful in advancing research and clinical practice in the context of PD-1/PD-L1-based immunotherapy and its relationship with cancer-related pain. For example, AI-based strategies can be implemented to analyze large structured and unstructured datasets, including patient records, imaging studies, and genomic data, to identify patterns and correlations between PD-1/PD-L1 immunotherapy and pain [78]. This can help in pinpointing potential biomarkers and risk factors. For this aim natural language processing (NLP) methods can be used to analyze medical literature and clinical notes to extract insights related to PD-1/PD-L1 immunotherapy and pain [79, 80].
Moreover, AI algorithms can be used to create predictive models that assess the risk of pain-related complications in patients undergoing PD-1/PD-L1 immunotherapy. This could include identifying patients at higher risk and providing them with targeted interventions. Unsupervised machine learning (ML) models can represent a suitable strategy for clustering. In other words, these models can help healthcare providers tailor treatment plans and pain management strategies to individual patients. Efforts to use AI for analyzing ICIs have already been undertaken. For example, in an ML investigation on thyroid-related adverse events in patients receiving PD-1 or PD-L1 therapies, Kim et al. [81] found that opioid use was associated with a roughly 4.0-fold decrease in the risk of thyroid-related adverse events compared to those not using opioids. Among the models assessed, random forest stood out as the most effective for predicting thyroid-related complications, with an area under the receiver operating characteristic curve (AUROC) of 0.77. Other attempts focused on the prediction of cardiac adverse events [82] and adrenal insufficiency [83].
Clinical decision support systems can be implemented for this aim [84]. They are a set of tools designed to help healthcare providers make informed decisions at the point of care. These tools use data analysis, AI, and evidence-based knowledge to assist in diagnosing, treating, and managing patient conditions more effectively and efficiently. Nevertheless, AI methods can be used for simulation and modeling [85]. Briefly, AI can simulate various treatment scenarios and predict potential outcomes, helping researchers and clinicians understand the impact of different approaches on pain management.
AI plays a transformative role in the preclinical research phase, particularly by accelerating the research process and improving precision. The scientific method traditionally relies on interconnected and chronological techniques. Researchers faced challenges related to time constraints and human error, both of which are intrinsically linked. However, advancements in technology, including automation and AI, have significantly reduced time delays and minimized human error. In recent decades, the search for cellular molecular targets has opened numerous application fields. For example, molecular biology delves deeply into cellular mechanisms and cell-ligand interactions. In the field of bioinformatics, advanced molecular docking techniques, which study molecular structure and identify potential drugs using computer-assisted design, are now crucial for precision and efficiency in medicine [86]. This technique aims to predict molecular interactions based on three-dimensional structures [87]. For these aims, AI provides revolutionary tools that enhance prediction, streamline research, and facilitate the repetition of experiments, allowing researchers to address errors and restart experiments quickly (Figure 4).
Time-saving with AI-based molecular docking. Parts of the figure were adapted from pictures provided by Servier Medical Art, licensed under CC BY 3.0
Various software programs enable the study of protein interactions, enabling virtual drug screening and unprecedented time savings in research. For instance, ArgusLab is a free software that simplifies the study of molecular interactions and helps with molecular modeling and drug design [88].
Finally, AI and its subfields, such as ML, can be employed to improve patient monitoring and symptom management significantly. AI-driven wearable devices and mobile applications can track patients’ pain levels and other symptoms in real-time, providing valuable data for healthcare providers. This continuous monitoring enables adjustments to pain management strategies, allowing for personalized and adaptive care tailored to each patient’s needs [89]. Despite the numerous benefits, it’s crucial to consider the ethical implications and potential risks associated with the use of AI and ML in healthcare. Since data privacy is a significant concern, sensitive patient information must be protected from unauthorized access and misuse. However, the use of AI in medicine presents other paramount ethical dilemmas related to informed consent and bias as it must necessarily ensure the accuracy of the information used [90, 91].
Research challenges are summarized in Table 2.
Research challenges and perspectives
| Target | Need | Future directions |
|---|---|---|
| Clinical implications and challenges | PD-1/PD-L1-based immunotherapy presents challenges in managing cancer-related pain and requires attention from healthcare providers. | Developing targeted pain management strategies to improve patient care |
| Patient monitoring and feedback are crucial for adjusting pain management strategies in real-time. | PROMs, PROs, and PREMs to gather data on patient experiences and outcomes | |
| Ameliorate diagnosis of pain phenomena (e.g., neuropathic pain) | Fostering collaboration across multidisciplinary teams to address pain management challenges | |
| Preclinical research | Mechanisms through which PD-L1/PD-1 immunotherapy modulates pain | Developing ad hoc pain modelsExploring the intersection of analgesic pathways and chronic pain processesIdentifying specific mechanisms and biomarkers to guide targeted pain management |
| Clinical research | The link between immunotherapy and pain conditions | Comprehensive data collection, longitudinal studies EBM analyses |
| Artificial intelligence | Artificial intelligence can be used to:
| |
Addressing ethical issues and risk of bias. PD-1/PD-L1: programmed cell death-1/programmed cell death ligand-1; PROMs: patient-reported outcome measures; PROs: patient-reported outcomes; PREMs: patient-reported experiences measures; EBM: evidence-based medicine
Cancer-related pain can hugely impact the quality of life of cancer patients. Understanding the complex interplay between immunoregulation and cancer pain is crucial to defining strategies to improve the quality of life of cancer patients. Novel evidence demonstrated that PD-1/PD-L1 axis plays an important role in the modulation of cancer pain through: i) neuroinflammation, ii) neuromodulation, iii) interacting with opioid receptors, and iv) interfering with bone processes. As a result, the PD-1/PD-L1 axis alterations mediated by anti-PD-1/PD-L1 immunotherapy should be deeply investigated. In this perspective, molecular pathological epidemiology may be a useful tool to achieve this purpose.
Immunotherapy itself can induce pain as a side effect through inflammatory responses or autoimmune reactions. For example, ICIs may induce or exacerbate neuropathic pain due to inflammation or direct effects on nerve cells. However, preclinical findings underscore the potential beneficial effects of immunotherapy on pain, for example in bone pain. Therefore, the impact on the PD-1/PD-L1 signal can modulate neuronal excitability, resulting in both analgesic and pro-nociceptive effects.
In addition, some evidence has reported that lifestyle factors can influence the efficacy of PD-1/PD-L1 immunotherapy as well as the mechanisms underlying cancer-related pain. Nevertheless, whether the modulation of cancer-related pain by lifestyle factors is mediated by PD-1/PD-L1 axis should be further investigated.
In this uncertainty, clinicians must optimize pain management in patients undergoing immunotherapy, aiming to improve their quality of life.
In this way, PROs, PROMs, and PREMs should play a major role in the clinical practice to improve the management of cancer-related pain in patients treated with immunotherapy.
Future challenges include deepening our understanding of pathophysiological dynamics and gathering data to develop personalized, and multidisciplinary therapeutic approaches tailored to different stages of the disease and various therapies. Different AI and ML strategies have the potential to play a pivotal role in this process by enabling advanced data analysis, modeling complex biological interactions, and identifying patterns within large datasets. This can lead to the development of predictive models and decision-support tools that help customize treatment plans for individual patients, enhance disease monitoring, and optimize therapeutic outcomes.
AI: artificial intelligence
CCL2: chemokine C-C motif ligand 2
CNS: central nervous system
DCs: dendritic cells
DRG: dorsal root ganglia
ERK2: extracellular signal-regulated kinase 2
ICIs: immune checkpoint inhibitors
IFN-γ: interferon-gamma
IL: interleukin
irAEs: immune-related adverse events
MAP: mitogen-activated protein
ML: machine learning
MMP-9: matrix metalloproteinase-9
MOP: mu opioid receptor
NGF: nerve growth factor
OPG: osteoprotegerin
PD-L1/PD-1: programmed cell death ligand-1/programmed cell death-1
PNS: peripheral nervous system
PREMs: patient-reported experiences measures
PROMs: patient-reported outcome measures
PROs: patient-reported outcomes
RANK: receptor activator of nuclear factor-kappa B
SCFAs: short-chain fatty acids
SDH: spinal cord dorsal horn
SHP-1: Src homology 2 domain-containing tyrosine phosphatase-1
TLR4: Toll-like receptor 4
WT: wild-type
The TRIAL Group: Ciro Emiliano Boschetti, Alessandra Bracigliano, Giuseppe Buongiorno, Lucia Cannella, Ester Calogero, Roberta Carella, Laura Cascella, Vincenzo Cascella, Francesco Casto, Alessio Cirillo, Ottavia Clemente, Vincenzo Damiano, Matteo De Simone, Rosario De Feo, Giuseppina Della Vittoria Scarpati, Simone D’Ambrosio, Ida D’Onofrio, Morena Fasano, Giulio Ferone, Pierluigi Franco, Daria Maria Filippina, Luigi Pio Guerrera, Franco Ionna, Davide Leopardo, Francesco Longo, Maria Grazia Maglione, Roberto Manzo, Luisa Marciano, Maria Grazia Massaro, Aurora Mirabile, Massimo Montano, Costanza Pagliuca, Luca Perna, Arianna Piccirillo, Monica Pontone, Fabiana Raffaella Rampetta, Vincenzo Ricci, Lorenzo Triuzzi, Pasquale Vitale, Alessia Zotta, Antonio Grimaldi
M Cascella, FS, and GV: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. BM, CG, LS, MM, and LF: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. OP, SP, and FG: Validation, Writing—review & editing. FP, AO, M Capuozzo, AG, and GS: Supervision. FM and AC: Software. AM and LL: Visualization. All members of The TRIAL Group: Supervision. All authors read and approved the submitted version.
Giustino Varrassi, the Editorial Board Member and a Guest Editor of Exploration of Immunology, had no involvement in the decision-making or the review process of this manuscript. The other authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Rucha A. Kelkar ... Giustino Varrassi
Mariateresa Giglio ... Filomena Puntillo
Antonella Ciaramella, Giancarlo Carli
