Abstract
Aim:
This study aims to identify the factors affecting the formation of neutralizing antibodies (NAbs) in healthy adults four weeks post-COVID-19 vaccination.
Methods:
A cross-sectional study was conducted among mass vaccination attendees using inactivated CoronaVac. Collected the peripheral blood serum four weeks following the second vaccine dose. Forty-four adults aged 26–85 were split into two groups based on age (≤ 60 years and > 60 years) and BMI (non-obese ≤ 25 kg/m2 and obese > 25 kg/m2). Variables like age, gender, BMI, and the presence of comorbidities were recorded. CD4/CD8 ratio and vitamin D levels were examined for their influence on NAbs formation. NAbs were measured using ELISA, T-cells via flow cytometry, and vitamin D through radioimmunoassay. Descriptive data analysis was performed as mean ± standard deviation to show the characteristics of the sample. Students’ t-tests and multivariate and univariate regression analyses were used to evaluate the data.
Results:
Significant variations in NAbs levels were observed with age (P = 0.013), BMI (P = 0.004), and comorbidities (P = 0.034). The elderly demonstrated higher NAb levels, potentially due to the high vitamin D levels compared to the adult group. The vitamin D levels strongly correlated with NAb titer (P < 0.001; R = 0.843). A collective correlation was found between NAb levels and the factors of age, BMI, and CD4/CD8 ratio (P = 0.033). A negative correlation existed between BMI and NAb levels (P = 0.018; R = –0.356) and between age and the CD4/CD8 ratio (P = 0.440; R = –0.119), but age alone did not correlate with NAb titer.
Conclusions:
Age, BMI, CD4/CD8 ratio, and comorbidities influence the production of post-vaccination NAbs. Sufficient vitamin D levels in the elderly significantly boost post-vaccination NAb levels. Maintaining a healthy body weight is also vital, as studies have revealed a significant and negative correlation between BMI and the level of NAbs, suggesting a possible need for adjusted vaccine doses in obese individuals.
Keywords
Neutralizing antibodies, post-vaccination, CD4/CD8 ratio, vitamin D, body-mass indexIntroduction
The COVID-19 disease pandemic has led to the development of several vaccines at an unprecedented pace because vaccines are an effective way to prevent the rapid spread of COVID-19 [1]. In Indonesia, the COVID-19 vaccination started in January 2021, and the first vaccine used was CoronaVac, a β-propiolactone-inactivated vaccine with aluminum hydroxide as an adjuvant. The vaccine’s efficacy was 65.3%, which met WHO requirements with a minimum vaccine efficacy of 50%. Community vaccination programs must notice the effectiveness of the COVID-19 vaccine against SARS-CoV-2, especially for the variants of concern (VOC). When vaccination was first carried out in Indonesia, the VOC was mainly the B.1.1.7 (alpha) variant, B.1.351 (beta) variant, and the P.1 (gamma) variant [2].
Most approved vaccines are delivered systemically, such as CoronaVac administered intramuscularly and will induce the formation of immunoglobulin G (IgG) antibodies in the serum. Recent studies indicate that systemic immunization routes can trigger both systemic and mucosal immune responses [3]. However, intramuscular vaccines administered to naive individuals did not induce significant mucosal IgA responses [4].
Two structural proteins have been used as target antigens for SARS-CoV-2: the nucleoprotein (NP) and the spike protein. While NP is located inside the virus or infected cells, its antibodies are unlikely to neutralize the virus due to its biological role and membrane shielding. In contrast, the spike protein, a trimeric glycoprotein essential for viral entry via the angiotensin converting enzyme-2 (ACE2) receptor, is the key target for neutralizing antibodies (NAbs) [5, 6]. The spike protein of SARS-CoV-2, especially the receptor binding domain (RBD) protein, is the primary and potential target for the induction of antibodies post-vaccination but carries certain risks, including the potential for antibody-dependent enhancement (ADE). The RBD is highly exposed to immune responses, leading to frequent mutations (variants of concern), which can reduce the efficacy of NAbs and increase the risk of ADE. During infection, antibodies bind to viral particles to neutralize them. However, when these antibodies are sub-neutralizing or non-neutralizing, they form immune complexes that mimic viral receptors and facilitate viral entry instead of blocking it. These immune complexes interact with Fcγ receptors (FcγRs) on the surface of APCs and enter the cell via endocytosis, bypassing its usual receptor (ACE2). In the APCs, the virus can escape the endosome and replicate, exploiting cells and expanding the range of cell types. The infection of APCs, such as macrophages, can lead to an inflammatory cascade, which causes excessive cytokine production and immune dysfunction; this process is a crucial feature of ADE and causes antibody titers to decrease over time, contributing to severe disease progression [7].
Not all antibodies can prevent the virus or pathogen from infecting cells. However, they may still mark the pathogen for destruction by other immune cells through opsonization, activating the complement system, or promoting other immune responses [8]. The NAbs are a subset of post-vaccination antibodies that can directly neutralize the pathogen by blocking the pathogen’s ability to enter and replicate within cells, and it will prevent the pathogen from establishing an infection. Vaccine-induced NAbs titers have been established to provide protection in non-human primates, using prototype DNA vaccines against the SARS-CoV-2 spike protein. In COVID-19 vaccination, NAbs might block the viral spike protein from attaching to the host ACE2 receptor, thereby stopping the infection [9–11]. NAbs are often considered one of the most critical components of protective immunity because they can prevent the pathogen from causing disease. A high level of NAbs is usually a good indicator of vaccine-induced protection, whereas other types of antibodies may indicate an immune response but not necessarily one that can prevent infection. That is why the NAb is typically used as a primary indicator of vaccine efficacy [10]. A previous study identified a strong non-linear relationship between vaccine neutralization rates and vaccine efficacy (Spearman r = 0.905; P = 0.0046). It was observed that the decline in neutralization titers over time is consistent across different vaccines. However, this decline impacts protection against SARS-CoV-2 infection in a non-linear manner, depending on the initial efficacy of the vaccine. For instance, a vaccine with an initial efficacy of 95% is expected to retain 77% efficacy after 250 days, while a vaccine with an initial efficacy of 70% would likely drop to 33% efficacy within the same timeframe. The neutralization titer was estimated to decrease with a half-life of 108 days [10].
A previous study showed that most individuals recovering from mild-to-moderate COVID-19 generate strong IgG antibody responses to the viral spike protein, with moderate-to-high antibody titers. These titers show a significant correlation with the neutralization of authentic SARS-CoV-2, with over 90% of seroconverters producing detectable NAbs. Notably, antibody levels remain stable for at least three months, with only modest declines observed at five months, aligning with patterns seen in other coronaviruses like SARS-CoV-1 and MERS-CoV. In COVID-19 patients, the NAb persists within 75–90 days of the onset of symptoms. Apart from the vaccine type, adjuvants in the vaccines also play a role in inducing immune cells to increase the antibody titers [12, 13].
Many factors affect the formation of NAbs, such as the characteristics of the individual, such as age, gender, BMI, history of infectious diseases, comorbid diseases, and, of course, the immune system status [12]. The study also examined the correlation between BMI and NAbs formation. Several previous studies have indicated that a high BMI is associated with severe COVID-19 and significantly increased morbidity and mortality. Additionally, obesity has been linked to reduced vaccine efficacy [14, 15]. Therefore, it is crucial to investigate whether obesity influences the formation of COVID-19 post-vaccination NAbs.
A person’s immune system profile, both cellular and humoral, plays an essential role in determining the formation of an antibody titer. Cellular and humoral immune responses interact to provide protection. CD4 cells will activate B lymphocyte cells, which will differentiate into plasma cells and produce antibodies. CD4 cells also activate CD8 cells, which play a role in cellular immunity [16]. The CD4/CD8 ratio is a crucial indicator of immune system balance and function. The normal CD4/CD8 ratio in healthy individuals must be better defined. Ratios between 1.5 and 2.5 are generally considered normal. However, some argue that the immune system is strong if the CD4/CD8 ratio is more than 1.0 [17, 18]. Deviations from this range can indicate various immune system issues. Previous studies found that the SARS-CoV-2 spike protein elicits NAbs and CD4 T cell responses after a single immunization in mice, and spike protein is a potent target for CD4 cells but not too strong for CD8 cells [13, 19].
In addition to the individual immune system factors, several studies found that vitamin D status correlates with the post-vaccination immune response to several diseases, such as hepatitis B, tetanus toxoid vaccine, and BCG vaccine [20–22]. Animal studies have shown better immunogenicity when given vitamin D together with inactivated polio vaccine, hepatitis B vaccine, and Haemophilus influenza vaccine. Elderly, obese, and chronic kidney disease patients are often deficient in vitamin D and often have an inadequate response to vaccines [23, 24]. Epidemiological data reports that several countries with a high prevalence of vitamin D deficiency experience an increased susceptibility to complications and death from COVID-19. The impact of vitamin D deficiency in people with COVID-19 causes longer hospitalization and a higher mortality rate. There is an inverse relationship between vitamin D concentrations and the severity of COVID-19 disease [25].
Considering the individual characteristics, comorbid diseases, immune system function, and vitamin D status, this research investigated factors influencing the formation of NAbs post-vaccination. This study’s potential impact expands our understanding of the variables associated with immune responses post-vaccination, ultimately contributing to developing strategies to enhance NAbs production, particularly in preparation for future pandemics.
Materials and methods
Study design
A cross-sectional study was conducted among healthy adult participants of a COVID-19 vaccination program in Bandung, Indonesia. The study focused on the level of NAbs post-vaccination four weeks after administering the second vaccine dose from individuals receiving the inactivated CoronaVac vaccine. The study starts from October 2021 until February 2022. The research complied with ethical standards, with approval from the Ethics Committee of Immanuel Hospital, Bandung, Indonesia (ethics approval number: 36/A01/EC/IX/2021).
Sample collection procedure
Participants from a mass COVID-19 vaccination event (second dose) held at a private institution were invited to participate in the study. Those meeting the inclusion criteria were informed about the study and offered the opportunity to undergo a NAb test four weeks following their second dose of the CoronaVac vaccine.
The inclusion criteria were as follows: male or female participants aged 18 years or older, with no prior history of SARS-CoV-2 infection, who had received two doses of the inactivated CoronaVac vaccine (3 μg β-propiolactone-inactivated SARS-CoV-2 in 0.5 mL aqueous suspension), produced by Sinovac Life Sciences (Beijing, China), administered four weeks apart.
Exclusion criteria were prior history of SARS-CoV-2 infection, pregnancy, autoimmune disease or immunodeficiency history, current use of immunosuppressant drugs, and having a household member infected with COVID-19 within the month prior to sample collection.
Of approximately 104 vaccination participants, 63 registered their interest in the study. Prior to enrollment, participants were provided with a detailed explanation of the study’s purpose and procedures. Personal identity and medical history were reviewed to confirm consistency between the first and second vaccination doses, ensuring both involved the CoronaVac vaccine. A thorough medical history was obtained, including information on comorbidities, chronic conditions, autoimmune disorders, medication usage, and any exposure to COVID-19 within the past month, either personally or through household contacts.
Participants who met the inclusion criteria and agreed to participate (44 participants) were asked to sign an informed consent form. They were then instructed to visit the Prodia Clinical Laboratory four weeks after receiving their second vaccine dose to draw 5 mL of cubital venous blood. The blood samples were collected and stored at minus 20 for further examination of neutralizing SARS-CoV-2 antibodies (NAb), CD4 and CD8 cell counts, and 25-OH-vitamin D concentration.
Procedure for examining neutralizing SARS-CoV-2 antibodies, CD4 and CD8 cells, and vitamin D concentration
The concentration of NAbs SARS CoV-2 was examined using the enzyme immunoassay (ELISA) method with the Anti SARS-CoV-2 Neutralizing Antibody Titre Serologic Assay Kit (SARS CoV-2 NeutraLISA from EUROIMMUN). This enzyme immunoassay provides semiquantitative in vitro determination of NAb in serum. NeutraLISA supports evaluating the individual immune response following SARS-CoV-2 infection or vaccination with RBD-based vaccines. The interpretation is: < 20 IU/mL is negative; ≥ 20–34 IU/mL borderline, and ≥ 35 IU/mL positive.
Examine absolute values of CD4 and CD8 cells using flow cytometry technique. Pipette 20 µL of BD Multitest™ CD3/CD8/CD45/CD4 reagent into a BD Trucount™ tube. Add 50 µL of the sample directly above the stainless steel holder in the Trucount™ tube. Vortex the tube gently for 5 seconds to mix. Incubate in a dark place at room temperature for 15 minutes. Add 450 µL of BD FACS™ lysing solution to the tube and vortex gently for 5 seconds to mix. Incubate again in the dark at room temperature for 15 minutes.
Analyze the sample using a BD FACSVia™ flow cytometer with the gating strategy as follows: For T helper cells (CD4+), the gate on cells that are positive for CD4 (APC) and negative for CD8 (PE). For T cytotoxic cells (CD8+), gate on cells that are positive for CD8 (APC) and negative for CD4 (PE). The results provide the absolute counts of CD4+ T helper cells and CD8+ T cytotoxic cells within the sample, and then the CD4/CD8 ratio is analyzed.
We are examining the concentration of 25-OH-vitamin D3 using the chemiluminescence radioimmunoassay method (DiaSorin, Stillwater, MN, USA) with the following interpretation: normal/sufficient: 30–100 ng/mL; insufficiency: 10–29 ng/mL; deficiency: < 10 ng/mL. All examinations were performed at the Prodia Clinical Laboratory, Bandung, Indonesia.
Analysis data
Descriptive data analysis was performed as mean ± standard deviation to show the characteristics of the sample. The 2-tailed Student’s t-test was used to compare the data between any two groups through the Shapiro-Wilk normality tests. The nonparametric Mann-Whitney U-test was used for data with asymmetric distribution. The multivariate regression test was calculated to determine the correlation between all the predictor factors (age, BMI, CD4/CD8 ratio, and vitamin D concentration) and the NAbs titer. The correlation between each variable with NAb titer was determined using the Pearson correlation coefficient when data were normally assumed or Spearman’s Rank correlation when scale data were non-normal, followed by a regression correlation test. Statistical analyses were conducted using SPSS software (version 16.0). With P < 0.05 considered significant.
Results
The total participants were 44 healthy adults who had received two doses of inactivated SARS-CoV-2 vaccine, CoronaVac intramuscularly, within four weeks apart and had no SARS-CoV-2 previous infection. The characteristics of the participants are summarized in Table 1.
Characteristic participants
| Participants | N | % | Mean | Range |
|---|---|---|---|---|
| Age | ||||
| Adults (≤ 60 yrs) | 32 | 72.73 | 42.44 ± 10.63 | 26–59 yrs |
| Elderly (> 60 yrs) | 12 | 27.27 | 65.58 ± 6.40 | 61–85 yrs |
| Gender | ||||
| Male | 24 | 54.55 | ||
| Female | 20 | 45.45 | ||
| BMI (kg/m2) | ||||
| Non-obese | 31 | 70.45 | 22.04 ± 2.27 | 17.04–24.98 |
| Obese | 13 | 29.55 | 28.20 ± 2.22 | 26.22–34.63 |
| Comorbid diseases | ||||
| Comorbid (–) | 26 | 59.09 | ||
| Comorbid (+) | 18 | 40.91 | ||
| Ratio CD4/CD8* | ||||
| Ratio ≥ 1.0 | 31 | 70.45 | 1.48 ± 0.32 | 1.00–2.58 |
| Ratio < 1.0 | 13 | 29.55 | 0.79 ± 0.14 | 0.52–0.97 |
| Vitamin D concentration | ||||
| Sufficient (30–100 ng/mL) | 3 | 6.82 | 34.97 ± 4.48 | 30.90–41.20 |
| Insufficient (10–29 ng/mL) | 26 | 59.09 | 16.05 ± 4.09 | 10.40–25.20 |
| Deficiency (< 10 ng/mL) | 15 | 34.09 | 7.63 ± 1.74 | 3.40–9.60 |
* The CD4/CD8 ratio in healthy individuals between 1.0 and 4.0 is generally considered normal
Participants’ characteristics were differentiated based on age, gender, BMI, comorbid diseases, vitamin D concentration, and immune system status based on the ratio CD4/CD8, which can affect the formation of post-vaccination NAb.
In this study, the age groups were divided into an adult group (≤ 60 years) and the elderly group (> 60 years). The youngest participant was a 26-year-old male, and the oldest was an 85-year-old female. The BMI is divided into the non-obese group (BMI ≤ 25 kg/m2) and the obese group (BMI > 25 kg/m2). This category is under the BMI classification by the Indonesian Ministry of Health. The comorbid diseases in this study referred to chronic diseases such as hypertension, cardiovascular disease, diabetes mellitus, chronic obstruction pulmonary disease, cancer, autoimmune, and other chronic diseases.
The results of vitamin D concentration are pretty surprising. Despite Indonesia’s year-round sunshine, only 6.82% of the participants had sufficient vitamin D concentration. A significant 34.09% were found to have vitamin D deficiency, with an average vitamin D concentration of 7.63 ± 1.74 ng/mL. The lowest vitamin D concentration was recorded in a 28-year-old woman at 3.4 ng/mL, while the highest was 41.2 ng/mL in a 61-year-old woman.
The CD4/CD8 ratio in healthy individuals between 1.0 and 4.0 is generally considered normal. In this study, almost 30% had a CD4/CD8 ratio of less than 1.0. A wide heterogeneity of the ratio CD4/CD8 exists because differences in gender, age, ethnicity, genetics, exposures, and infections may all impact the ratio [17].
Furthermore, the participants underwent a comprehensive examination, which included the measurement of the anti-SARS-CoV-2 NAb titer, the absolute value of CD4 and CD8 lymphocytes, and 25-OH-vitamin D3 concentration. This thorough examination allowed for a detailed analysis of the participant’s immune system status and vitamin D levels.
NAbs SARS-CoV-2 titers and vitamin D concentration based on individual characteristics
Table 2 shows data on NAbs titers and vitamin D concentrations based on individual characteristics. Statistical analysis evaluated differences in NAb concentrations across various variables. The Shapiro-Wilk test was initially applied to assess the normality of data distribution for each variable. For variables exhibiting a normal distribution, comparisons between the two groups were conducted using an independent t-test. Conversely, for variables demonstrating non-normal distributions, the nonparametric Mann-Whitney U-test was employed to perform the analysis.
NAbs titer and vitamin D concentration based on individual characteristics
| Variables | NAb (IU/mL) | Vitamin D (ng/mL) | |||||
|---|---|---|---|---|---|---|---|
| Mean | Range | P | Mean | Range | P | ||
| Age | ≤ 60 yrs | 35.24 ± 24.65 | 0.50–95.58 | 0.013a | 12.76 ± 6.12 | 3.40–30.90 | 0.026b |
| > 60 yrs | 42.92 ± 30.26 | 0.50–91.77 | 19.02 ± 9.27 | 7.60–41.20 | |||
| Gender | Male | 32.89 ± 23.11 | 0.50–95.58 | 0.289b | 13.08 ± 3.85 | 5.10–19.90 | 0.231b |
| Female | 42.67 ± 29.23 | 0.50–91.77 | 16.14 ± 10.31 | 3.40–41.20 | |||
| BMI (kg/m2) | Non-obese | 43.64 ± 27.70 | 0.50–91.77 | 0.004a | 15.18 ± 8.21 | 5.10–41.20 | 0.487b |
| Obese | 22.29 ± 17.70 | 0.50–61.32 | 12.77 ± 5.72 | 3.40–24.70 | |||
| Comorbid | (–) | 44.40 ± 27.30 | 0.50–95.58 | 0.034a | 13.35 ± 6.82 | 5.10–32.80 | 0.210b |
| (+) | 27.13 ± 23.18 | 0.50–78.19 | 16.08 ± 8.44 | 3.40–41.20 | |||
| Ratio CD4/CD8* | ≥ 1.0 | 40.55 ± 25.85 | 0.50–95.58 | 0.223a | 14.60 ± 7.16 | 5.10–32.80 | 0.777b |
| < 1.0 | 29.65 ± 26.52 | 0.50–91.77 | 14.15 ± 8.68 | 3.40–41.20 | |||
a Independent t-test; b Mann-Whitney test; * The CD4/CD8 ratio in healthy individuals between 1.0 and 4.0 is generally considered normal. NAbs: neutralizing antibodies
The results showed that there were significant differences in NAb concentration based on age (P = 0.013), BMI (P = 0.004), and the presence of comorbid diseases (P = 0.034). One of the consequences of aging is a decline in immune function. Older people often respond less efficiently to antigens that enter their bodies, including during vaccination [20]. However, in this study, it turns out that the elderly group (> 60 years) showed significantly higher NAb titers (42.92 ± 30.26 IU/mL) compared to the adults group (35.24 ± 24.65 IU/mL). When the NAb concentration was assessed based on NAb interpretation (negative: < 20 IU/mL; borderline: ≥ 20–34 IU/mL; positive: ≥ 35 IU/mL), in both groups, the mean NAb was considered as NAb positive (≥ 35 IU/mL). In the elderly group, 50% revealed positive NAb titer; however, in the adult group, only 38.23% did. As with NAb titers, vitamin D concentrations were also significantly (P = 0.026) higher in the elderly group (19.02 ± 9.27 ng/mL) compared to the adult group (12.76 ± 6.12 ng/mL). However, the vitamin D concentration of both groups was included in vitamin D insufficiency (10–29 ng/mL). In the adult group, 37.5% revealed vitamin D deficiency (< 10 ng/mL), and only 3.12% had sufficient vitamin D concentration (30–100 ng/mL). In the elderly group, 16.67% had sufficient vitamin D, and 16.67% had vitamin D deficiency. The correlation between vitamin D concentration and NAb titers will be analyzed further.
Based on gender, the NAb titer was higher in females (42.67 ± 29.23 IU/mL) than in males (32.89 ± 23.11 IU/mL) but not significantly different (P = 0.289). Likewise, the mean vitamin D concentrations are higher in females, and both are included in the insufficiency category. Gender also influences the immune system’s function due to hormonal differences between men and women [23].
Based on BMI, obese participants showed significantly lower NAb concentrations or borderline NAb (22.29 ± 17.70 IU/mL) compared to non-obese or positive NAb (43.64 ± 27.70 IU/mL) with a P-value of 0.004. However, there was no significant difference in vitamin D concentrations (P = 0.487). Several previous studies have stated that there is a relationship between adipose tissue metabolism and immune cell function. Leptin can be responsible for decreased B cell function in obesity [24, 26, 27].
The next factor studied was the presence of comorbid diseases on NAb titers. In this study, what is meant by comorbid diseases are heart disease, hypertension, diabetes mellitus, respiratory system diseases, impaired kidney function, autoimmune disease, and cancer. The participants without comorbid diseases significantly (P = 0.034) showed higher NAb titers (44.40 ± 27.30 IU/mL) compared to participants with comorbid diseases (27.13 ± 23.18 IU/mL). Comorbid diseases are generally chronic diseases that can affect the immune system and the formation of post-vaccination NAb. High numbers of comorbidities and specific comorbidities could impact the immune response of COVID-19 patients. Lymphocyte count and CD4/CD8 ratio were negatively correlated with cardiovascular diseases, chronic hepatitis B, cancer, and also with the amount of comorbidities in COVID-19 patients [28]. The ratio of CD4 and CD8 cells provides valuable insights into a person’s immune system status. This ratio is a crucial indicator of immune system balance and function. A healthy individual’s CD4/CD8 ratio is considered normal when the value is between 1.0 and 4.0; in a healthy immune system, there are usually one to three CD4+ T cells for every CD8+ T cell. Deviations from this range can indicate various immune system issues [17]. This study revealed no significant difference in NAbs titer between individuals with a CD4/CD8 ratio < 1.0 (29.65 ± 26.52 IU/mL) compared to a CD4/CD8 ratio ≥ 1.0 (40.55 ± 25.85 IU/mL), likewise with the vitamin D concentration.
Analysis factors influenced the NAbs post-vaccination formation
To determine whether there is a correlation between age, BMI, CD4/CD8 ratio, and vitamin D concentration towards the NAbs titer, a multivariate regression correlation test was conducted, preceded by a Shapiro-Wilk test for normality. The results showed that all data were normally distributed (P > 0.05), except for vitamin D data. The results are shown in Table 3 (age, BMI, ratio CD4/CD8) and Table 4 (vitamin D).
The multivariate regression correlation test between age, BMI, and CD4/CD8 ratio on neutralizing antibodies (NAbs) titer was preceded by the Shapiro-Wilk normality test
| Shapiro-Wilk test | Age | BMI (kg/m2) | Ratio CD4/CD8 | Vitamin D | NAb |
|---|---|---|---|---|---|
| Mean | 47.02 | 23.86 | 1.29 | 14.47 | 37.33 |
| Standard deviation | 15.41 | 3.65 | 0.44 | 7.73 | 26.83 |
| Asymptotic significance | 0.058 | 0.200 | 0.182 | 0.034 | 0.068 |
| Predictor | R | R square | P | ||
| Age, BMI, ratio CD4/CD8 | 0.441 | 0.195 | 0.033 | ||
| Multiple correlation test | Coefficients | P | |||
| Constant | 69.462 | 0.029 | |||
| Age | 0.225 | 0.371 | |||
| BMI | –2.577 | 0.018 | |||
| Ratio CD4/CD8 | 14.589 | 0.099 | |||
Linear regression correlation analysis between neutralizing antibodies (NAbs) titer with BMI, vitamin D, and comorbid diseases
| Predictor | R | R square |
|---|---|---|
| BMI | –0.356 | 0.028 |
| Vitamin D | 0.843 | 0.711 |
| Comorbid diseases | –0.320 | 0.103 |
| Regression correlation test | Unstandardized coefficients | P |
| Constant | 99.912 | |
| BMI | –2.623 | 0.018 |
| Constant | –4.769 | |
| Vitamin D | 11.137 | < 0.001 |
| Constant | 44.399 | |
| Comorbid diseases | –17.274 | 0.034 |
The Shapiro-Wilk normality test demonstrated that the data of age, BMI, ratio CD4/CD8, and NAbs titer were all within normal distribution, except for the vitamin D concentration data. That is why Spearman’s Rank correlation test analyzes the correlation test for vitamin D, while others are done using a multivariate regression correlation test. The multivariate regression correlation test showed that age, BMI, and CD4/CD8 ratio moderately correlated with NAb titer (P = 0.033; R = 0.441). The R square value of 0.195 means that the effect of age, BMI, and CD4/CD8 ratio together on NAbs formation is 19.5%.
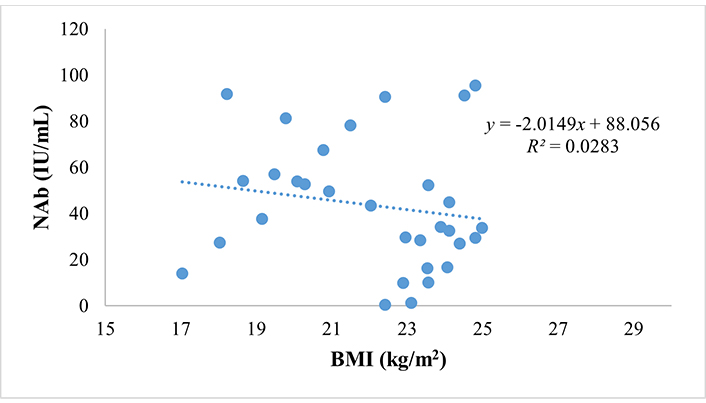
BMI is a significant factor among the three variables, with a P-value < 0.05, while age and the CD4/CD8 ratio have a P > 0.05. The impact of BMI on NAbs titer is substantial. We used Pearson’s linear regression correlation tests to demonstrate this, revealing that BMI significantly influences NAbs titer (P = 0.018) with a negative and weak correlation (R = –0.356). This means that the higher the BMI, the lower the NAb titer. The predictive relationship between BMI and NAbs titer is shown in the scatter plot in Figure 1. The value of R square = 0.0283 determines that the influence of BMI on decreasing NAb titer is only 2.83%.
Furthermore, other factors analyzed for correlation with NAbs titer were vitamin D concentration and comorbid diseases; the results are listed in Table 4.
Our analysis of the influence of comorbid diseases on NAbs has revealed a significant albeit weak correlation (P = 0.034; R = –0.320). The influence of comorbid diseases on NAb formation is 10.3% (R square = 0.103), underscoring the need for caution and further investigation in this area.
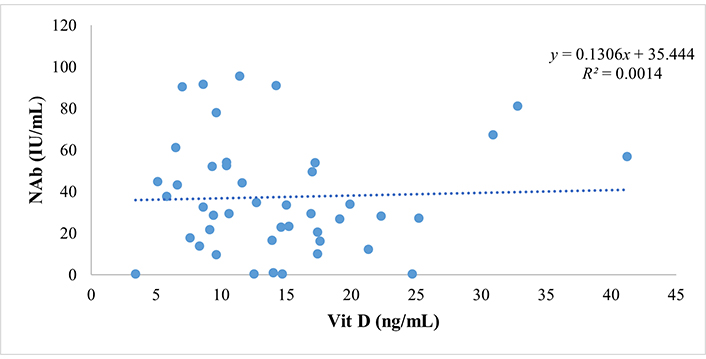
Spearman’s correlation test revealed that vitamin D concentration significantly and strongly correlated with the NAb titer (P < 0.001 and R = 0.843). This means the higher the vitamin D concentration, the higher the NAb titer, with a 71.1% influence. An increase in vitamin D concentration will increase the NAb titer. This finding underscores the potential role of vitamin D in enhancing the immune response post-vaccination. The predictive relationship between vitamin D and NAb titer is shown in the scatter plot in Figure 2.
The overall analysis results show that NAbs post-vaccination were influenced by age, BMI, and CD4/CD8 ratio together at 19.5%, comorbid diseases at 10.3%, and vitamin D concentration at 71.1%. The vitamin D status has the most effect on the formation of post-vaccination NAb. Our linear regression correlation test aimed to determine whether the mechanism of vitamin D in increasing NAb formation is through increasing the CD4/CD8 ratio. However, the results showed no correlation (P > 0.05) between vitamin D level and CD4/CD8 ratio.
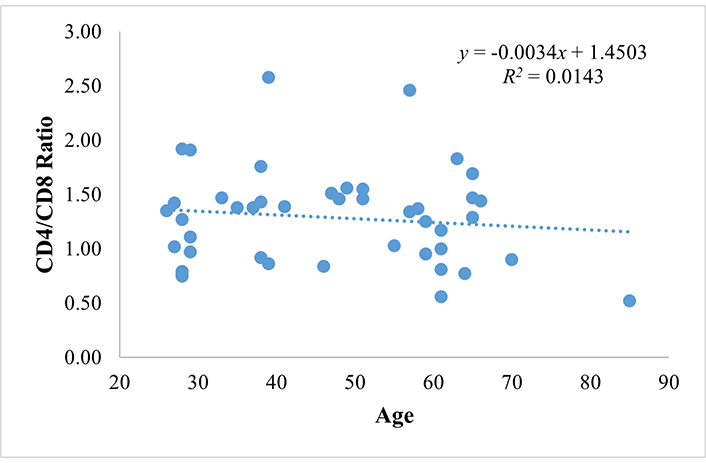
Furthermore, we analyzed the correlation between CD4/CD8 ratio and age. This study showed that the mean of the CD4/CD8 ratio in the adult group (1.35 ± 0.40) is higher than that of the elderly group (1.14 ± 0.43). However, the regression correlation test showed no correlation (R = –0.119; P = 0.440), between age and the CD4/CD8 ratio, and the results are shown in Table 5.
Regression correlation analysis between age and CD4/CD8 ratio
| Predictor | R | R square |
|---|---|---|
| Age | –0.119 | 0.0143 |
| Regression correlation test | Coefficients | P |
| Constant | 1.450 | 0.440 |
| Age | –0.003 |
The CD4/CD8 ratio is influenced only 1.43% by age (R square = 0.0143), with the predictive relationship shown in Figure 3.
Previous studies showed that the CD4/CD8 ratio declines with age, reflecting the effects of immunosenescence, and cytomegalovirus (CMV) infection also can reduce the CD4/CD8 ratio [29, 30]. The different results in this study are likely due to the number of research participants being too small, and further research is needed with an adequate number of research subjects.
Discussion
As individuals age, their immune responses naturally decline, a phenomenon known as immunosenescence. The shrinking of primary lymphoid organs such as the bone marrow and thymus, a reduction in B cells, T cells, and memory cells, and a diminished function of mature lymphocytes in secondary lymphoid tissues characterize this gradual deterioration of immune function. Meanwhile, the production of pro-inflammatory cytokines increases. These changes contribute to a weakened immune response to vaccinations in elderly populations [20, 31]. Typically, the elderly exhibit a less robust response to immune challenges than younger individuals, and advancing age is often associated with a reduced formation of antibodies following vaccination. However, this study found that the elderly group (42.92 ± 30.26 IU/mL) showed significantly higher NAb levels than the adult group (35.24 ± 24.65 IU/mL). In the elderly group, 50% of participants showed positive NAb titers, compared to only 38.23% in the adult group.
The higher levels of NAbs in the elderly group compared to the adult group could also be due to cross-reactivity. As we know, COVID-19 antibody titers can show cross-reactivity due to prior exposure to other coronaviruses, such as those causing common colds (e.g., HCoV-OC43 or HCoV-229E). This cross-reactivity arises because some parts of the spike or nucleocapsid proteins of SARS-CoV-2 share structural similarities with endemic human coronaviruses. While these antibodies may bind to SARS-CoV-2, they often have limited neutralizing capability. Studies suggest that these antibodies might help reduce severity in some cases but are often non-neutralizing, meaning they cannot effectively prevent the virus from entering cells. This phenomenon complicates serological testing and interpretations of immunity. This condition may also explain how severely COVID-19 disease affects older people versus younger people [32, 33]. While cross-reactive antibodies may influence the antibody level, the primary factors for differences in severity are immune system aging, inflammation levels, and the presence of comorbidities. These facts highlight the importance of vaccination and prevention, especially for older populations.
The variations in immune system function among individuals within the same age group can be attributed to multiple factors, such as the gut microbiome, vitamin D levels, and the presence of comorbidities, all of which can influence NAb formation. Therefore, age alone does not determine immune system function [31, 34]. These findings imply that aging is not the sole factor influencing post-vaccination NAb formation. Instead, considering other factors, such as vitamin D concentration and comorbid conditions, is crucial, which also affect NAb production in elderly individuals. A deeper understanding of these factors could inform strategies to enhance immune responses in aging populations.
During the pandemic, older individuals often prioritized boosting their immune systems by maintaining a balanced diet, taking vitamins, and ensuring adequate rest. This behavior could explain the higher vitamin D levels observed in the elderly group. Conversely, the adult group may have experienced stress related to work and financial difficulties, which could negatively impact immune function.
Gender was also examined as a factor influencing NAb titers post-vaccination. In this study, females exhibited higher NAb titers (42.67 ± 29.23 IU/mL) than males (32.89 ± 23.11 IU/mL), although this difference was not statistically significant. Generally, females tend to display more robust adaptive immune responses than males and lower infection susceptibility and responsiveness to vaccination [35, 36]. This factor is mainly due to the influence of sex steroid hormones, such as estradiol, which enhance humoral and cell-mediated immune responses to antigenic stimulation, vaccination, and infections. Additionally, genetic factors may play a role, as several genes related to innate and adaptive immune responses are located on the X chromosome [23]. High testosterone levels in males have been associated with lower NAb responses following influenza vaccination, suggesting an immunosuppressive role for testosterone in the context of vaccination [37, 38]. Despite these differences, gender-specific factors are often overlooked in research on immune responses and are rarely considered in the design or dosing of vaccines for elderly individuals [39].
The study also examined the impact of BMI on NAb formation, revealing that high BMI, or obesity, has emerged as an independent risk factor for infection and is related to severe COVID-19, even though the independent relative risk of obesity is higher than that of hypertension and type 2 diabetes [14, 40]. NAb titers in non-obese individuals (43.64 ± 27.70 IU/mL) were significantly higher than in obese individuals (22.29 ± 17.70 IU/mL). Clinical and epidemiological data suggest that obese individuals are more susceptible to infectious diseases and tend to experience more severe infections compared to non-obese individuals [27]. Martínez-Colón et al.’s study [15] found that adipose tissue can serve as a potential reservoir for SARS-CoV-2. They found viral replication in adipocytes and detected SARS-CoV-2 RNA in the cytoplasm of adipocytes in human autopsy specimens, which causes the secretion of inflammatory factors that lead to more severe clinical symptoms. The targets are mature adipocytes and a small proportion of adipose tissue macrophages that cause immune activation and secretion of inflammatory factors associated with severe COVID-19 symptoms [15].
Obesity is associated with high leptin concentrations, which lead to increased production of inflammatory cytokines and pro-inflammatory immune cells, resulting in suboptimal immune responses [41, 42]. Elevated leptin levels can impair the function of helper T-lymphocytes and B-lymphocytes, decreasing IgG production and contributing to diminished B cell function in obesity [43]. Additionally, obesity-induced chronic inflammation can trigger the development of metabolic and other chronic diseases [44]. Previous studies have demonstrated that obesity is associated with impaired immune responses to influenza vaccination, although the effectiveness of COVID-19 vaccines did not significantly differ between individuals with and without obesity [45, 46].
Comorbidities, such as hypertension, diabetes mellitus, cardiovascular disease, chronic obstructive pulmonary disease, and cancer, also impact immune function. This study identified a weak negative correlation between comorbidities and NAb formation. Individuals with comorbid conditions may experience reduced immune function, making them more susceptible to COVID-19 infection [28, 47]. Specific comorbidities, such as diabetes mellitus, can alter immune profiles, shifting the balance from regulatory T cells to pro-inflammatory Th1 and Th17 cells, thereby affecting vaccine responses. Cardiovascular diseases, cancer, and chronic hepatitis B can reduce the CD4/CD8 ratio, an important indicator of immune system balance and function [48].
The CD4/CD8 ratio serves as a clinical marker for assessing immune function, especially in the context of infections and immune disorders. In healthy individuals, this ratio typically ranges from 1.0 to 4.0. One infection that notably affects the CD4/CD8 ratio is CMV infection. Most people are infected with CMV at some point in their lives, and it usually remains latent in healthy individuals. Chronic CMV infection can reduce the CD4/CD8 ratio, primarily due to increased CD8+ T cells, particularly CMV-specific CD8+ T cells.
In some cases, this leads to an inverted ratio (below 1.0), indicating a cytotoxic shift in the immune response. This inversion is widespread in older adults as CMV prevalence rises with age. Persistent CMV infection is linked to immune senescence, marked by aging-related immune decline and diminished functionality [49].
A low or inverted CD4/CD8 ratio is associated with poorer health outcomes, particularly in elderly or immunocompromised individuals, with CMV as a significant factor contributing to this immune imbalance. A low CD4/CD8 ratio among older adults is often regarded as a hallmark of immunosenescence. Previous studies indicate that the CD4/CD8 ratio declines with age, reflecting the effects of immunosenescence, increased susceptibility to infections, and reduced vaccine efficacy [29, 49].
In our study, the CD4/CD8 ratio was lower in the elderly group (1.14 ± 0.43) compare to the adult group (1.35 ± 0.40). However, the statistical analysis showed no significant difference and no correlation. This may be due to the inadequate number of research participants. In relation to past CMV infection, unfortunately, antibody tests specific to CMV were not performed, so the relationship between the CD4/CD8 ratio and CMV seropositivity remains uncertain. This problem highlights a limitation of our study and the potential for further discovery of the correlation between the CD4/CD8 ratio, CMV infection, and the NAbs level. However, the result showed a correlation between NAbs and vitamin D levels. In this study, vitamin D status emerged as a key factor influencing NAb formation. Although Indonesia is a tropical country with abundant sunlight, vitamin D deficiency remains prevalent. In the adult group, 40.62% of participants were vitamin D deficient, likely due to limited sun exposure and a lack of regular vitamin D supplementation.
In contrast, only 16.67% of elderly participants were vitamin D deficient, as many regularly consumed vitamin D supplements. Approximately 90% of the elderly group reported daily vitamin D supplementation (1,000–5,000 IU), particularly during the COVID-19 pandemic, compared to only 20% of adults who reported regular vitamin D intake. The higher vitamin D levels observed in the elderly group (19.02 ± 9.27 ng/mL) compared to the adult group (12.76 ± 6.12 ng/mL) may reflect the elderly’s greater attention to immune health during the pandemic. Adequate vitamin D levels can help mitigate the effects of age-related immune changes [50], and this study confirmed a strong correlation between vitamin D levels and NAb concentrations, with vitamin D accounting for 71.1% of the variation in NAb levels. The higher NAb concentrations in the elderly group may thus be attributed to their higher vitamin D levels rather than age alone.
Previous studies reported a significant negative correlation between average population serum 25(OH)D levels and high COVID-19 cases and mortality [25, 51]. Research using the BNT162b2 vaccine found that post-vaccination IgG antibodies measured 8 weeks post-immunization were negatively associated with age, and higher IgG levels were revealed in individuals with a concentration of 25(OH)D > 50 nmol/L [52]. Vitamin D deficiency is associated with lower antibody responses following vaccination. Individuals with vitamin D deficiency had a weaker hemagglutination inhibition response to influenza vaccination and lower seroconversion rates following hepatitis B vaccination [22, 53]. Additionally, patients receiving vitamin D supplementation demonstrated higher anti-tetanus IgG responses than those receiving a placebo [54]. However, there are still conflicting results considering the association between vitamin D serostatus and antibody response to vaccination [53]. The precise mechanisms underlying vitamin D’s role in enhancing antibody production and mucosal immunity remain incompletely elucidated. Future research focusing on gene expression, cytokine responses, and system-level interactions is essential to provide a more comprehensive understanding of this association [53].
While vitamin D status influences the production of post-vaccination NAbs, it does not affect the CD4/CD8 ratio. Theoretical models suggest that aging can reduce the CD4/CD8 ratio. This statement reflects a decline in immune function, making older individuals more susceptible to infections and less responsive to vaccinations [17]. However, no correlation was observed between age and the CD4/CD8 ratio in this study, and the CD4/CD8 ratio is influenced only 1.43% by age. Maintaining adequate vitamin D levels may mitigate the effects of aging and the associated decline in the CD4/CD8 ratio.
Conclusion
Vaccination is a crucial strategy in preventing infectious diseases, with post-vaccination NAbs as a key measure of vaccine effectiveness. Age, BMI, CD4/CD8 ratio, and comorbidities play a role in post-vaccination NAb formation. While aging can lead to a decline in immune system function, maintaining adequate Vitamin D levels significantly improves immune system function, particularly in producing post-vaccination NAbs. Equally important is maintaining a healthy body weight, as it is a crucial factor in supporting a strong immune system and the production of post-vaccination NAbs. The negative correlation between BMI and NAb suggests a possible need for adjusted vaccine doses in obese individuals, however, the authors acknowledge the study’s sample size had limited power to detect a meaningful effect. It is noteworthy that further studies with a sufficient number of participants are needed to evaluate the immune response to vaccines more thoroughly.
Abbreviations
| ACE2: | angiotensin converting enzyme-2 |
| ADE: | antibody-dependent enhancement |
| CMV: | cytomegalovirus |
| IgG: | immunoglobulin G |
| NAbs: | neutralizing antibodies |
| NP: | nucleoprotein |
| RBD: | receptor binding domain |
| VOC: | variants of concern |
Declarations
Acknowledgments
We thank Prodia Clinical Laboratory for examining the blood samples.
Author contributions
HR: Conceptualization, Investigation, Data curation, Formal analysis, Methodology, Writing—original draft. FT, GP, and AAP: Writing—review & editing. SF: Data curation, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The studies involving human participants were reviewed and approved by the Ethics Committee of Immanuel Hospital, Bandung, Indonesia (ethics approval number: 36/A01/EC/IX/2021).
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Funding
This study was supported by the Universitas Kristen Maranatha, Bandung, Indonesia, under the B scheme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.