Affiliation:
1Department of Pediatrics, Medicine Hospital, Faculty of Medicine, Istanbul Atlas University, 34303 Istanbul, Turkey
ORCID: https://orcid.org/0000-0002-1614-6149
Affiliation:
2Department of Microbiology, Faculty of Medicine, Istanbul Atlas University, 34303 Istanbul, Turkey
ORCID: https://orcid.org/0000-0003-3472-2020
Affiliation:
3Department of Medical Biochemistry, Faculty of Medicine, Istanbul Atlas University, 34303 Istanbul, Turkey
ORCID: https://orcid.org/0000-0001-8893-2926
Affiliation:
4Department of Public Health, Cerrahpaşa Faculty of Medicine, Istanbul University-Cerrahpaşa, 34303 Istanbul, Turkey
ORCID: https://orcid.org/0000-0002-5925-2128
Affiliation:
3Department of Medical Biochemistry, Faculty of Medicine, Istanbul Atlas University, 34303 Istanbul, Turkey
Email: huzun59@hotmail.com
ORCID: https://orcid.org/0000-0002-1347-8498
Explor Immunol. 2025;5:1003181 DOI: https://doi.org/10.37349/ei.2025.1003181
Received: September 27, 2024 Accepted: January 02, 2025 Published: January 23, 2025
Academic Editor: Margherita Sisto, University of Bari Medical School, Italy
The article belongs to the special issue Chronic Inflammation and Autoimmunity
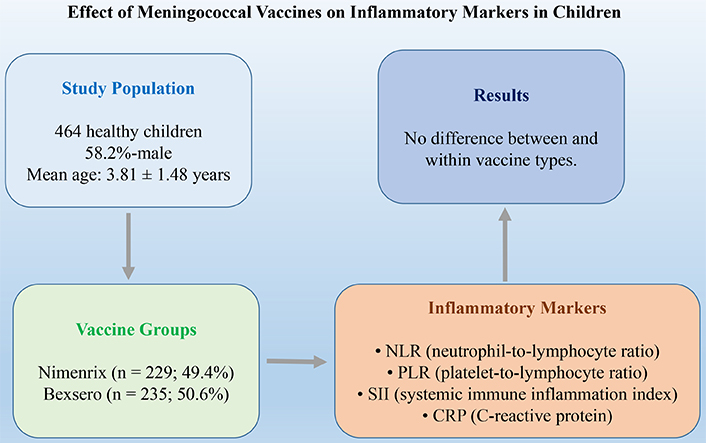
Aim: Immunization with meningococcal vaccine (MV) is the most effective measure to control and prevent the transmission of meningococcal infections. In this study, in order to support the appropriate use of various MVs in the prevention of meningococcal meningitis (MM), the effects of MVs, especially single-dose and inter-booster administered, on inflammatory parameters in < 5-year-old children were investigated.
Methods: A total of 464 healthy children were included in this study. The data of those who received the first 2 doses at 2-month intervals and the next dose between 8–12 months were included. Nimenrix® (Pfizer) administered as a single dose to children from 12 months of age. Bexsero® (GSK) was administered as 2 + 1 doses under 2 years of age and 2 doses 2 months apart over 2 years of age. Neutrophil, lymphocyte, monocyte, platelet counts, C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), derived NLR (dNLR), platelet-to-lymphocyte ratio (PLR), systemic inflammation response index (SIR-I), and systemic immune inflammation index (SII) were evaluated.
Results: Of the 464 participants, 58.2% were male, with a mean age of 3.81 years, and both sex ratios and ages were similar across the Nimenrix and Bexsero groups. The laboratory and inflammatory parameters of the two vaccine groups were similar. In both vaccine groups, changes in laboratory parameters before and 3-months after vaccination were similar. The changes in laboratory parameters over time between vaccine groups and their interactions were not significant.
Conclusions: The NLR, dNLR, PLR, SIR-I, and SII are useful biomarkers indicating the inflammatory response of Nimenrix and Bexsero vaccines. Inflammatory markers can be used as both a safety endpoint and a protection endpoint for MVs (Nimenrix and Bexsero). However, further studies involving larger patient cohorts as well as detailed laboratory data on specific markers of inflammation are needed to draw comprehensive conclusions regarding the inflammatory response following vaccination.
Invasive meningococcal diseases are one of the major causes of mortality and morbidity worldwide. The disease is clinically rapid and aggressive due to the difficulty in controlling endotoxin-associated vascular damage caused by the causative microorganism [1]. Worldwide, an estimated 1.2 million meningococcal infections occur annually and 135,000 of these cases result in death [2]. Looking at the death statistics of the Turkish Statistical Institute after 2009, the number of deaths related to meningococcal disease was reported as 154. Serious sequelae such as deafness, convulsions, limb amputation, and mental retardation are observed in 5–30% of survivors [3].
Vaccination is the best way to protect the patient against this aggressive disease that leaves little time for intervention after the onset of symptoms [4]. Since the vast majority of invasive meningococcal are encapsulated and the disease may be caused by one of several serogroups, vaccine studies have targeted capsular polysaccharides of meningococci [5]. Since most of the disease occurs in previously healthy individuals with no risk factors, vaccination is important in these age groups [6, 7]. Blood samples including complete blood count, coagulation parameters, biochemistry, inflammatory markers such as C-reactive protein (CRP), procalcitonin, and blood culture should be obtained quickly [8, 9].
Neutrophils, lymphocytes, and platelets are involved in the control of inflammation and systemic inflammation is associated with changes in the quantity and composition of circulating blood cells such as neutrophilia, lymphopenia, and thrombocytosis [10]. Mean platelet volume (MPV), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have recently been investigated as novel inflammatory markers in many diseases [11]. MPV is a potential marker of platelet count and activity and correlates with inflammation and inflammation severity [12]. NLR may be useful for predicting the activity of autoimmune and inflammatory diseases [13]. PLR is used as an index for the inflammatory state in various diseases. It is suggested that these markers can be used in various clinical situations to identify patients and the prognosis of the disease or to predict health status [14, 15].
Meningococcal diseases can be fatal or cause permanent brain damage. Therefore, the most effective way to combat the disease is to prevent transmission and control the disease before it develops. Since inflammatory parameters may guide the safety of the vaccine, there is a need for objective, reliable, inexpensive, and easily applicable laboratory parameters that can be useful as indicators of protection in predicting the indicators for safety. In the literature review, there is no study suitable for our purpose. In this study, in order to support the appropriate use of various meningococcal vaccines (MVs) in the prevention of meningococcal meningitis (MM), the inflammatory effects of MVs, especially single-dose and inter-booster administered, on inflammatory parameters in 5-year-old children were investigated.
Ethical approval of this study was obtained by the Non-Interventional Ethics Committee of the Medical Faculty of Istanbul Atlas University (04.03.2024; No: E-22686390-050.99-40431). The study was performed in accordance with the Declaration of Helsinki, and informed consent was obtained from the families of all patients prior to their inclusion in the study.
The presenting complaints, clinical and laboratory findings, treatment, and follow-up of patients admitted to the Pediatric Outpatient Clinic between 2018 and 2024 within the scope of meningococcal routine vaccination were evaluated retrospectively. A flow chart of the selection of cases is shown in Figure 1.
The data of those who received the first 2 doses at 2-month intervals and the next dose between 8–12 months were included. The MenACWY-TT conjugate vaccine (Nimenrix®, Pfizer) was administered as a single dose to children from 12 months of age. The 4-component meningococcal serogroup B vaccine (4CMenB, Bexsero®, GSK) was administered as 2 + 1 doses under 2 years of age and 2 doses 2 months apart over 2 years of age. No one in our hospital had a febrile convulsion 3-months after vaccination in our hospital.
Four hundred and sixty-four healthy children aged 3.81 ± 1.48 years who applied for routine vaccination were included in the study. In Turkey, children of that age also receive the following vaccinations:
The first dose of hepatitis B vaccine is administered before the baby is discharged from the hospital after birth.
Month 1: The second dose of hepatitis B vaccine is administered according to the vaccination schedule.
Month 2: The bacille Calmette-Guérin (BCG) vaccine (tuberculosis vaccine), the pneumococcal conjugate vaccine (PCV) and 5-component vaccine against diphtheria, tetanus, pertussis, poliomyelitis (inactivated), and Haemophilus type b are given during the routine follow-up of the baby.
Month 4: 5 mixed vaccines and PCV vaccines are given.
Month 6: Hepatitis B vaccine, 5-mixed vaccine, and PCV are administered. In addition, live polio vaccine is given orally.
Month 12: The last dose of PCV, measles, mumps, rubella, and varicella (chickenpox) are administered.
Month 18: 5-mixed vaccine, oral live polio, and hepatitis A vaccines are given.
Month 24: Hepatitis A vaccine is repeated.
Patients with chronic disease, chronic diarrhea, heart disease, malnutrition, central nervous system infection, respiratory system infection, patients who had used antibiotics in the last month, or patients who had been treated in any center before admission were excluded from the study. At the same time, those who had had an allergic reaction to meningococcal or a similar vaccine and those with known allergies to any substance in the vaccine were excluded from the study.
In our hospital, vaccinated children are routinely followed up and the side effects of vaccines are evaluated after 3 months. The effects of vaccines on inflammatory parameters were evaluated with CRP and new generation inflammatory biomarkers.
Venous blood samples were taken from all patients at the time of admission. For CBC, 0.5–2 mL of blood was drawn into purple capped ethylenediaminetetraacetic acid (EDTA) tubes and measured in an automatic blood count device (Sysmex XN-1000™, Roche Diagnostics GmbH, Mannheim, Germany) within 1 h at the latest.
The derived NLR (dNLR) was calculated as follows: neutrophil count/(white cell count–neutrophil count).
The NLR was calculated as follows: neutrophil count/lymphocyte count.
The PLR was calculated as follows: platelet count/lymphocyte count.
The systemic immune inflammation index (SII) was calculated with the formula as follows: (neutrophil count × platelet count)/lymphocyte count.
The systemic inflammation response index (SIR-I) was calculated with the formula as follows: (neutrophil count × monocyte count)/lymphocyte count.
The serum CRP levels were measured using the nephelometric method (IMMAGE® 800 Beckman Coulter, Inc., CA 92821, USA).
IBM SPSS (The Statistical Package for the Social Sciences) version 21.0 software was used for the evaluation and analysis of the data. Descriptive statistics were expressed as frequencies (n) and percentages (%) for categorical variables and means ± standard deviations (SD) or medians (25th percentile–75th percentile) for numerical variables. The normality of continuous variables was assessed using histograms and Q‒Q plots. Furthermore, hypothesis tests were conducted to determine the relationships between variables. The chi-square test was used to compare categorical variables. Independent samples t tests or Wilcoxon tests were applied to compare continuous variables between two independent groups. Paired samples t tests or Wilcoxon tests were applied to compare continuous variables between two dependent groups. Finally, two-way repeated-measures ANOVA was used to examine interactions between groups. A p value < 0.05 was considered to indicate statistical significance.
In the study group, 50.6% (n: 235) were vaccinated with Bexero and 49.4% (n: 229) with Nimenrix. Of the 464 participants, 58.2% were male, and the sex ratio was similar between the vaccine groups (Nimenrix: 55.5% male/44.5% female vs. Bexsero: 60.9% male/39.1% female; p = 0.239). The mean age of the participants was 3.81 ± 1.48 years, and the ages were similar between the vaccine groups (Nimenrix: 3.77 ± 1.46 years vs. Bexsero: 3.85 ± 1.51 years; p = 0.570).
The most common local and systemic adverse events in children administered Nimenrix and Bexsero were tenderness and redness (erythema) at the vaccination site. None of the patients had fever, cough, wheezing, rhinorrhea, shortness of breath, history of previous the paediatric and neonatal intensive care unit (PICU/NICU) admission, and hospitalisation before vaccination. While 11.9% of the Bexsero group had fever 3 months after vaccination, none of the Nimenrix group had fever. Three months after vaccination, none of the patients in both groups had cough, wheezing, rhinorrhea, shortness of breath, and hospitalization or PICU/NICU.
There were no statistically significant differences between the Nimenrix and Bexsero groups in any of the laboratory parameters before and 3 months after vaccination. All inflammatory parameters were similar between the two groups (Table 1).
Comparison of pre- and post-vaccination laboratory parameters within vaccine types and over time
| Laboratory parameters | Vaccine type | p value | ||
|---|---|---|---|---|
| Nimenrix (n: 229; 49.4%) | Bexsero (n: 235; 50.6%) | |||
| Mean ± SD or Median (25P–75P) | Mean ± SD or Median (25P–75P) | |||
| WBC (103/µL) | Before vaccination | 8,030 (6,820–9,000) | 8,080 (6,910–9,200) | 0.565* |
| After vaccination | 7,680 (6,720–8,830) | 7,880 (6,590–8,920) | 0.804* | |
| p value | 0.186Ω | 0.102Ω | ||
| HGB (g/dL) | Before vaccination | 11.1 (10.6–12.2) | 11.1 (10.5–12.2) | 0.530* |
| After vaccination | 11.4 (10.8–12.2) | 11.4 (10.8–12.2) | 0.858* | |
| p value | 0.113Ω | 0.015Ω | ||
| HCT (%) | Before vaccination | 33.3 (31.8–35.9) | 33.1 (31.2–35.3) | 0.223* |
| After vaccination | 34.1 (32.5–36.3) | 34.0 (32.4–36.3) | 0.998* | |
| p value | 0.058Ω | 0.001Ω | ||
| PLT (103/µL) | Before vaccination | 316,721 ± 59,404 | 316,836 ± 65,539 | 0.984† |
| After vaccination | 312,886 ± 62,948 | 309,030 ± 67,329 | 0.524† | |
| p value | 0.478¶ | 0.186¶ | ||
| LYM (103/µL) | Before vaccination | 4.67 ± 1.38 | 4.70 ± 1.52 | 0.833† |
| After vaccination | 4.52 ± 1.26 | 4.64 ± 1.65 | 0.366† | |
| p value | 0.156¶ | 0.680¶ | ||
| Neutrophil (103/µL) | Before vaccination | 2.55 ± 1.1 | 2.63 ± 1.22 | 0.477† |
| After vaccination | 2.67 ± 1.09 | 2.51 ± 1.04 | 0.122† | |
| p value | 0.290¶ | 0.263¶ | ||
| Monocyte (103/µL) | Before vaccination | 0.96 (0.73–1.25) | 0.90 (0.65–1.25) | 0.298* |
| After vaccination | 0.76 (0.62–1.09) | 0.81 (0.63–1.25) | 0.387* | |
| p value | 0.004Ω | 0.170Ω | ||
| PLR | Before vaccination | 69.01 (54.78–84.47) | 68.46 (54.41–84.35) | 0.855* |
| After vaccination | 69.57 (57.12–83.53) | 69.17 (53.87–86.06) | 0.522* | |
| p value | 0.672Ω | 0.862Ω | ||
| NLR | Before vaccination | 0.55 (0.33–0.82) | 0.57 (0.33–0.83) | 0.801* |
| After vaccination | 0.57 (0.36–0.81) | 0.52 (0.34–0.74) | 0.162* | |
| p value | 0.374Ω | 0.739Ω | ||
| SII | Before vaccination | 171.28 (107.19–245.07) | 173.71 (108.16–251.85) | 0.938* |
| After vaccination | 178.07 (116.04–269.14) | 154.93 (103.77–256.46) | 0.106* | |
| p value | 0.403Ω | 0.405Ω | ||
| dNLR | Before vaccination | 0.48 (0.28–0.67) | 0.47 (0.28–0.7) | 0.893* |
| After vaccination | 0.50 (0.34–0.75) | 0.43 (0.29–0.73) | 0.095* | |
| p value | 0.118Ω | 0.748Ω | ||
| SIR-I | Before vaccination | 0.48 (0.34–0.74) | 0.47 (0.29–0.84) | 0.761* |
| After vaccination | 0.52 (0.26–0.9) | 0.47 (0.23–0.9) | 0.538* | |
| p value | 0.244Ω | 0.859Ω | ||
| CRP (mg/L) | Before vaccination | 1.25 (0.76–2.76) | 1.25 (0.85–2.55) | 0.955* |
| After vaccination | 1.35 (0.95–2.35) | 1.45 (0.95–2.61) | 0.264* | |
| p value | 0.297Ω | 0.458Ω | ||
WBC: white blood cell; HGB: hemoglobin; HCT: hematocrit; PLT: platelet; LYM: lymphocyte; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; SII: systemic immune inflammation index; dNLR: derived NLR; SIR-I: (neutrophil count × monocyte count)/lymphocyte count; CRP: C-reactive protein; SD: standard deviation. †: Independent samples t test was used to compare the mean values. *: Mann-Whitney U test was used to compare median values. Ω: Wilcoxon test for to compare median differences. ¶: Paired samples t test for to compare the mean differences
In the Nimenrix group, only the monocyte count decreased significantly 3-months after vaccination [0.96 (0.73–1.25) vs. 0.76 (0.62–1.09); p = 0.004]. Changes in all other parameters before and 3-months after vaccination were not statistically significant. In the Bexsero group, there was a significant increase in hemoglobin [11.1 (10.5–12.2) vs. 11.4 (10.8–12.2); p = 0.015] and hematocrit [33.1 (31.2–35.3) vs. 34 (32.4–36.3)] levels, while changes in other parameters were not significant. Changes in inflammatory parameters were not significant in either group (Table 1).
Vaccination recommendations against Neisseria meningitidis, which can cause epidemics all over the world, have started to be included in many guidelines, especially in Europe and US [16–20]. Although effective protection has been achieved with 4CMenB [21] and MenACWY-TT [22], their effects on clinical protection are not clearly known. Hervé et al. [23] reported vaccine-related injection site local adverse events (moderate to severe transient reactogenicity) have been reported to occur after both the first and subsequent doses. Extrinsic and intrinsic factors can impact the reactogenicity profile, tolerability and immunogenicity of vaccines in a given individual. They include host characteristics, such as age, gender race/ethnicity, body mass, general health and pre-existing immunity, and vaccine administration and composition factors, such as route and site of administration, injection technique, type of antigen, vaccine formulation, and type of adjuvant [23]. In the current study, the most common local and systemic adverse events in children administered Nimenrix and Bexsero were tenderness and redness (erythema) at the vaccination site. While 11.9% of the Bexsero group had fever 3 months after vaccination, none of the Nimenrix group had fever. Three months after vaccination, none of the patients in both groups had cough, wheezing, rhinorrhea, shortness of breath, history of previous PICU/NICU, and hospitalization. Changes in inflammatory parameters were not significant in either group. Although MVs are not included in the routine vaccination schedule in Turkey and some other countries, according to the results of the study, the safety of the vaccine in particular, it is recommended that it be included in the routine vaccination schedule as soon as possible.
The most common local and systemic side effects in infants and children are vaccination site tenderness and erythema, fever, and sensitization, while in adolescents and adults, vaccination site pain, malaise, and headache are the most common. In addition, it has been reported that fever is observed at a higher rate in infants when administered together with other routine vaccines [24–26]. McQuaid et al. [26] reported that the 4CMenB vaccine is immunogenic and was fairly well tolerated by 5-year-old children, although injection-site pain was noteworthy. In the current study, the most common local and systemic adverse events in children administered 4CMenB and MenACWY-TT were tenderness and redness (erythema) at the vaccination site. While 11.9% of the Bexsero group had fever 3 months after vaccination, none of the Nimenrix group had fever. Although side effects (fever and local reactions) are observed more frequently in 4CMenB compared to MenACWY-TT vaccines, they are within acceptable limits. Our results confirm existing evidence regarding the safety of 4CMenB vaccination in babies under 2 years of age [27, 28]. Prophylactic paracetamol administration could represent a protective factor against fever, especially during the first 24 h after vaccination [28]. Fever reaction was higher in 4CMenB administration with routine infant vaccines and a significant decrease in fever reaction was observed with prophylactic paracetamol administration [29]. Inflammatory markers can be both a safety endpoint and a protection endpoint for both vaccines.
Recently, the determination of systemic inflammation has been facilitated by the use of new biomarkers that can be easily calculated with whole blood parameters. NLR has also been shown to be important in determining the prognosis of infectious diseases including respiratory syncytial virus (RSV), COVID-19, and bacteremia [30–33]. Wang et al. [34] demonstrated that the prevalence of latent tuberculosis infection (LTBI) was as high as 18.8% in patients with end-stage kidney disease or kidney transplant. BCG vaccination and high NLR might have protective effects against LTBI in patients with renal failure or transplant. The present study demonstrated that there is no significant difference in PLR, NLR, and dNLR between Bexsero group and Nimenrix group 3-month post vaccination period following administration of vaccines. The results of the study suggest that the prediction of inflammatory status after MVs by these inexpensive and routinely monitored parameters may be of benefit to physicians working in countries with limited resources.
SIR-I and SII markers, which can be obtained by the ratio of simple hemogram parameters to each other, are the subject of research in many diseases associated with inflammation. Wang et al. [35] suggested that SII may be an effective indicator for predicting the severity of Mycoplasma pneumoniae pneumonia (MPP) in children. SII is more sensitive and specific than NLR, PLR, and SIR-I in evaluating the condition of MPP. In current study, SIR-I and SII levels, used as a combination of all these parameters, did not significantly differ between vaccine groups. The results of our previous study [36] showed that breastfed and RSV-vaccinated children were less prone to inflammation because their NLR, PLR, and SII ratios were lower. The vaccine does not prevent the disease 100%. However, it can prevent severe disease with dehydration or death. The other inflammatory markers SIR-I and SII are similarly useful biomarkers. Inflammatory markers can be used as both a safety endpoint and a protection endpoint for Bexsero and Nimenrix vaccines. Serum bactericidal activity is the validated marker of protection for MVs. Inflammatory markers can be used as both a safety endpoint and a protection for MVs.
CRP is widely measured clinically as an end-point marker of systemic inflammation that predicts elevated risk for incident various diseases. To the best of our knowledge, this study is the first to document differences in CRP response to MV in relation to symptoms and baseline levels of CRP. In current changes in CRP levels were also not significant in either group. McDade et al. [37] reported that influenza vaccination produces a mild CRP response in the Philippines. Lower CRP at baseline was associated with larger CRP response to vaccination in the entire sample, and among participants without recent symptoms of infection.
A study on an Italian military cohort demonstrated the safety of multiple vaccinations, showing that adverse effects were generally mild and well-tolerated [38]. This aligns with findings in pediatric populations, where multiple vaccinations similarly exhibit a favorable safety profile, with mild, manageable side effects across age groups.
As a retrospective study, the study only involved healthy individuals, so the conclusions cannot be employed for severe and critical cases. Obviously, these indices are traditional markers, but they are cheap, convenient, and perhaps a better choice.
The antibody response of vaccinated children could not be monitored after vaccination.
Limited by the sample size, differences between different vaccine doses have not been discussed and large clinical trials are needed to confirm the protective effect of different doses and types of vaccines.
The inclusion of Bexsero and Nimenrix vaccines in routine immunization programs in many countries provides an ideal opportunity to evaluate the impact of these vaccines in a real-world setting and our results will guide the assessment of inflammation in the administration of these vaccines. Fast, simple, and convenient, these hematologic indices are markers that can be used to predict the severity of inflammation in vaccination. MM can also occur in children without additional risk factors. Predicting the inflammatory status even in children without an additional risk factor with NLR, dNLR, PLR, SIR-I, and SII obtained from routine whole blood samples after vaccination is important in terms of patient approach. Serum bactericidal activity is the validated marker of protection for MVs. Inflammatory markers can be used as both a safety endpoint and a protection endpoint for Bexsero and Nimenrix vaccines. Local and systemic adverse events were not observed in children followed up 3 months after vaccination. Although meningococcal infections are one of the most feared infectious diseases due to their high mortality and epidemics, vaccination is not yet widely practiced. In MM, where serogroup changes continue dynamically, it will be possible to reduce mortality and morbidity with surveillance and immunization in accordance with surveillance. However, further studies involving larger patient cohorts as well as detailed laboratory data on specific markers of inflammation are needed to draw comprehensive conclusions regarding the inflammatory response following vaccination.
BCG: bacille Calmette-Guérin
CRP: C-reactive protein
dNLR: derived neutrophil-to-lymphocyte ratio
LTBI: latent tuberculosis infection
MM: meningococcal meningitis
MPP: mycoplasma pneumoniae pneumonia
MPV: mean platelet volume
MVs: meningococcal vaccines
NLR: neutrophil-to-lymphocyte ratio
PCV: pneumococcal conjugate vaccine
PICU/NICU: paediatric and neonatal intensive care unit
PLR: platelet-to-lymphocyte ratio
RSV: respiratory syncytial virus
SII: systemic immune inflammation index
SIR-I: systemic inflammation response index
OO: Conceptualization, Investigation, Resources, Writing—original draft, Writing—review & editing, Project administration. NE: Conceptualization, Investigation, Resources, Writing—original draft, Writing—review & editing. SD: Validation, Formal analysis, Investigation, Data curation, Writing—original draft, Writing—review & editing, Project administration. US: Software, Validation, Formal analysis, Data curation, Writing—original draft, Writing—review & editing, Project administration. HU: Conceptualization, Methodology, Investigation, Resources, Writing—original draft, Writing—review & editing, Visualization, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.
Ethical approval of this study was obtained by the Non-Interventional Ethics Committee of the Medical Faculty of Istanbul Atlas University (04.03.2024; No: E-22686390-050.99-40431). And the study was also in accordance with the Declaration of Helsinki.
The informed consent was obtained from the families of all patients prior to their inclusion in the study.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 6473
Download: 47
Times Cited: 0
Yuto Sasaki ... Tadashi Matsuda
Aleksandra Kozlova ... Maria Zakharova
Jihye Heo ... Jea-Hyun Baek
Vasiliy Ivanovich Reshetnyak, Igor Veniaminovich Maev
