Abstract
Aim:
The objectives of our study were to evaluate a range of circulating biomarkers in COVID-19-related long-term neurological dysfunction.
Methods:
The study involved 30 patients with post-COVID syndrome (PCS) and 28 patients after COVID-19 without PCS. The third cohort consisted of 29 patients with acute COVID-19 of varying severity. The severity of COVID-19 was classified as mild and moderate to severe. The Montreal Cognitive Assessment (MOCA) and the SAGE test were used to study cognitive functions. The Hospital Anxiety and Depression Scale (HADS), the Sheehan Anxiety Scale, and the Beck Depression Inventory were used to study affective functions. The levels of serum cytokines and IgM, IgG, IgA to the SARS-CoV-2 coronavirus were determined using the Vector-Best test systems (Novosibirsk, Russia). We also studied the IgG subclasses to the spike protein of the SARS-CoV-2.
Results:
А mild to moderate COVID-19 infection primarily increases the risk of affective disorders and asthenia and, to a lesser extent, the development of cognitive impairment. The levels of IFN-α, IL-6, as well as serum antibodies to the SARS-CoV-2 among patients with PCS were significantly higher compared to convalescents without PCS. IgM to the SARS-CoV-2 was detected in the blood of patients with PCS during 2–7 months after the disease. After moderate and severe COVID-19, IgG2 and IgG4 were predominant in the blood of patients with PCS and neurological symptoms. The levels of IL-1, IL-4, IL-6, IL-8 in the blood serum of patients with PCS were higher after moderate and severe COVID-19 compared to patients who had mild COVID-19.
Conclusions:
The obtained data on an elevated level of cytokines and IFN-α in the blood of PCS patients can suggest the hypothesis about the participation of chronic inflammation in neurological disorders. The main limitation of the study is the relatively small sample size, which limits the statistical analyses.
Keywords
SARS-CoV-2, COVID-19, post-COVID syndrome, cytokines, antibodiesIntroduction
The emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes coronavirus disease-2019 (COVID-19), led to more than 7 million deaths worldwide since 2019. The COVID-19 causes generalized damage to many organs and systems of the body. After the end of the acute phase of the disease, 10–30% of patients develop post-acute sequelae from SARS-CoV-2 infection, which is called long-COVID or post-COVID syndrome (PCS) [1, 2]. With PCS, the symptoms of COVID infection last for at least three months and this can happen after severe, moderate or mild, or even asymptomatic COVID-19 [3]. Common clinical symptoms of PCS include weakness, fatigue, cough, sub-febrile temperature, headache, loss of smell or taste, sleep disturbances, muscle and joint pain. PCS also involves a variety of pulmonary, neurological, cardiovascular, renal, gastrointestinal, endocrine, and thromboembolic complications [4–9].
The most important consequences of the COVID-19 infection include neurological symptoms, which represent a serious global problem. The risk of neurological and psychiatric diagnoses associated with COVID-19 is thought to have increased following the emergence of the Delta variant [10]. The SARS-CoV-2 virus has a direct neurotropic effect on the brain, which is one of the target organs for coronaviruses. SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies were detected in the cerebrospinal fluid of patients with acute encephalopathy caused by COVID-19 [11, 12]. Autopsies of the brains of patients who died from COVID-19 have revealed pathological features associated with the SARS-CoV-2 in cortical neurons [13, 14].
SARS-CoV-2 can damage neurons by directly entering the central nervous system (CNS), but even if the virus does not directly enter the CNS, peripheral cytokines involved in the host antiviral response, especially interleukin 6 (IL-6), IL-8, interferon (IFN) and tumor necrosis factor (TNF) can trigger an inflammatory response by increasing the permeability of the blood-brain barrier and participate in neuroinflammation [15, 16]. Studies have shown that psychiatric disorders, including mood disorders and schizophrenia, are closely associated with inflammation. Proinflammatory cytokines can potentially influence emotional behavior and cognitive function by decreasing brain monoamine levels, activating neuroendocrine responses, enhancing excitotoxicity such as increased glutamate levels, and impairing brain plasticity [17]. This may be one of the potential mechanisms for the development of mental disorders in patients with COVID-19 [18, 19].
SARS-CoV-2 virus can trigger a cytokine storm and lead to autoimmune dysregulation [20, 21]. When SARS-CoV-2 replicates in the body, an immune response is activated, which is mediated by membrane-bound immune receptors and downstream signaling pathways [22]. This response includes massive infiltration of monocytes and macrophages that secrete cytokines, chemokines and other inflammatory mediators that contribute to the cytokine storm which can lead to significant tissue damage, organ dysfunction, and respiratory failure during the acute phase of COVID-19 [22, 23]. The results of meta-analyses revealed an increase in the level of cytokines in the blood of patients with first-episode psychosis and exacerbation of schizophrenia, including IL-6, IL-17, IL-8, IFN-γ, transforming growth factor-β (TGF-β) [24].
After the acute phase of inflammation when the pathogen has been eliminated, the phase of inflammation suppression begins. This includes such processes as decrease in pro-inflammatory cytokine production and increase in anti-inflammatory cytokine production, cessation of neutrophil infiltration at the site of inflammation, induction of neutrophil apoptosis and their absorption by macrophages, reprogramming of macrophages from M-1 (pro-inflammatory) activation type to M-2 (proliferative), return of immune cells, particularly dendritic cells and macrophages that have not undergone apoptosis, to the lymphatic and blood vessels, development of immunosuppressive cells at the previous site of inflammation and tissue damage repair [25]. Molecules that stimulate an anti-inflammatory response include IL-10, TGF-α, and other specialized pro-resolving mediators [26–29]. In SARS-COVID-19, along with the development of a pro-inflammatory response, a compensatory anti-inflammatory response also occurs [30]. In particular, the expression of anti-inflammatory cytokines, such as IL-4 and IL-10 increases during and after the acute period of COVID infection [30, 31]. As a result of insufficient suppression by anti-inflammatory cytokines, most patients with long-COVID experience unbalanced long-term production of a number of proinflammatory cytokines [30]. By analyzing cytokine profiles in people who have recovered from COVID-19 and are experiencing neurological symptoms, specific biomarkers may be identified for early detection or prediction of the course of neurological manifestations. This may help tailor treatment plans and monitor recovery or disease progression over time.
The relationship between viral clearance and antibody response in long-COVID is also an area of interest [32–34]. In the study, it was shown that patients with long-COVID have a high specific T-cell response to SARS-CoV-2, which did not decrease for 8 months, in the absence of naive T- and B-lymphocytes [34]. The reason for this stable level of specific immune response in patients with PCS may be the ongoing persistence of SARS-CoV-2 virus. As has been shown, the persistence of the Spike protein and SARS-CoV-2 viral RNA fragments which can be detected in extracellular vesicles may be related to PCS [33]. Thus, the presence and action of antibodies in long-COVID may have implications for treatment strategies and patient management. For example, this may help to guide the decision to prescribe immunomodulatory therapy or other measures to limit persistent symptoms.
The aim of the study was to evaluate the levels of serum cytokines and indicators of the humoral immune response to SARS-CoV-2 in patients with PCS with identified neurological symptoms.
Materials and methods
Contingents and samples
This is a cohort study that included 30 blood sera from patients after COVID-19 with PCS. The second cohort consisted of 28 blood sera from COVID-19 convalescents without signs of PCS. These two cohorts were observed in the Clinic of the Federal State Budgetary Scientific Institution “IEM” and the Medical Research Center in 2023. The third cohort included 29 archived blood samples from patients of the Vsevolozhsk Interdistrict Clinical Hospital who were hospitalized with acute COVID-19 in 2020. The exclusion criteria were oncological diseases or immunodeficiency states.
Methods for assessing the neuropsychological status in patients with PCS
The neuropsychological status was assessed in 30 patients from the Clinic of the FSBSI “IEM” and the Medical Research Center who were observed in 2023. The severity of COVID-19 was classified as mild and from moderate to severe. Cognitive functions, severity of anxiety, depression, and asthenia were evaluated. For cognitive functions, the Montreal Cognitive Assessment (MOCA) and the Self-Administered Gerocognitive Exam (SAGE) Dementia and Self-Control Test were used. Given the different sensitivity and specificity of anxiety and depression tests [35], for the study, in addition to the most common in practice Hospital Anxiety and Depression Scale (HADS), the Sheehan’s Anxiety Scale, and the Beck Depression Inventory were used. Asthenia was assessed using MFI-20.
Enzyme-linked immunosorbent assay
Blood samples were tested by enzyme-linked immunosorbent assay (ELISA) for the presence of immunoglobulin G (IgG), IgM, IgA antibodies to SARS-CoV-2 using Vector-Best test systems (Vector, Novosibirsk, Russia). The seroprevalence rate was calculated according to the manufacturer’s instructions. Additionally, the tested samples were tested by ELISA for the presence of IgG subclass antibodies specific to the SARS-CoV-2 S-protein, in duplicate. The test principle is based on the indirect ELISA method. For this, 96-well Nunc MaxiSorp plates (Thermo Fisher Scientific, Waltham, USA) were sensitized with 2 μg/mL of recombinant S-protein containing (455–659 aa), obtained at the Institute of Experimental Medicine as described previously [36]. Before antibody detection, serum samples were heated at 56°C for 30 minutes. After 3-fold washing, falling serial dilutions of sera were added to the wells of the sensitized plates in 1:4 increments. After 1.5 hours of incubation at 37°C, the wash solution was used to remove unbound antibodies. Horseradish peroxidase-labeled rabbit anti-human IgG antibodies (Polygnost, Leningrad Region, Russia) were used as conjugates. The final ELISA titers were determined as the highest dilution at which the optical density at 450 nm (OD450) exceeded the mean OD450 value plus 3 standard deviations (SD) of the control wells. The mean values of the control wells were determined for each dilution of 4 to 6 negative blood sera obtained before SARS-CoV-2 emergence.
Concurrent ELISA
SARS-CoV-2 Surrogate Virus Neutralization Test Kit (AtaGenix, Wuhan, China), which is a type of competitive ELISA, where the protein-protein interaction between the receptor-binding domain (RBD) of the viral glycoprotein and the cell surface receptor ACE2 is blocked by neutralizing antibodies against the SARS-CoV-2 RBD. The test detects circulating antibodies against SARS-CoV-2 that block the interaction between RBD and ACE2. The percentage of neutralization is calculated based on OD450 according to the manufacturer’s instructions.
Laboratory data
The content of IL-1β (IL-1), IL-4, IL-6, IL-8, IL-10, TNF-α, or IFN-α was determined using ELISA kits (Vector-best, Novosibirsk, Russia) according to the manufacturer’s instructions. All blood tests were performed on unheated blood samples. Serotonin content was determined using ST/5-HT(5-hydroxytryptamine) ELISA (Wuhan Fine Biotech Co., Ltd., Wuhan, China).
Statistical processing of results
Statistical analysis was performed using the Prism 8 package (GraphPad Software, San Diego, USA). Preliminary analysis of equality of variance was performed using the Shapiro-Wilk W-test. When the data was not normally distributed, a comparison between two independent groups was performed using the nonparametric Mann-Whitney test. For nominal data, Fisher’s exact two-sided test was used for the same purposes. The presence of a statistical relationship between variables was assessed using correlation analysis in Python 3 with “Corr” function of Pandas library and Spearman correlation coefficient (rs). The strength of the relationship between variables was evaluated according to the ‘Chaddock scale’: correlation coefficient (rs) from 0.1 to 0.3 (weak); from 0.3 to 0.5 (moderate); from 0.5 to 0.7 (noticeable); and from 0.7 to 0.9 (high). Differences were significant at P < 0.05.
Results
The main data on the observed patient cohorts
The main indicators of the examined patient groups are presented in Table 1.
Main characteristics of the study patient groups※
| Characteristic | Group 1 (PCS) | Group 2 (Convalescents without PCS) | Group 3 (Acute COVID-19) |
|---|---|---|---|
| Number of people | 30 | 28 | 29 |
| Age (years)※※ | 41.5 (23.3; 55.75) | 69.00 (61.0; 75.0) | 62.0 (55.0; 73.0) |
| Men | 8 (26.7%) | 5 (17.8%) | 14 (48.3%) |
| Women | 22 (73.3%) | 23 (82.2%) | 15 (51.7%) |
| Number of days between symptom onset and examination※※ | 45.0 (27.8; 68.3) | 510.0 (390.0; 900.0) | 7.0 (4.25; 9.0) |
| Proportion of patients seropositive for IgM | 6 (20%) | 1 (3.6%) | 17 (58.6%) |
| Proportion of patients seropositive for IgG | 22 (73.3%) | 18 (64.2%) | 14 (48.3%) |
| IL-6 levels※※ | 22.24 (19.57; 25.78) | 0.12 (0.00; 1.83) | 33.86 (13.44; 104.30) |
| TNF-α levels※※ | 6.46 (0.53; 13.48) | 0.045 (0.00; 1.52) | 3.38 (0.06; 9.01) |
| IFN-α levels※※ | 12.98 (9.26; 16.89) | 7.02 (3.16; 10.63) | 15.84 (9.30; 21.14) |
※ Reference values for IL-6: 1.3–6.8 pg/mL, for TNF-α: 0–8.21 pg/mL, for IFN-α: < 10 pg/mL. ※※ means medians (lower quartile; upper quartile) [Me (Q1; Q3)]. PCS: post-coronavirus disease syndrome; IgG: immunoglobulin G; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; IFN-α: interferon-α; COVID-19: coronavirus disease-2019
The statistical significance of differences between the examined groups of patients is presented in Table 2.
The statistical significance of differences between the examined groups of patients※
| Characteristic | Group 1 (PCS vs. convalescents without PCS) | Group 2 (PCS vs. acute COVID-19) | Group 3 (Convalescents without PCS vs. acute COVID-19) |
|---|---|---|---|
| Age (years) | < 0.0001 | < 0.0001 | 0.13 |
| Men (%) | 0.31 | 0.015 | < 0.001 |
| Number of days between symptom onset and examination | < 0.0001 | < 0.0001 | < 0.0001 |
| Proportion of patients seropositive for IgM | 0.062 | < 0.001 | < 0.001 |
| Proportion of patients seropositive for IgG | 0.32 | 0.62 | 0.36 |
| IL-6 | < 0.0001 | 0.31 | < 0.0001 |
| TNF-α | < 0.0001 | 0.14 | 0.0012 |
| IFN-α | < 0.0001 | < 0.0001 | < 0.0001 |
PCS: post-coronavirus disease syndrome; IgG: immunoglobulin G; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; IFN-α: interferon-α; COVID-19: coronavirus disease-2019
The age of patients with PCS was significantly lower than that of convalescents without PCS (Tables 1 and 2). The proportions of positive patients for IgM content were statistically significantly higher among acute COVID-19 patients compared to two other groups (< 0.001), and the proportions of individuals seropositive for IgG did not differ in the three groups. The levels of IL-6, TNF-α, and IFN-α levels among convalescent patients without PCS were statistically significantly lower than those among patients with PCS or acute COVID-19 (Tables 1 and 2). IL-6 and IFN-α levels were significantly elevated in patients with PCS and were close to those levels in patients with acute COVID-19. At the same time, TNF-α levels in patients with PCS were even higher in patients with PCS than in patients with acute COVID-19 cases (Table 1).
Since PCS is a consequence of a cytokine storm in COVID-19, we studied the serum levels of early cytokines in COVID-19 patients with mild or medium severe to moderate and severe forms of the disease cases of COVID-19. Figure 1 shows that mean IL-6 value levels, even in mild cases of acute COVID-19 were above reference value levels.
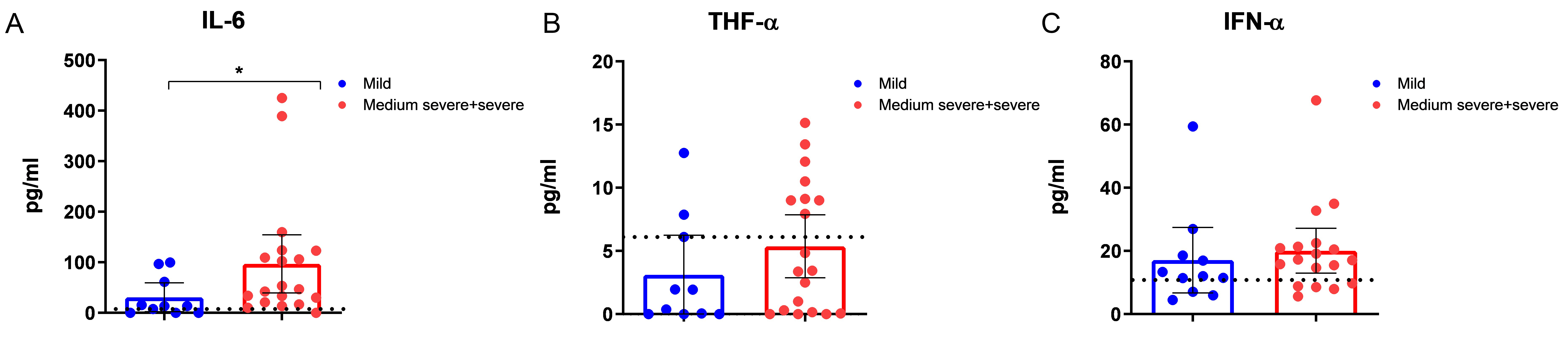
Content of early cytokines and interferon-α in the blood serum of patients with acute COVID-19 of varying severity (mild: n = 10, medium severe + severe: n = 19). (A) Content of IL-6 (IL-6 values reference range 1.3–6.8 pg/mL); (B) content of TNF-α (TNF-α values reference range 0–8.21 pg/mL); (C) content of IFN-α (IFN-α values reference range 0–10 pg/mL). Mean values and 95% confidence intervals (CI) are presented. Dotted lines indicate reference values. IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; IFN-α: interferon-α; COVID-19: coronavirus disease-2019
TNF-α levels were increased mainly in severe COVID-19, and IFN-α levels were increased in both mild and moderate to severe COVID-19 forms (Figure 1).
As shown in Figure 2, statistically significantly higher levels of IL-6 and IFN-α were observed in patients with unfavorable disease outcomes compared to patients who subsequently recovered (Figure 2A).
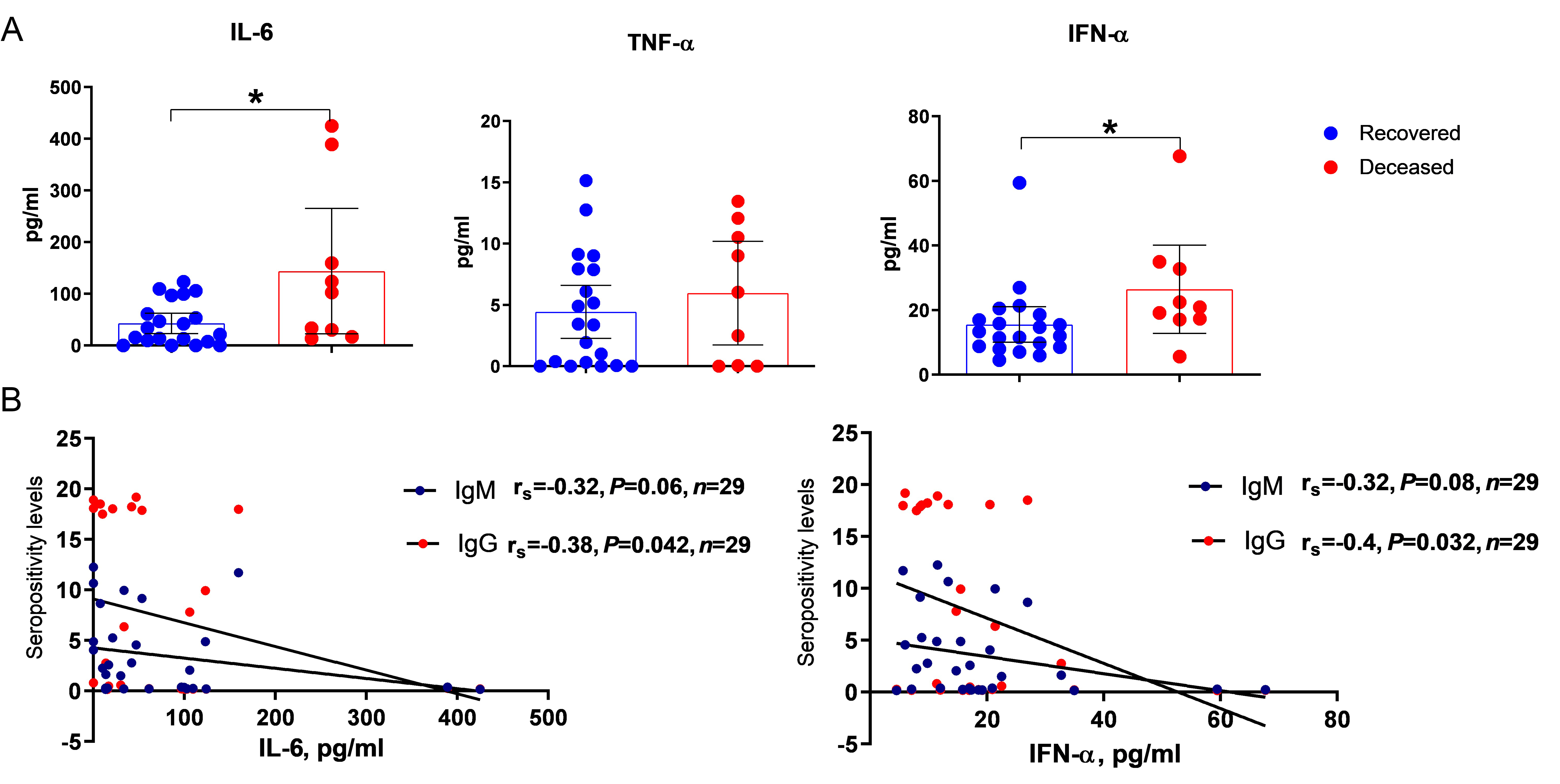
Results of the analysis of early cytokines and interferon-α among COVID-19 patients. (A) Content of early cytokines and interferon-α in the blood serum of patients with COVID-19 with different disease outcomes (recovered: n = 18, deceased: n = 10). Mean values and 95% confidence intervals (CI) are presented; (B) correlation analysis of IL-6 or IFN-α and IgM/IgG to SARS-CoV-2 in the same blood serum. * P < 0.05. IgG: immunoglobulin G; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; IFN-α: interferon-α; COVID-19: coronavirus disease-2019
A weakly negative correlation of IL-6 and IFN-α levels with seropositivity rates for serum IgG to the SARS-CoV-2 virus was shown (Figure 2B). This may indicate a positive effect of early production of IgG antibodies on systemic inflammation processes and may have prognostic significance for the outcome of the disease.
Blood test parameters in patients with PCS after mild or moderate to severe COVID-19
Figure 3A shows that the values of several neuropsychological tests were higher in patients who had moderate and severe COVID-19 compared to mild COVID-19, although the differences were not statistically significant. Serum cytokine levels (except IL-10) were higher after moderate to severe COVID-19 compared to mild COVID-19, in this case, the differences were also not statistically significant (Figure 3B).
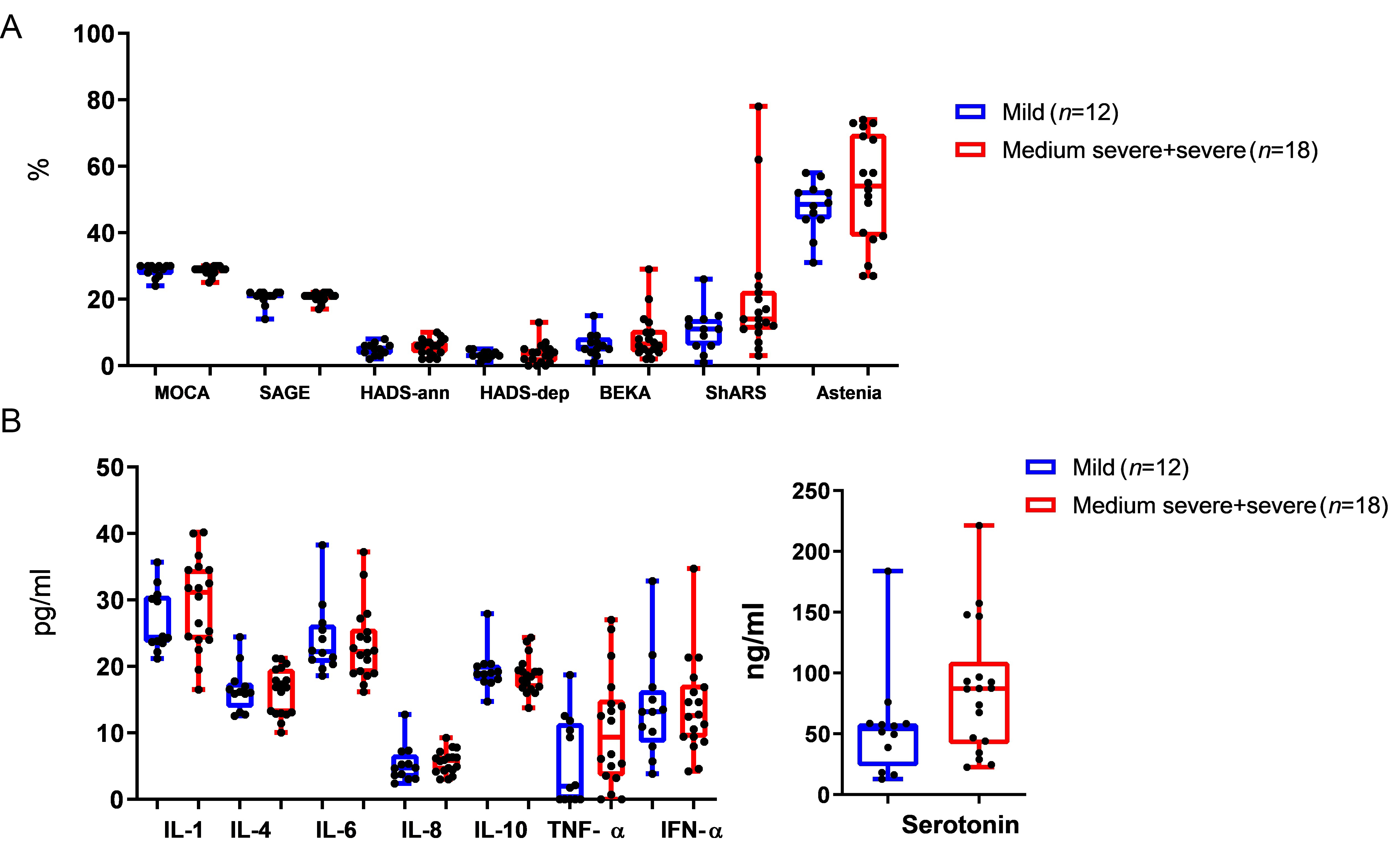
Clinical and blood test data in patients with PCS after COVID-19 of varying severity. (A) Anxiety and depression indicators; (B) comparative analysis of cytokine and serotonin values in serum. Medians (Me) and 95% confidence intervals (CI) are presented. IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; IFN-α: interferon-α; COVID-19: coronavirus disease-2019; PCS: post-coronavirus disease syndrome; HADS-ann: Hospital Anxiety and Depression Scale-anxiety; HADS-dep: Hospital Anxiety and Depression Scale-depression; ShARS: Sheehan Anxiety Rating Scale
At the same time, IL-10 levels were slightly reduced in moderate to severe post-COVID, although the results were not statistically significant (Figure 3B). Serotonin levels were also higher in patients with moderate to severe COVID-19. The differences were also not statistically significant.
Comparison of IgG subclasses to the SARS-Cov-2 S-protein showed that IgG2 predominates in patients with PCS, with elevated levels of IgG1 and IgG4 found among those with moderate to severe COVID-19 (Figure 4).
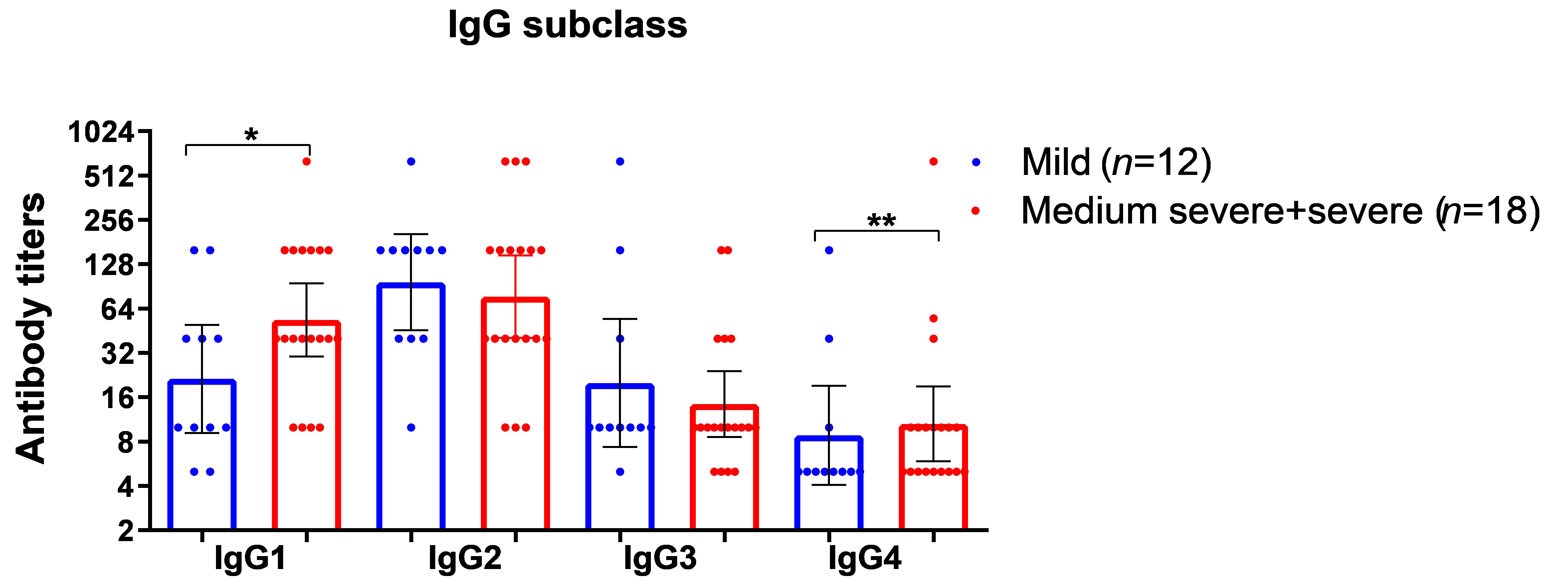
Results of the study on IgG subclass levels detected in patients with PCS after COVID-19 of varying severity. Geometric means and 95% confidence intervals (CI) are presened. * P < 0.05, ** P < 0.01. IgG: immunoglobulin G; COVID-19: coronavirus disease-2019; PCS: post-coronavirus disease syndrome
The levels of cytokines and serotonin in the serum of PCS patients and convalescent controls without PCS
In PCS patients, levels of IL-1, IL-4, IL-8, and IL-10 were significantly higher than those in convalescents without PCS (Figure 5).
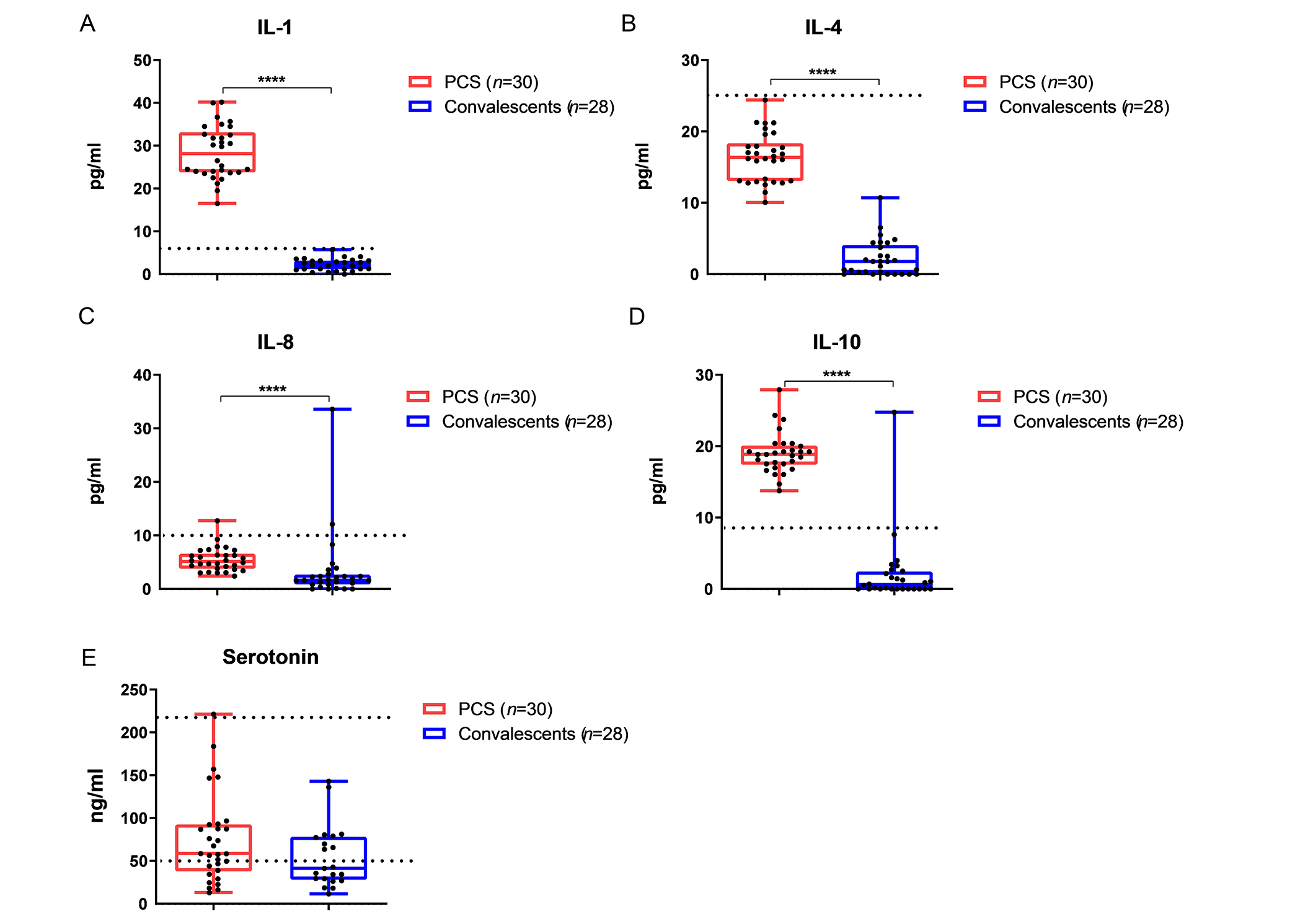
Comparative analysis of the content of cytokines and serotonin in sera of PCS patients and convalescents without post-COVID syndrome. (А) Content of IL-1, the reference range is from 0 to 4.9 pg/mL; (B) content of IL-4, the reference range is from 0 to 25 pg/mL; (C) content of IL-8, the reference range is from 0 to 9.1 pg/mL; (D) content of IL-10, the reference range is from 0 to 9.1 pg/mL; (E) content of serotonin, normal serotonin concentrations range is from 50 to 220 ng/mL. **** P < 0.0001. Medians (Me) and 95% confidence intervals (CI) are presented. Dotted lines indicate reference values or reference intervals. COVID-19: coronavirus disease-2019; PCS: post-coronavirus disease syndrome; IL-1: interleukin-6
With regard to IL-1, PCS patients had higher IL-10 levels than convalescents without PCS (Figure 5A). IL-4 and IL-8 values did not exceed normal ranges in either PСS or control groups (Figure 5B and 5C). As well as IL-1 levels, IL-10 levels were higher among PCS patients compared to convalescents without PCS (Figure 5D). Serotonin levels did not differ significantly between PCS patients and control groups. Mean serotonin levels were slightly higher in PCS patients than in controls (Figure 5E), but the number of patients with serotonin levels below reference values and within the reference interval was similar in both groups (Figure 5E).
The levels of humoral immunity levels in long-COVID patients and controls without PCS
Humoral immune responses to SARS-CoV-2 were significantly increased in PCS patients compared to controls in terms of IgG, IgM, and IgA compared to these indices in convalescents without PCS (Figure 6). The differences between the two examined groups of patients in terms of anti-RBD determined by concurrent ELISA were not statistically significant (Figure 6).
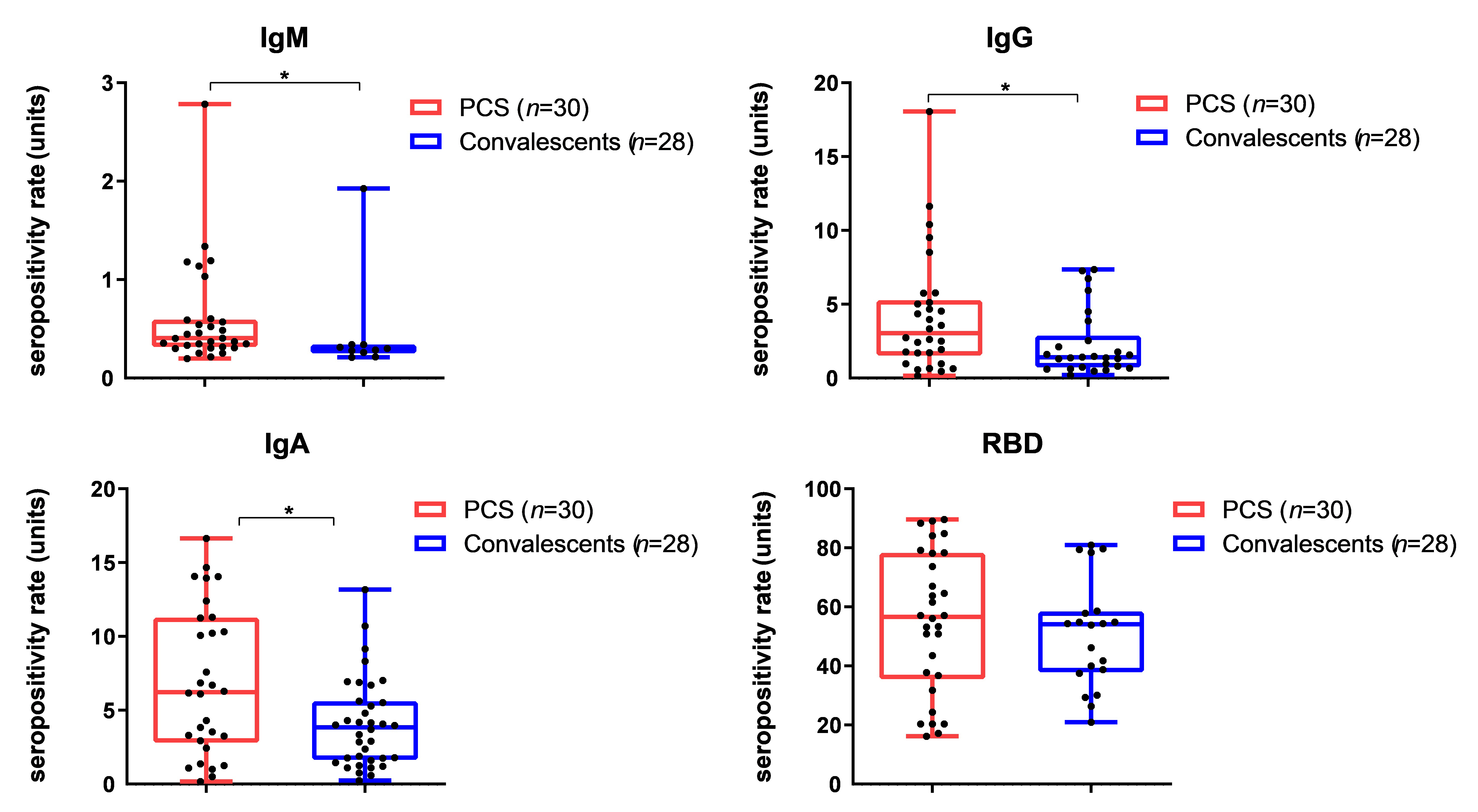
Humoral immunity levels in PCS patients versus controls. Control group consisted of 28 blood sera from convalescents without PCS with COVID-19 history. Medians (Me) and 95% confidence intervals (CI) are presented. * P < 0.05. IgM: immunoglobulin G; PCS: post-coronavirus disease syndrome; COVID-19: coronavirus disease-2019; RBD: receptor-binding domain
Correlation analysis on clinical and laboratory parameters in PCS patients
A noticeable positive correlation between the age of patients and HADS depression scores (rs = 0.5) was shown, as well as a moderate positive correlation between age and asthenia grades (rs = 0.4) (Figure 7). The data from tests for assessing the cognitive and mental functions of patients with PCS correlated well with each other (Figure 7) (rs = 0.5–0.7). The severity of cognitive and neurological impairments in most cases weakly or moderately positively correlated with the content of IL-1 (rs = 0.3–0.4) (Figure 7). The HADS anxiety scores were negatively correlated with IL-6 (rs = –0.5) (Figure 7).
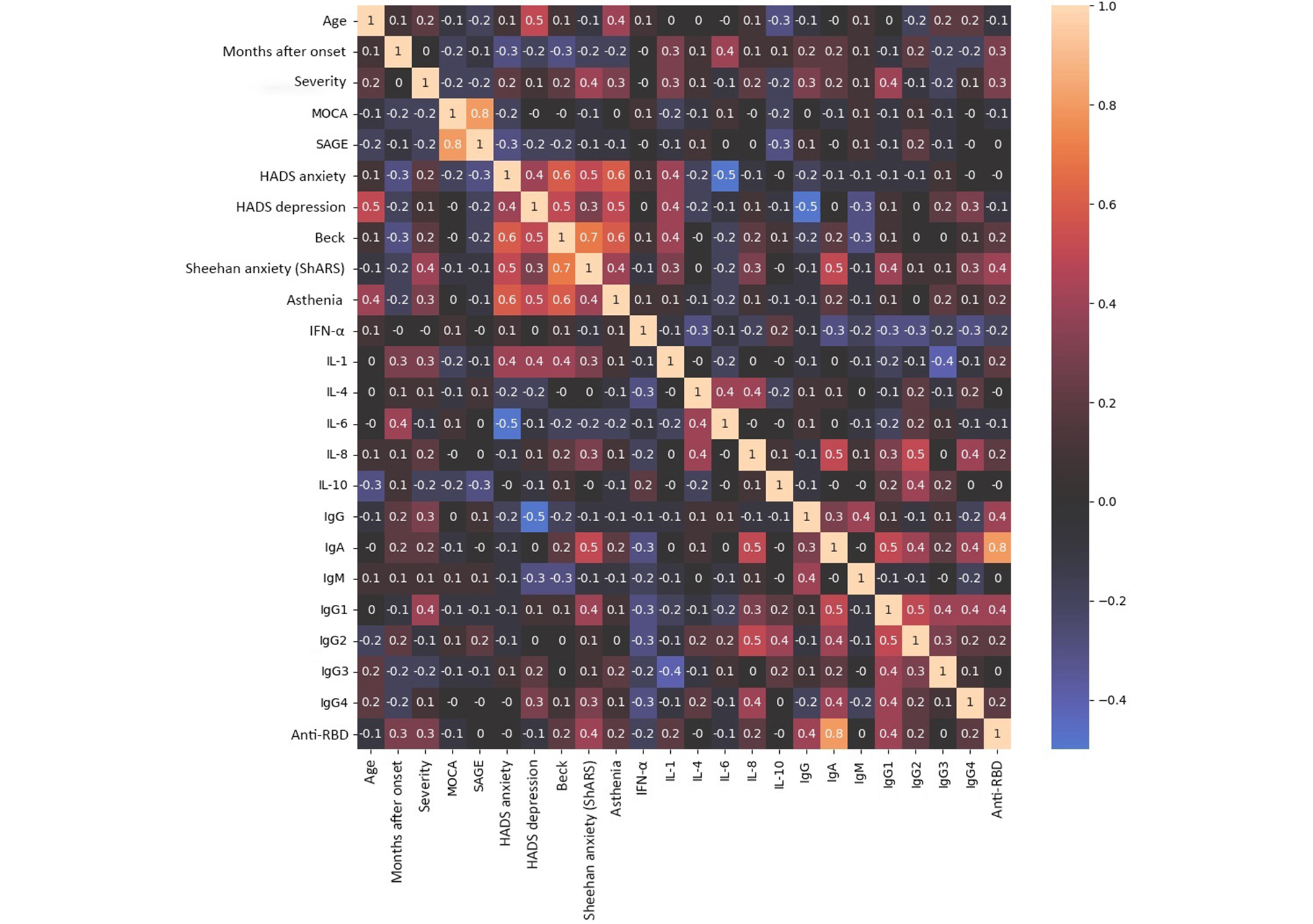
Correlation analysis of clinical data and blood test parameters in patients with post-COVID syndrome (n = 30). The data were normalized using the mean normalization method (Z-normalization). The pattern of feature intensity was obtained using the built-in functions of the Seaborn library in Python 3. Cells contain the values of the Spearman correlation coefficient (rs). A negative value of zero means a very small number that has no statistical significance. IgM: immunoglobulin G; COVID: coronavirus disease; IFN-α: interferon-α; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; RBD: receptor-binding domain; SAGE: Self-Administered Gerocognitive Exam; HADS: Hospital Anxiety and Depression Scale; MOCA: Montreal Cognitive Assessment
IgG1 antibody titers to the S-protein were medium to noticeably positively correlated with other classes and subclasses of antibodies (rs = 0.4–0.5) (Figure 7). A strong correlation was found between IgA and anti-RBD antibody levels (rs = 0.8) (Figure 7). Moderate to noticeable correlation of IgA, IgG2, and IgG4 levels with IL-8 values were also shown rs = 0.4–0.5 (Figure 7).
As expected, the levels of IgG, IgG1, and anti-RBD antibodies showed a weak to moderate positive correlation with COVID-19 severity (rs = 0.3–0.4) (Figure 7). Anti-RBD antibody levels were positively correlated with the number of months since disease onset (Figure 7). Anti-S IgG1 levels were moderately positively correlated with disease severity and Sheehan Anxiety Scale values (rs = 0.4) (Figure 7). The levels of IgA were noticeably correlated with Sheehan anxiety scale indices (rs = 0.5). At the same time, IgG levels were negatively correlated with Sheehan Anxiety Scale indices (rs = –0.1) (Figure 7).
Discussion
The late effects of COVID-19 (PCS) among which neurological symptoms are the most common long-term clinical manifestations have become a major global health problem [37]. There are various research methods for studying cognitive functions that allow for a comprehensive assessment of aspects of cognitive processes. One of the main methods for studying cognitive functions in clinical practice is neuropsychological testing. These are specialized tests designed to assess cognitive functions taking into account their neurological basis. Neuropsychological tests may include assessment of various aspects of memory, attention, executive functions, spatial and gnostic skills, and other cognitive processes [38]. These tests may also include assessment of deficits in cognitive functions associated with specific neurological conditions or diseases [37]. An important feature of using this method for diagnosing cognitive impairment is its ease of use, speed, and assessment of a wide range of cognitive indicators. The MOCA and the Mini-Mental State Examination (MMSE) are most often used in clinical practice. Due to its lower sensitivity, it was decided not to use the MMSE scale for the study, and the SAGE test was used. The peculiarity of this test is that it is completed independently by the patient. When assessing cognitive functions using the MOCA and SAGE scales in patients in the post-COVID period, a moderate decline in cognitive functions was detected in only two patients out of the total number of patients in the group. At the same time, no correlation was found with the severity of the disease, which is at odds with the data of some studies [39].
When assessing affective disorders, anxiety disorders were detected according to the HADS scale in 5 patients from the PCS group. Depressive disorders were found in 7 patients from the post-COVID-19 group, while 2 patients had moderate depressive disorder. An increase in the level of asthenia was detected in all patients in the post-COVID period according to the MFI-20 scale. This is consistent with the data available in the literature [40, 41]. A history of mild to moderate COVID-19 infection primarily increases the risk of developing affective disorders and asthenia and, to a lesser extent, the development of cognitive impairment.
Neuroinflammation may play a role in the various neurological symptoms and cognitive impairments observed in post-COVID patients. Neurofilament light chain (NFL) protein and glial fibrillary acidic proteins (GFAPs) that maintain the stability of neuronal axons and astrocytes may serve as circulating biomarkers associated with neuronal degeneration and injury [42]. Long-COVID patients with elevated blood NFL and GFAP levels had increased headaches and persistent neuropathic pain [43]. Peluso et al. [34] reported that serum levels of NFL and GFAP in acute COVID-19 patients were positively correlated with IL-6, TNF-α, and CCL2, which can induce immune cell activation and neuroinflammation. This indirect mechanism suggests that proinflammatory cytokines/chemokines may exacerbate significant neuronal injury. Previous studies have identified specific changes in cytokine profiles in long-COVID-19 patients. For example, some studies noted ongoing increases in cytokines such as IL-6 and IL-8. These markers indicate ongoing inflammation and may correlate with the severity and type of long-term COVID symptoms [44]. Prolonged action of IL-6, TNF-α, and C-reactive protein (CRP) was also involved in the systemic and neurological long-term sequelae of COVID-19 [45]. The present study found that IL-6 and IFN-α levels exceeded reference values and were similar to those of acute COVID-19 patients (Table 1), indicating ongoing inflammatory processes. The decrease in these indicators in convalescents without PCS was explained by their lack of neurological symptoms or longer time since the onset of the symptoms. If neurological disorders are detected in the post-COVID period, this data should be considered when prescribing anti-inflammatory therapy.
Our study showed that the severity of cognitive and neurological impairment in most cases was weakly or moderately positively correlated with IL-1 levels. IL-1 is considered a key mediator of immunity and inflammation [46]. In COVID-19, the hyperinflammatory IL-1 family of cytokines, including IL-1α, IL-1β, IL-18, IL-33, and IL-36, have been shown to be the main cause of inflammatory activity, and high levels of these cytokines are directly correlated with disease severity. Therefore, IL-1 family blockers can be considered as effective therapeutic agents for the treatment of COVID-19 [47]. Elevated levels of IL-1 in patients with PCS may reflect ongoing immune activation. It has been shown that chronic inflammation mediated by TNF-α release also contributes to cognitive dysfunction [48].
Another mechanism for PCS development involves chronic inflammation induced by type I IFN [49]. Our data on the increased levels of IFN-α in the most severe cases of COVID-19 and persistent elevations in IFN-α levels in patients with PCS and neurological disorders may support the theory of chronic inflammation caused by type I IFN in PCS. Some studies suggest that there may be a decrease in serotonin levels, which can impair the activity of the vagus nerve and worsen hippocampal reactions and memory [49], but in our study, we did not observe a decrease in serotonin levels in patients with PCS (Figure 5). The highest levels of serotonin were found in acute COVID-19 patients, and the lowest levels were in pre-COVID-19 samples from healthy patients collected in 2018 (Figure S1).
IL-8, also known as CXCL8, is a chemokine that plays an important role in the immune response by recruiting and activating neutrophils, which are critical for inflammation and infection-fighting, and may have a prognostic role in COVID-19 disease [50]. IL-8 has been linked to chronic inflammatory demyelinating polyneuropathy, thus it has been proposed as a possible biomarker of acute immune responses against the nervous system [51].
Our study found a tendency towards lower levels of IL-10 levels in PCS after moderate to severe COVID-19, which could indicate a decreased anti-inflammatory cytokine profile in more severe COVID-19. In the past, individuals with persistent post-COVID symptoms have exhibited lower levels of IL-4 and IL-10, which may indicate that these cytokines could serve as diagnostic markers for long-COVID, a condition characterized by an inflammatory response with reduced anti-inflammatory response mediated by IL-4 and IL-10 [52]. From another perspective, IL-4 contributes to neuroinflammation by recruiting immune cells into the CNS [53]. In our study, both IL-4 and IL-10 levels were higher in patients with PCS than in convalescent patients without PCS, although IL-4 levels were within normal ranges and IL-10 was above normal levels. These findings should be further explored with larger sample sizes.
In addition to the cytokine profile, IgM may provide insight into the immune response during and after infection and understanding the dynamics of viral clearance. Our study showed that several PCS patients had IgM antibodies to the SARS-CoV-2, and overall, the level of IgM seropositivity was higher among these patients (Figure 6). IgM is one of the first antibodies produced by the immune system in response to infection. In the context of COVID-19, it typically appears within days to weeks of symptom onset [54]. The presence of IgM is commonly indicated in recent infections [55]. In our study, IgM was detected on average 2 months after COVID-19, both after mild (in 2 people) and after moderate and severe (in 4 people). Whether the presence of IgM in the post-COVID period among patients diagnosed with neurological disorders for more than 2 months indicates the persistence of the virus is still unclear.
IgG antibodies are associated with long-term immunity [56]. Understanding the specific IgG subclasses produced in response to COVID-19 is critical to assessing the duration of immunity following natural infection or vaccination. The fact that in the present study the levels of IgG, but not IgA, negatively correlated with the Sheehan Anxiety Scale scores may be due to IgG exhibiting neutralizing properties to the SARS-CoV-2 virus. Also, in acute COVID-19, levels of IgM and IgG antibodies correlated negatively with levels of IL-6 and IFN-α (Figure 2B). All this together may indicate a positive role of antibody response in the pathogenesis of COVID-19. This does not allow us to exclude a positive role for individual classes and subclasses of antibodies in PCS.
Different IgG antibody subclasses (e.g., IgG1, IgG2, IgG3, and IgG4) have different functions and properties. Studying these antibody subclasses can provide insight into strength and persistence of immune memory following infection or vaccination. During the COVID-19 pandemic, some studies have shown that certain COVID-19 patients developed IgG4-associated diseases including parenchymal organs, lymph nodes, and pleural lesions [57]. The present study showed that after moderate and severe COVID-19, IgG2 and IgG4 were predominant in the blood of examined patients with PCS and neurological symptoms. The positive correlations between IgA, IgG2, and IgG4 levels with a cytokine-like IL-8 suggest further investigation to the role of IgG4 in post-COVID with neurological features. In summary, this study emphasizes the importance of addressing innate and adaptive immune responses in long-COVID patients with neuroinflammation.
The main limitation of the study is the relatively small sample size, which limits the statistical analyses. In addition, we studied only a limited number of cytokines. However, the study does include a control group of convalescent patients without PCS or neurologic manifestations.
Data on the increase in IFN-α during the most severe course of COVID-19 and the persistence of high levels of IFN-α in the blood during PCS, as well as the detection of IgM antibodies to SARS-CoV-2 in post-COVID serum, were obtained. Elevated levels of cytokines, especially early ones, were higher in patients with PCS compared to those who recovered from COVID-19 without PCS. In PCS patients, IgM antibodies were detected, which could indicate the persistence of the virus. No increase in serotonin was detected in PCS patients compared to convalescent controls.
Abbreviations
| CNS: | central nervous system |
| GFAP: | glial fibrillary acidic protein |
| HADS: | Hospital Anxiety and Depression Scale |
| IFN-α: | interferon-α |
| IgM: | immunoglobulin M |
| IL: | interleukin |
| MMSE: | Mini-Mental State Examination |
| MOCA: | Montreal Cognitive Assessment |
| NFL: | neurofilament light chain |
| OD450: | optical density at 450 nm |
| PCS: | post-coronavirus disease syndrome |
| RBD: | receptor-binding domain |
| SAGE: | Self-Administered Gerocognitive Exam |
| TGF: | transforming growth factor |
| TNF: | tumor necrosis factor |
Supplementary materials
The supplementary figure for this article are available at: https://www.explorationpub.com/uploads/Article/file/1003184_sup_1.pdf.
Declarations
Acknowledgments
The authors acknowledge Valentina Smelova, Analytics Development Manager (IP ‘Valentina Smelova Consulting’) for her excellent support in statistical processing and graphical representation of the data obtained.
Author contributions
YD: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Funding acquisition. ZM: Conceptualization, Investigation, Writing—review & editing. OT: Investigation, Formal analysis. TS, GM, and AL: Data curation, Formal analysis. PK and GL: Methodology, Investigation. IK: Investigation, Validation. SP: Data curation, Project administration. EF: Data curation, Validation. AS: Validation, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Local Ethics Committee of the Federal State Budgetary Scientific Institution “IEM” (protocol 1/23 dated 04/20/2023).
Consent to participate
All study participants signed written informed consent.
Consent to publication
Not applicable.
Availability of data and materials
The authors cannot share datasets as this is not permitted by the rules of the Institute of Experimental Medicine.
Funding
The work was supported by budget funds of the Federal State Budgetary Scientific Institution “Institute of Experimental Medicine”, topic FGWG-2003-0002. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.