Affiliation:
Allergy Department, Special Hospital for Pulmonary Diseases, 10000 Zagreb, Croatia
Email: vvukicevi@sfzg.unizg.hr
ORCID: https://orcid.org/0000-0001-8145-6840
Explor Immunol. 2025;5:1003188 DOI: https://doi.org/10.37349/ei.2025.1003188
Received: June 19, 2024 Accepted: March 02, 2025 Published: March 20, 2025
Academic Editor: Manuela Neuman, University of Toronto, Canada
The article belongs to the special issue Hypersensitivity Syndrome Reactions versus Allergy and Drug
Cutaneous reactions present a diagnostic challenge, mainly when multiple factors, such as infections, medications, and environmental triggers, contribute to the clinical picture. Erythema multiforme (EM) is an acute, self-limiting mucocutaneous disorder that is most commonly triggered by herpes simplex virus (HSV) but can also be associated with drug-induced hypersensitivity reactions. Diagnosing EM becomes even more complex in patients taking photosensitizing medications, such as doxycycline, which can cause phototoxic or photoallergic reactions. Differentiating between drug-induced and infection-associated EM, as well as distinguishing it from more severe conditions like Stevens-Johnson syndrome (SJS), is crucial for appropriate management. This case report presents a case of a 57-year-old Caucasian female with a history of penicillin allergy who developed a phototoxic reaction to doxycycline following sun exposure. She was treated with silver sulfadiazine for her skin lesions but subsequently developed EM, with target-like lesions predominantly on the legs and a concurrent herpes simplex labialis infection. Laboratory findings were unremarkable, and there was no mucosal involvement. Given the suspected drug-induced nature of the reaction and the presence of HSV, a cautious approach was taken. Treatment with oral prednisone led to the resolution of symptoms without recurrence. Patch testing for doxycycline and silver sulfadiazine was omitted due to the risk of severe cutaneous adverse drug reactions (SCARs) and their non-essential status. Instead, penicillin testing was prioritized due to its clinical importance, and the patient successfully passed the oral amoxicillin challenge. This case highlights the diagnostic challenges of differentiating between drug-induced, infection-triggered, and photosensitivity-related cutaneous reactions. A careful evaluation of medication history, infection status, and clinical presentation is essential to guide the management of this condition.
Cutaneous reactions present a multifaceted challenge, particularly when complicated by concurrent infections, medication utilization, and environmental triggers such as sun [1]. Distinguishing adverse drug reactions (ADRs) from other dermatological conditions such as viral exanthema, collagen vascular disease, neoplasia, bacterial infection, psoriasis, and autoimmune blistering disease, among others, requires precision, especially given the potential gravity of reactions necessitating immediate identification and discontinuation of the offending drug [2]. Erythema multiforme (EM) is an acute, self-limiting inflammatory mucocutaneous disorder characterized by a polymorphic eruption, typically presenting as target-shaped lesions [3]. It is most commonly triggered by infections, with herpes simplex virus (HSV) being the primary cause in 70–80% of cases [4]. However, less frequently, EM can be associated with medications, particularly antibiotics such as sulfonamides and tetracyclines, as a manifestation of a type IV hypersensitivity reaction [4]. Furthermore, EM can be confused with other more serious conditions such as severe cutaneous adverse drug reaction (SCAR) called Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) because although these are two different conditions, initial skin manifestations are similar, making early diagnosis difficult [5].
Additionally, diagnosing skin eruptions becomes even more challenging when patients on photosensitizing medications, such as tetracyclines, are exposed to UV radiation, leading to photosensitizing drug reactions, primarily phototoxicity and, less commonly, photoallergy [6]. Phototoxicity is a non-immunologic reaction causing direct skin damage, such as sunburn-like rash, less often pseudoporphyria, and lichenoid reactions. Symptoms appear within 48 hours and require high drug doses [6]. In contrast, photoallergy, a type IV hypersensitivity, occurs only in sensitized individuals after minimal drug and UV exposure, leading to eczematous eruptions within 24–48 hours after re-exposure [7].
This case report aims to illustrate the complexities and challenges involved in diagnosing and managing different cutaneous reactions, mainly when multiple factors contribute to the clinical picture. By examining a patient who experienced a phototoxicity reaction to doxycycline and sunburn lesions of the skin followed by EM after treatment with silver sulphadiazine and herpes simplex infection, this report aims to highlight the difficulties in differentiating between various types of ADRs and infection-driven skin manifestations.
A 57-year-old Caucasian female patient with a medical history of penicillin allergy sought care at our allergy clinic in June 2023. The patient presented with sunburn-like eczema on sun-exposed skin, particularly on her shins, but was otherwise in good health and did not report any other complaints. She had been taking doxycycline for a urinary infection caused by Ureaplasma urealyticum for the past seven days. Otherwise, she was healthy and did not take any other regular treatments. Feeling well after five days of doxycycline, she decided to go hiking in the mountains over the weekend. The following day, she noticed painful sunburns on her skin, especially her shins. She sought care from her general practitioner, who referred her to our allergy department for further evaluation. Based on the case history, we concluded it was a phototoxic reaction from sun exposure and doxycycline therapy. The patient was given topical therapy with silver sulfadiazine and advised to stop using doxycycline and limit sun exposure. Two weeks later, she returned with diffuse red papules that evolved into flat atypical target-shaped lesions on her legs, primarily her shins (Figure 1). During the examination, crusts on her upper lip (Figure 2), remaining from a recent herpes simplex infection that started ten days prior, were noted. However, there were no signs of mucosal involvement, facial oedema, lymphadenopathy, or respiratory tract infection.
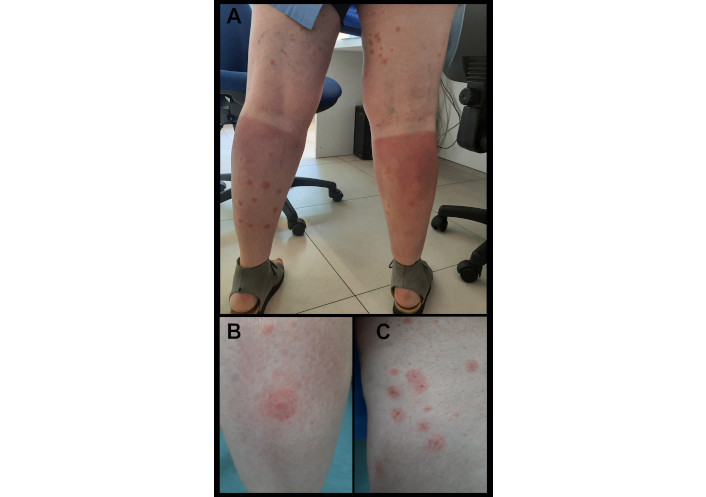
Clinical presentation of erythema multiforme. (A) Shows erythema multiforme on the legs, combined with signs of eczema caused by phototoxicity to doxycycline. (B) and (C) Close-up photos of erythema multiforme lesions
The laboratory test results were within normal limits. There was no sign of eosinophilia, lymphocytosis, or end-organ damage.
Oral corticosteroid treatment with prednisone (40 mg for five days) was initiated, resulting in improvement of the lesions. A tapering dose schedule eventually resolved the symptoms within three weeks. In the follow-up consultation three months later, there was no recurrence of the skin lesions, and the lesions completely dissolved without leaving any scars. Additionally, the patient was tested for penicillin due to a childhood history of allergy to penicillin of unknown presentation, and she passed the oral amoxicillin challenge tests. Drug testing for silver sulfadiazine and doxycycline was omitted due to the potential risk of inducing SCAR and the fact that these medications are not considered essential. Given their non-essential nature and the availability of alternative drug options, it was deemed safer to avoid testing them and opt for alternative medications that would pose a lower risk of SCARs in the future. Figure 3 presents a timeline of events.
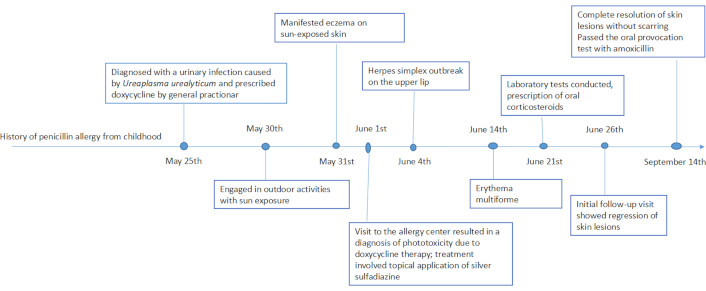
Chronology of events in a case report: doxycycline phototoxicity and erythema multiforme
The diagnostic challenges associated with the onset of EM present a significant hurdle, particularly during the initial stages of presentation. Distinguishing between microbial pathogens, such as Mycoplasma pneumonia and herpes viruses, and drug-related triggers can be complex, especially in cases complicated by concurrent infections, pharmacological interventions and sun exposure [8, 9]. In our case report’s context, potential triggers range from herpes virus infections to doxycycline or silver sulfadiazine involvement. However, a distinct form of EM, known as photosensitive erythema multiforme (PEM), is a rare condition characterized by lesion distribution on sun-exposed areas. It has been reported at sites of sunburn, following episodes of polymorphic light eruption or herpes labialis, and in association with drug exposure [10, 11], as observed in our case. However, there is a case report of photosensitive recurrent EM, unrelated to drug exposure or herpes labialis, that was reproduced through light testing, and the patient’s lesions responded to both oral prednisone and hydroxychloroquine [12]. Therefore, it is important to remain cautious, as this condition could be a manifestation of systemic lupus erythematosus (SLE), called Rowell syndrome [13]. However, since no other clinical symptoms of SLE were present in our patient, this diagnosis was promptly ruled out.
Given the suspected drug-induced nature of the reaction in our case and documented evidence indicating that EM can be triggered by doxycycline [14] and silver sulfadiazine [15], patch testing was considered as a diagnostic approach.
Patch testing is the safest option for testing for type IV cutaneous ADR to suspected culprit drug, and should be conducted approximately three months after the resolution of the eruption [16, 17]. Since commercial patch test preparations exist for only a few drugs, most tests require the drug in various recommended concentrations and vehicles [16]. However, although literature supports the good sensitivity of patch testing for silver sulfadiazine [18–21] and doxycycline [22, 23], particularly in cases of suspected photoallergy [24, 25], these tests were omitted in this case due to the potential risk of SCARs [16] and the non-essential nature of these medications. Instead, after three months, we prioritized testing for penicillin, as it is a more clinically important drug. Furthermore, doxycycline, a member of the tetracycline class of antibiotics, is notably recognized for its propensity to induce phototoxic reactions, especially at higher doses [26, 27]. However, these reactions usually resolve after discontinuation of the drug, eliminating the need for further testing [27].
Notably, although Mycoplasma pneumonia has been linked to EM [28], the association with Ureaplasma urealyticum remains unestablished in current literature, despite both belonging to the same Mycoplasma family [29]. Therefore, in this case, we have excluded Ureaplasma urealyticum from the potential etiological factors contributing to EM.
The pathogenic pathways differ between infection-triggered and drug-induced EM. In infection-induced cases, autoreactive T-cells response to cells containing the HSV-DNA polymerase gene, ultimately leading to keratinocyte lysis and epidermal damage mediated by immune cell recruitment [30, 31]. Conversely, drug-induced EM involves tumour necrosis factor-alpha, perforin, and granzyme B, contributing to epidermal destruction [31]. Consequently, clinical presentations vary between herpes virus-induced and drug-induced reactions, with distinct lesion distribution and mucosal involvement patterns [4]. In HSV-triggered EM, typically target lesions predilection sites are in the acral extremities, while mucosal lesions are absent or minimal, with no or mild prodromal signs/symptoms. The symptoms are acute, self-limited, and sometimes recurrent, with no reported mortality [4]. In contrast, drug-triggered EM is typically presented first with the prodromal flu-like syndrome and followed by blistering lesions on acral extremities, with mucosal involvement (predominantly orally) [4]. Additionally, trauma and sun exposure may influence the lesion distribution in EM, as evidenced by cases where lesions appear in previously sunburned areas [11]. Vigilance in recognizing warning signs indicative of SCARs is crucial to preempt the progression to life-threatening conditions like SJS. SCARs represent a severe manifestation of ADRs, characterized by limited treatment options and increased mortality risk [32–34].
In this case, differentiating the specific trigger agent for the reaction posed a challenge, emphasizing the cautious approach required in diagnostic testing to prevent exacerbation or adverse outcomes. Due to the favourable clinical response, additional testing, such as a skin biopsy, was not performed to differentiate the underlying cause. Herpes simplex infection was diagnosed based on clinical presentation, and PCR testing for HSV was not conducted. Additionally, since there were no signs of respiratory tract infection, testing for Mycoplasma pneumoniae was deemed unnecessary. All these tests require a long turnaround time for results, and as clinicians, we often need to rely on the clinical presentation to guide decision-making. Given the complexities involved, careful consideration was given to penicillin allergy testing, guided by the patient’s historical medical records and the necessity for accurate delabeling in cases of multiple drug allergies [35, 36].
ADR: adverse drug reaction
EM: erythema multiforme
HSV: herpes simplex virus
SCAR: severe cutaneous adverse drug reaction
SJS: Steven-Johnson syndrome
SLE: systemic lupus erythematosus
I express my sincere gratitude to those who contributed to this case report. First and foremost, we extend our heartfelt thanks to the patient for consenting to the publication of this report and for their cooperation throughout the process. My appreciation goes to the Special Hospital for Pulmonary Diseases, Zagreb, Croatia, specifically to the staff of the allergy unit, for providing the necessary facilities and support that made this case study possible. I thank everyone who, directly or indirectly, played a role in the successful completion of this case report.
VVL: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
The author declares that she has no conflicts of interest.
According to the policy of our hospital—Special Hospital for Pulmonary Diseases, ethical committee approval is not required for case reports, provided that written informed consent is obtained from the patients.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
All data for this study are included in the manuscript. For further discussion regarding this report, please contact the corresponding author.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4770
Download: 43
Times Cited: 0
Shambo S. Samajdar ... Shashank R. Joshi
