Affiliation:
1Talwar Research Foundation, New Delhi 110068, India
2Qiagen Sciences Inc., Germantown, MD 20874, USA
Affiliation:
1Talwar Research Foundation, New Delhi 110068, India
3uniQure USA, Lexington, MA 02421, USA
Explor Immunol. 2021;1:398–405 DOI: https://doi.org/10.37349/ei.2021.00026
Received: June 19, 2021 Accepted: September 29, 2021 Published: December 31, 2021
Academic Editor: Dominique J. Charron, Hospital Saint Louis, France
The article belongs to the special issue Human Reproduction: Involvement of the Immune System
This article is a tribute and homage to Gerard Chaouat who invited me to contribute this article. My years in France have remained very memorable to me. Reviewed briefly is the vaccine that was made against human chorionic gonadotropin (hCG) to prevent unwanted pregnancy in sexually active women. It has now been developed as a genetically engineered recombinant vaccine and passed onto industry for its production under good manufacturing practices (GMP) conditions for confirmatory trials. The trials have received the approval of the Drugs Controller General of India. The trials have started but have been interrupted by the coronavirus disease 2019 (COVID-19) pandemic. This vaccine is likely to have another highly beneficial application in the treatment of cancers expressing ectopically hCG.
Human chorionic gonadotropin (hCG) is not made normally by a woman and its appearance in urine or blood is taken as an indication of the on-set of pregnancy. With these established criteria, we made vaccine-inducing antibodies against hCG, which should be safe in a non-pregnant female devoid of hCG. As hCG plays an important role in the establishment of pregnancy, vaccine-inducing antibodies neutralizing hCG should prevent pregnancy. This was proven to be true by our phase II clinical trials conducted on the initial vaccine developed by us [1]. To enhance immunogenicity, β-hCG was linked non-covalently to α-subunit of ovine luteinizing hormone (LH). This heterospecies dimer (HSD) linked to tetanus toxoid (TT) was more immunogenic and induced higher antibody titers than hCGβ-TT [2]. It was then taken through phase I safety followed by phase II efficacy trials with the approval of ethics and drugs regulatory authorities. All women immunized generated antibodies. On the decline of titers, they were given booster injections to maintain antibody titers above 50 ng/mL. They all remained protected from becoming pregnant. Only 1 pregnancy occured in 1224 cycles [1]. Antibody response in 4 women of proven fertility who were sexually active, is shown in Figure 1.
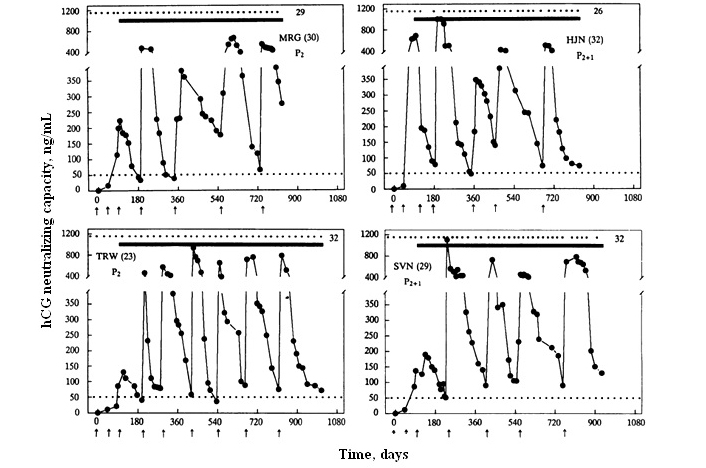
Anti-hCG response to the HSD vaccine in 4 sexually active women of proven fertility. MRG 30-yr-old and TRW 23-yr-old had 2 children each; MJN 32-yr-old and SVN 29-yr-old had 2 children each and 1 elective termination of pregnancy. All of them remained protected from becoming pregnant over 26–32 cycles. Dots at the top edge represent the menstrual events that remained regular, solid lines denote the period over which they were exposed to pregnancy. Arrows indicate the day on which the vaccine was given. Booster injections were given to keep antibody titers above 50 ng/mL
Note. Reprinted from “A vaccine that prevents pregnancy in women” by Talwar GP, Singh O, Pal R, Chatterjee N, Sahai P, Dhall K, et al. Proc Natl Acad Sci U S A. 1994;91:8532–6 (https://doi.org/10.1073/pnas.91.18.8532). © 1994 National Academy of Sciences.
All women continued to ovulate as indicated by progesterone in the luteal phase. Menstrual regularity was maintained. Antibodies generated by the vaccine did not intervene with normal reproductive functions. They remained sexually active.
The block of fertility was reversible. With the decline of antibodies titers, women desiring further a child, conceived at antibody titers below 10 ng/mL and gave birth to normal children fully comparable to others in the family born before they joined the trials [3].
I retired as director of the National Institute of Immunology (NII), New Delhi, India, and my successor asked me to leave the vaccine with NII. However, nothing was done. In 2006, I received a grant under Indo-US Bilateral Program to revive the vaccine. We now thought of making a genetically engineered recombinant vaccine so that it can be made at low cost with consistent characteristics, and made accessible to all, wherever they are, including France.
In the new vaccine, the β-subunit of hCG was linked to B subunit of heat-labile enterotoxin (LTB) of Escherichia coli as a carrier (Figure 2). The carrier chosen in this design of the vaccine does not evoke carrier-induced-immuno-suppression which TT as carrier did on continuous repeated use for long periods [4]. The B subunit of enterotoxin does not have these disadvantages. LTB has also adjuvant properties and evokes a mucosal response.
Keeping all these properties in mind, LTB was used to replace the previous carriers TT and diphtheria toxoid (DT) [5].
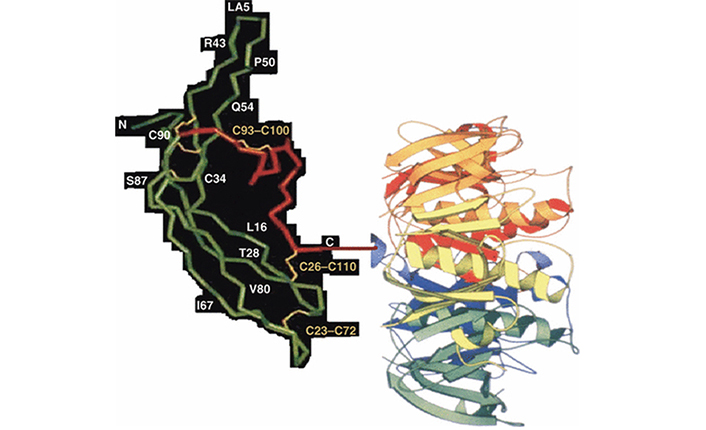
Recombinant anti-hCG vaccine hCGβ-LTB. The carrier LTB is linked at the C-terminal amino acid glutamine of hCGβ. Reproduced from [6]
Note. Reprinted from “Possibility and potential of a vaccine against human chorionic gonadotropin for family planning” by Purswani S, Lohiya NK, Talwar GP. Curr Sci. 2010;99:169–76 (https://www.currentscience.ac.in/Volumes/99/02/0169.pdf). © 2010 Current Science Association.
The hCGβ-LTB vaccine adsorbed on alhydrogel was used along with heat-killed Mycobacterium indicus pranii (MIP formerly known as Mw), a vaccine which we had developed originally against leprosy. MIP is a potent potentiator of humoral and cell-mediated immune responses [7]. The atomic microscopic image of MIP is shown in Figure 3. Figure 4 shows bioneutralization capacity of hCG by antibodies generated by hCGβ-LTB vaccine given with and without MIP.

Atomic force microscopic image of MIP
Note. Adapted from “An immunotherapeutic vaccine originally developed against leprosy is effective not only against leprosy but also against tuberculosis, anogenital warts and some cancers–a potent invigorator of immune response” by Talwar GP, Sonar K, Gupta JC, Puruswani S, Bhaskar S, Panda AK, et al. Int J Infect Dis. 2020;1:1–9 (https://unisciencepub.com/storage/2020/07/An-Immunotherapeutic-Vaccine-Originally-Developed.pdf). © 2020 GP Talwar.
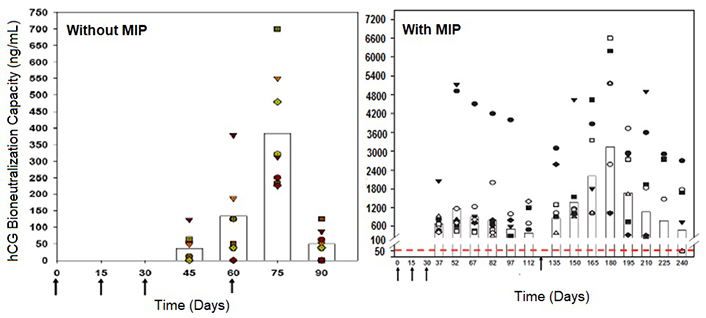
Enhancement of antibody response to the hCGβ-LTB vaccine in Balb/c mice by MIP. Mice were immunized intra-muscularly with 2μg of the vaccine adsorbed on alum with or without MIP. Primary immunization consisted of 3 injections given at fortnightly intervals followed by a booster on day 60 or 120. The symbols represent the titers in a given mouse. Bars give the geometrical means
Note. Reprinted from “Making of vaccines against human chorionic gonadotrophin for control of fertility of women without impairment of ovulation and menstrual regularity” by Talwar GP, Gupta JC, Nand KN, Thapa R, Mehta M. Reproductiv Immunol Open Acc. 2016;1:4 (https://doi.org/10.21767/2476-1974.100004). CC BY.
Besides enhancing substantially antibody titers generated by the genetically engineered hCGβ-LTB vaccine, MIP brings in additional advantages to the body. It is effective against tuberculosis and several cancers [8, 9].
Very recently 2 clinical trials with MIP on coronavirus disease 2019 (COVID-19) infected patients have been published. In one conducted at Fortis Hospital in Mumbai, 117 patients suffering from COVID-19, were given treatment with MIP (Mw), 0.3 mL intradermal injections every day for 3 days, It led to rapid recovery of 116 patients. No systemic side-effects were observed of using Mw, MIP [10]. Another randomized trial with MIP (Mw) has been carried out in critically ill patients infected with COVID-19 in Delhi. The trial was on 42 subjects (22 patients treated with MIP, 20 with placebo). On days 14 and 21, subjects on the Mw (previous code of MIP) arm had a better clinical status than placebo [11]. No adverse events related to MIP use were observed.
We obtained the approval of the Review Committee of Genetic Manipulations (RCGM) for our recombinant vaccine after extensive toxicology studies. With the permission of the Drugs Controller General of India (DCGI) and ethics committees, a combined phase I/II trial has started at the All India Institute of Medical Sciences, New Delhi, and Sir Gangaram Hospital, New Delhi, India. Before the unforeseen COVID epidemic, 9 women received the primary injections consisting of 2 injections at fortnight intervals of the DNA version of the recombinant vaccine followed by 2 injections of the protein version of the recombinant vaccine. As the recombinant vaccine is first made in DNA form, followed by its expression as protein, we have tested in mice, the immunogenicity of the combined use of the DNA form of the vaccine for priming, followed by the protein version of the vaccine. This mode of immunization generates a higher antibody response in mice (Figure 5) [12], hence the adoption of this regime for the clinical trial.
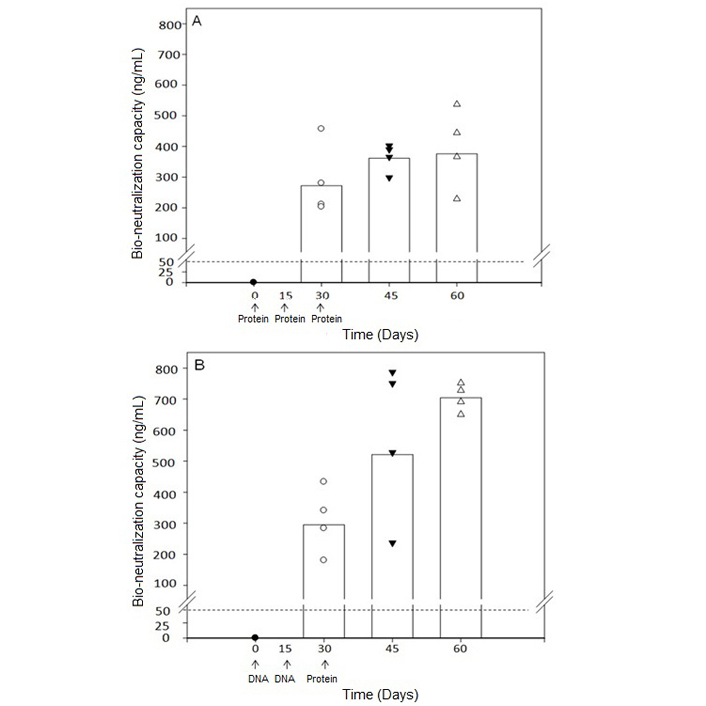
Bioneutralization capacity of anti-hCG antibodies. (A) Immunization with protein form of the vaccine for the three primary injections; (B) immunization with DNA form of the vaccine for the first two injections followed by protein form of the vaccine. A dotted line of 50 ng/mL is the protective threshold of antibodies for preventing pregnancy in humans
Note. Reprinted from “Priming with DNA enhances considerably the immunogenicity of hCG β-LTB vaccine” by Nand KN, Gupta JC, Panda AK, Jain SK, Talwar GP. Am J Reprod Immunol. 2015;74:302–8 (https://doi.org/10.1111/aji.12388). © 2014 Wiley Blackwell.
An unexpected observation was the formation of nodules at the site of immunization in 3 women. Experiments were carried out in mice, where again nodules were seen after 2 injections of the DNA version followed by 2 injections of the protein version of the vaccine, all given intradermally with MIP as adjuvant (unpublished observations). We hypothesized that repeated use of the adjuvant in all 4 injections may have caused the formation of nodules. Employing MIP only in the first DNA version of the vaccine followed by the remaining one injection of DNA and two of the protein versions of the recombinant vaccine without MIP was seen to avoid the formation of nodules in mice. This mode of immunization will be followed in the clinical trial.
The recombinant vaccine has been passed on to M/s Bharat Biotech, who are making the recombinant vaccine under GMP conditions and supplying it free of charge for the clinical trial. The clinical trial has been interrupted by the COVID-19 epidemic. It will be resumed on the removal of the restrictions.
A number of papers have appeared reporting the ectopic synthesis of hCG by a variety of cancers at the advanced stage: lung cancer [13], bladder carcinoma [14], colorectal carcinoma [15], pancreatic carcinoma [16], breast cancer [17], cervical carcinoma [18], oral cancers [19], vulva/vaginal cancers [20], prostate cancer [21] and gastric carcinomas [22]. At this stage, the tumors are highly metastasizing and aggressive. We confirmed in mice that antibodies generated against hCG bind selectively to the tumor secreting hCG and not elsewhere in the body (Figure 6) [23]. What is amazing and highly interesting is that antibodies against hCG exercise impressive cytotoxicity on tumor cells expressing ectopically hCG (Figure 7).
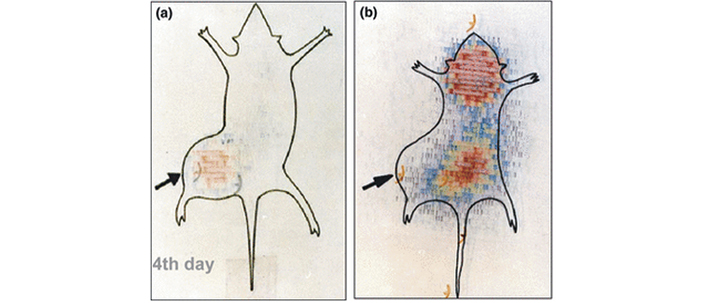
Imaging and selective delivery of radiations to tumors expressing hCG. (a) Whole-body scan of a JEG-3 tumor-bearing nude mouse on 4th day after injection of 131I anti-hCG monoclonal antibody; (b) whole-body scan of a JEG-3 tumor-bearing nude mouse injected with 131I irrelevant monoclonal antibody on day 4
Note. Adapted from “Immunological approaches against human chorionic gonadotropin for control of fertility and therapy of advanced-stage cancers expressing hCG/subunits” by Talwar GP, Gupta JC, Shankar NV. Am J Reprod Immunol. 2011;66:26–39 (https://doi.org/10.1111/j.1600-0897.2011.01002.x). © 2011 Wiley Blackwell.
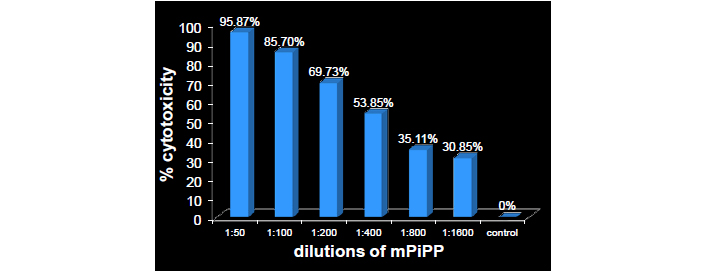
Dose-dependent cytotoxicity exercised by the monoclonal anti-hCG-antibody PiPP on lung cancer cells (A549)
Note. Reprinted from “Immuno-Interception of Human Chorionic Gonadotropin has Two Applications of Extraordinary Utility” by Talwar GP, Gupta JC. Perceptions Reprod Med. 2019;3:PRM.000565 (http://dx.doi.org/10.31031/prm.2019.03.000565). © 2018 Talwar GP.
Thus an additional unforeseen use of anti-hCG vaccine along with humanized antibodies will be in the treatment of such cancers.
A recombinant vaccine against hCG has been made and passed onto the industry. It is highly immunogenic. Its safety has been established and approval has been received from the DCGI and ethics committees for confirming its ability to prevent pregnancy by another trial. The trial which started around 24 months back is suspended due to the COVID-19 pandemic. An additional, but highly useful application of this vaccine is expected in advanced stage cancers expressing ectopically hCG.
COVID-19: coronavirus disease 2019
hCG: human chorionic gonadotropin
LTB: heat-labile enterotoxin
MIP: Mycobacterium indicus pranii
TT: tetanus toxoid
GPT developed the concept and coordinated the research work cited in the review. JCG, SP, KNN, and PP contributed to the development of the recombinant hCG vaccine and its evaluation. HKV undertook studies on immunotherapy of cancers cells. KME contributed to making available the GMP grade vaccine for the clinical trial.
The authors declare no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Part of the work reviewed in this article received grants from the Indian Council of Medical Research and the Department of Biotechnology, Ministry of Science and Technology, Govt of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 6604
Download: 51
Times Cited: 0
Gustaaf Albert Dekker, Pierre Yves Robillard
Kushaan Khambata ... Satish K. Gupta
Julia Szekeres-Bartho
Shigeru Saito ... Sayaka Tsuda
Noémie Abisror ... Arsene Mekinian
Ruchi Sachdeva, Rahul Pal
Shibin Cheng ... Surendra Sharma
Betcy Susan Johnson, Malini Laloraya
Alison McCallion ... Chandrakant Tayade
Pier Luigi Meroni ... Francesco Tedesco
Marijke M. Faas
Alaa Kazhalawi ... Nathalie Lédée
Chiara Agostinis ... Roberta Bulla
Mickey V. Patel ... Charles R. Wira
Thanh Luu ... Joanne Kwak-Kim
