Affiliation:
Department of Physiology, Midnapore College, Midnapore, Paschim Medinipur, West Bengal 721101, India
Email: saptadip174@gmail.com
ORCID: https://orcid.org/0000-0001-6741-699X
Explor Immunol. 2021;1:90–111 DOI: https://doi.org/10.37349/ei.2021.00009
Received: April 02, 2021 Accepted: June 02, 2021 Published: June 30, 2021
Academic Editor: Masutaka Furue, Kyushu University, Japan
The article belongs to the special issue Cross Talk Among Skin Cells and Immune Cells
Skin is the largest organ of the body having multifunctional activities. It has a dynamic cellular network with unique immunologic properties to maintain defensive actions, photoprotection, immune response, inflammation, tolerogenic capacity, wound healing, etc. The immune cells of the skin exhibit distinct properties. They can synthesize active vitamin D [1,24(OH)2D3] and express vitamin D receptors. Any difficulties in the cutaneous immune system cause skin diseases (psoriasis, vitiligo, atopic dermatitis, skin carcinoma, and others). Vitamin D is an essential factor, exhibits immunomodulatory effects by regulating dendritic cells’ maturation, lymphocytes’ functions, and cytokine production. More specifically, vitamin D acts as an immune balancing agent, inhibits the exaggeration of immunostimulation. This vitamin suppresses T-helper 1 and T-helper 17 cell formation decreases inflammatory cytokines release and promotes the maturation of regulatory T cells and interleukin 10 secretion. The deficiency of this vitamin promotes the occurrence of immunoreactive disorders. Administration of vitamin D or its analogs is the therapeutic choice for the treatment of several skin diseases.
Vitamin D belongs to the fat-soluble group in the common classification of vitamins. A healthy diet meets the daily requirements of vitamins. They are essential for body growth, tissue repair, disease prevention, and immunomodulation [1, 2]. Despite the diet, niacin and vitamin D are synthesized in the body. Amino acid tryptophan is the precursor for niacin synthesis. On the other hand, human skin can produce vitamin D. Normally, vitamin D increases intestinal calcium absorption, reduces urinary calcium loss resulting in the maintenance of calcium homeostasis in the body. Vitamin D regulates calcium metabolism in the bone as the vitamin D receptors (VDRs) are present in osteoblast cells. Calcitriol affects osteoblast proliferation, differentiation, and mineralization. Vitamin D stimulates the production of bone matrix proteins for mineralization. Moreover, vitamin D regulates the expression of receptor activators of nuclear factor kappa B (NF-κB; RANK) ligand (RANKL) and osteoprotegerin (OPG). RANKL binds with RANK and then advances the maturation and differentiation of osteoclast. Vitamin D tunes the osteoblastic and osteoclastic activity during bone remodeling. This traditional concept of vitamin D’s functions has been expanded in more areas. Vitamin D has crucially involved in lymphocyte homing characteristics in the skin and gut, and immunomodulatory actions including regulation of inflammatory response. Vitamin D deficiency causes rickets, osteomalacia, and osteoporosis increases susceptibility to infection, and the risk of autoimmunity [3–5].
Skin is the largest organ of the body and performs various functions, including disease prevention, initiation of the immune response, tolerogenic activity, wound healing, and angiogenesis. Epidermis, dermis, and hypodermis are three distinct parts of the human skin. The epidermis has multiple (five) layers: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, stratum corneum (inner to outer direction). These layers have specific cellular characteristics and form the superficial covering of the body. The innermost part of the epidermis is abundantly richer with keratinocytes, melanocytes, and Langerhans cells (LCs) [6]. Keratinocytes are the major functional cell of the skin. Melanocytes synthesize melanin, which protects DNA from ultraviolet (UV) radiation [7]. LCs are the specific class of dendritic cells (DCs) that are associated with skin immunity. Moreover, T lymphocytes, particularly CD8+ lymphocytes are also the common resident of the skin [6]. Skin-associated lymphoid tissue (SALT) is connected with immune defense. SALT provides local immune responses [8, 9], protects the body from pathogenic invaders, and sometimes involves the pathophysiology of cutaneous diseases [10, 11].
Vitamin D is predominantly linked with skin immunity. Like other fat-soluble vitamins, the isoprene compound is the initial precursor of vitamin D (Figure 1). Biochemically vitamin D is a class of secosteroids. Plant steroid ergocalciferol is known as vitamin D2, while cholecalciferol (vitamin D3) is essentially present in the human. 7-dehydrocholesterol is converted to vitamin D3 in the epidermal layer of human skin after exposure to UVB radiation having a wavelength between 290 nm and 320 nm of sunlight [12] (Figure 1). The formation of 1,25(OH)2D3 makes the active form of vitamin D, which binds with intracellular VDR for physiological action (Figure 1). Vitamin D maintains a balance between immunostimulatory and immunoregulatory actions. It promotes T-cell homing activity in the epidermis [13, 14]. The presence of VDR in the immune cells (macrophages, DCs, T- and B-cells) drives the immunomodulatory actions of vitamin D. It exerts anti-inflammatory effects, tolerogenic capacity, and prevents autoimmunity. The deficiency of vitamin D promotes autoimmune diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), hashimoto thyroiditis (HT), and multiple sclerosis (MS) [15, 16].
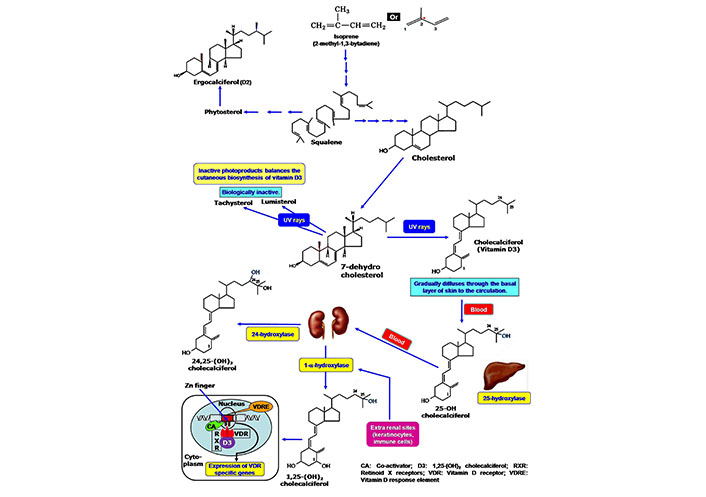
Cutaneous synthesis of Vitamin D, its activation and mode of action. Cholesterol is the primary precursor molecule of vitamin D, which is synthesized from isoprene compound. In the epidermal cells of the skin, cholesterol is present as 7-dehydrocholesterol that is converted to vitamin D on exposure to UVB irradiation. At the subsequent activation stage, hepatic 25-hydroxylase converts the vitamin D to 25-hydroxycholecalciferol. Another hydroxylation reaction produces 1,25-dihydroxycholecalciferol as an active component by the action of enzyme 1-α hydroxylase of renal cells. The formation of 24,25-dihydroxycholecalciferol lowers the active form of vitamin D. The cellular activity of vitamin D is started by the interaction with the intracellular VDR. The vitamin D-VDR complex binds to the VDRE of DNA by using the Zn-finger system in the presence of the co-activator. VDRE: vitamin D response element
Skin is a very sensitive part of the body. It acts as an interface between the invaders and the protectors. Several pathogenic organisms, environmental toxicants, and others have given threatening from outside the skin. However, beneath the skin, a large number the immune-competent cells make a protective barrier. These cells can trigger innate response, adaptive immunity, as well as inflammatory response. Although, the inflammatory response is protective; it is the cause of several skin diseases [psoriasis, atopic dermatitis (AD), vitiligo, etc]. Vitamin D shows immunosuppressive action in skin inflammation, balances the cytokines levels, increases tolerogenic capacity, and anti-inflammatory cytokines. Inadequate vitamin D enhances the risk of skin diseases like psoriasis, ichthyosis, vitiligo, photoreactivity, AD, hair loss, and melanoma. The present review has focused on the immunomodulatory action of vitamin D with particular reference to cutaneous immunity and the impact of vitamin D on skin diseases.
There are limited numbers of natural foods that contain a sufficient amount of vitamin D. Some animal products, such as fats, eggs, and milk supply vitamin D through diet. However, exposure to sunlight induces vitamin D synthesis in human skin from the precursor molecule 7-dehydrocholesterol (provitamin D3) to fulfill the daily requirements. 7-dehydrocholesterol is present in the cell membrane of epidermal basal and suprabasal keratinocytes, as well as dermal fibroblasts. The 7-dehydrocholesterol is converted to D3. After synthesis in the cutaneous surface, the plasma membrane releases vitamin D3 to the systemic circulation (Figure 1) where it binds with vitamin D-binding protein (DBP) for its circulation [17]. The cutaneous synthesis has been varied from individual to individual. The aged people have thinner skin that reduces the production of vitamin D [18, 19]. Ardawi et al. [20] reported that obesity decreased the vitamin D levels in the individual. Adipocytes can take up vitamin D3 due to its lipid solubility and subsequently be stored in subcutaneous or omental fat for further use [21]. Ergosterol from plants and fungi is used as the commercial source of this vitamin [22, 23]. Commercial vitamin supplementation is sometimes mixed with vitamin D3 and vitamin D2. Biologically vitamin D2 has less affinity for the vitamin DBP in the plasma and shows a short half-life [24].
The native form of vitamin D is not the active component. 25-hydroxylase [cytochrome P450 (CYP)27A1/CYP2R1/CYP2D25] a class of CYP, catalyzes the hydroxylation reaction at the C-25 position of the vitamin D in the liver [25]. Another hydroxylation occurs in the kidney where 1-α-hydroxylase incorporates a hydroxyl group to the 25-OH vitamin D (calcidiol) to form 1,25(OH)2 vitamin D (calcitriol or 1,25-dihydroxy cholecalciferol), which exerts all the biological activities of vitamin D. 1-α-hydroxylase is a specific type of parathyroid hormone-dependent CYP monooxygenase 25-hydroxyvitamin D3 1 alpha-hydroxylase (CYP27B1) enzyme. However, 24-hydroxylase (CYP24) includes another hydroxyl group at the C-24 position of the active vitamin D to produce 24,25-OH vitamin D, which is functionally inactive. A regulatory feedback mechanism maintains the levels of active vitamin D. High levels of 1,25(OH)2 vitamin D repress the expression of 1-α-hydroxylase in the kidney and alternatively, induce the expression of CYP24 for inactivation of vitamin D. Thus, circulating vitamin D is in a tight regulation system [4, 12, 26]. Besides the kidney, CYP27B1 is also expressed in the extra-renal tissues, including the brain, lungs, placenta, and macrophages. A separate regulatory mechanism maintains the activity of CYP27B1 in the extra-renal site. Cytokines like interferon (IFN)-gamma (IFN-γ), interleukin (IL)-1, or tumor necrosis factor-alpha (TNF-α) regulate the expression of CYP27B1 in place of parathyroid hormone. Additionally, macrophages produce a variant form of inactive CYP24 that continues the prolonged activity of vitamin D in the immune compartment [27]. Shahriari et al. [28] reported that a reduced number of active nephrons and high levels of fibroblast growth factor (FGF)-23 affect the circulating calcitriol levels.
Vitamin D is one of the non-plant steroid-derived natural antioxidants that has been synthesized in the human cell. This endogenous cholesterol-derived component fights against the stress response to protect the cells [29, 30]. This cholesterol-derived molecule shows functional characteristics similar to steroid hormone instead of fat-soluble vitamins. The action of vitamin D depends on the expression of VDR. Immune cells like macrophages, DCs, T-cells, and B-cells express VDR [4, 15]. Vitamin DBP help to transport vitamin D through circulation. At the cellular level, vitamin D binds with VDR (a nuclear receptor superfamily member). The mechanism of action is similar to retinoic acid and thyroid hormone. 1,25 dihydroxy cholecalciferol migrates into the nuclei of the target cells and binds with high-affinity VDRs. After binding vitamin D with VDR, a conformational change occurs, allowing its dimerization with retinoid X receptor (RXR). 11-cis retinoic acid is the ligand of RXR. The heterodimer complex of VDR-RXR binds to vitamin D response element (VDRE) in association with co-activators. The interaction of VDR-RXR heterodimer with DNA is mediated through zinc finger (Zn-finger). Finally, vitamin D modulates the activity of the promoter regions of vitamin D-targeted genes for their expression [31, 32].
The skin forms the superficial covering of the entire body, comprise the epidermis and the dermis. Beneath the dermis, the layer is called the hypodermis. The epidermis is the outmost region. It is a thin layer, composed of stratified squamous epithelium that is divided into several layers. The deepest layer is called the stratum basale, which acts as the germinal center. The stratum basale consists of a single layer of undifferentiated cells with constant mitotic activity [33]. The cells are in contact with the dermis. Four types of cells comprise the stratum basale: keratinocytes (columnar type), melanocytes, tactile cells (Merkel cells), and nonpigmented granular dendrocytes (LCs). The keratinocytes of the stratum basale constantly divide and produce new cells that are pushed towards the surface of the epidermis. These cells produce a specific type of protein called keratins (K5 and K14), making the skin waterproof. When the keratinocytes are pushed away from the vascular bed of the dermis, the cells suffer from deprivation of nutrient and oxygen supply. This condition starts the keratinization process where the cells are filled with keratin along with the nuclear-disintegrated state. Finally, keratinocytes reach the outer surface of the skin. They appear as dead scales at the superficial layer containing full of keratin enclosed by a loose cell membrane.
Melanocytes are specialized epithelial cells responsible for the synthesis of melanin pigment. Tactile cells act as a sensory receptor for tactile perception. LCs are associated with immune defense. Above the stratum basale, the other layers are stratum spinosum, stratum granulosum, stratum lucidum, stratum corneum. The stratum spinosum contains several layers of cells. These cells arise from the keratinocytes of the basal stratum after differentiation. They exhibit a spiny appearance on their surfaces due to the spine-like extensions. The stratum granulosum has three or four layers of flattened type granular cells (keratinocytes) that contain fibers of keratin (keratohyalin) and blackened granules in their cytoplasm. Stratum lucidum is a thin, clear layer, exists only in the lips and in the thickened skin of the soles and palms. The stratum corneum is the uppermost layer of the skin. It is composed of 25 to 30 layers of keratinized flattened, scale-like dead cells. Several appendages like hair follicles, eccrine sweat glands, sebaceous glands, and apocrine glands are associated with the skin. These structures are involved in various physiological functions like regulation of body temperature, prevention of infection, maintenance of moisture, and smoothness of the skin.
Keratinocytes are actively participating in vitamin D metabolism. They synthesize vitamin D from 7-dehydrocholesterol and express CYP27A1 and CYP27B1 for activation of vitamin D to produce 1,25(OH)2 cholecalciferol. These cells express VDR that ensures the action of vitamin D through an autocrine/paracrine manner [34]. Vitamin D differentially regulates the proliferation and differentiation of keratinocytes. VDR in association with co-activators like VDR-interacting protein (DRIP) and steroid receptor co-activator (SRC) promotes the differentiation process of keratinocytes [35]. Gniadecki [36] observed the stimulatory and inhibitory effects of 1,25(OH)2D3 on keratinocyte proliferation in a cell culture study. He reported that a low concentration of 1,25(OH)2D3 (10–11 mol/L or less) in association with calcium (0.15 mmol/L) restricts the cell cycle in the late G1 phase, resulting in inhibition of keratinocyte proliferation. Reduction in the expression of c-Myc, cyclin D, and elevated levels of cell cycle inhibitors p21cip, and p27kip inhibit the cell cycle [37]. High concentrations of calcium (1.8 mmol/L) and 1,25(OH)2D3 (greater than 10–11 mol/L) stimulates cell growth by pushing the cell in the S phase of the cell cycle [36]. Moreover, elevated levels of calcium and activation of phospholipase C-γ1 are essential for the differentiation of keratinocytes. Calcitriol induces the synthesis of involucrin, transglutaminase, loricrin, and filaggrin in keratinocytes during differentiation [34, 38]. Xie et al. [39] had indicated the occurrence of impaired epidermal differentiation and the appearance of inappropriate levels of involucrin, profilaggrin, and loricrin in VDR knockout mice. Vitamin D acts as an anti-apoptotic agent at physiological concentrations. This vitamin rescues the keratinocytes from the adverse effects of pro-apoptotic stimuli like ceramide, UV radiation, and TNF-α [34]. VDR-dependent synthesis of glucosylceramide (GlcCer) critically maintains the permeability barrier of the epidermis. VDR SRC2, or SRC3 is essential for GlcCer production. Silencing either VDR, SRC2 or SRC3 reduces GlcCer synthesis by altering the expression of fatty acid elongase and ceramide glucosyltransferase. This silencing adversely affects the formation of epidermal GlcCer species, lamellar body, and the expression of the lipid transporter ATP-binding cassette transporter protein 12, resulting in an abnormal permeability barrier. VDR null mice also exhibit defective barrier function [35]. Thus, VDR and its co-activator are indispensable for the epidermis-specific sphingolipid synthesis and maintenance of barrier.
Epidermis, dermis, cutaneous appendages, and subcutaneous tissue are the structural part of the skin [40]. The skin has a complex immune system. DCs, LCs, mast cells, B- and T-lymphocytes, and keratinocytes comprise the immune compartment of the skin [10, 41]. LCs are the specialized subset of DCs that are abundantly present in the epidermal region of the skin. The surface marker of LCs of mice and humans is langerin [42]. The cutaneous immune system provides body defense against pathogens (bacteria, virus, protozoa, and others) and antigens (protein, polysaccharides, glycoprotein, and others). Varieties of immune cells [T-cells, B-cells, antigen-presenting cells (APCs)] and different biomolecules (cytokines, chemokines) have been associated with the immune network. Impaired immune reactions dampen the self tissue. Any mistake in the regulatory process exaggerates inflammatory response and promotes the consequence of many diseases.
Keratinocytes play an important role in the immune response of the skin. They can potentially recognize pathogenic organisms through pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [10]. Microbial lipopolysaccharides (LPS), flagellins, unmethylated cytosine-phosphate-guanine (CpG) DNA sequences, teichoic acids, mannose-rich oligosaccharides act as PAMPs [43, 44]. The keratinocytes express cell surface and cytoplasmic receptors for the detection of microbial products. These molecules are termed pattern recognition receptors (PRRs). These are cell surface Toll-like receptors (TLRs), intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). The epidermal keratinocytes express TLR1, TLR2, TLR4, TLR5, and TLR6, along with endosomal (TLR3 and TLR9) [43]. The activation of TLRs induces innate and adaptive immunity. The TLRs of keratinocytes trigger the dermal immune response as the compartment of the dermis has associated with the immune network containing varieties of immune cells like CD4+ T-lymphocytes, γδT-lymphocytes, APCs, natural killer (NK) cells, and mast cells [9]. Activation of T-helper (Th)1 cells promotes the release of IFN-alpha (IFN-α) and IFN-beta (IFN-β) [44]. NLRs are essential for the recognition of irritants and toxins. Activation of NLRs stimulates pro-inflammatory signaling through inflammasomes that finally activate a specific protein apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD; ASC), and pro-caspase-1 followed by induction of immune response [45, 46]. Moreover, keratinocytes synthesize cytokines, including IL-1, IL-6, IL-10, IL-17, IL-18, IL-22, and TNF-α (Figure 2) [6]. To increase the communication and co-operation with other cells, keratinocytes express chemokines, such as C-X-C motif ligand (CXCL)1 and CXCL8, CXCL9, CXCL10, CXCL11, and CC chemokine ligand (CCL) 20 (Figure 2). They attract immune cells like LCs. CXCL1 and CXCL8 increase neutrophil infiltration in the epidermis [47]. Keratinocytes can increase the CD4+ and CD8+ T-cells responses by modulating the expression of Th1 (IL-1, IFN-γ, TNF-α) or Th2 (IL-4, IL-5, IL-6) cytokines [48]. Melanin synthesizing melanocytes can induce local immune responses, trigger phagocytosis, and produces IL-1β, IL-6, TNF-α, IL-8, IFN-γ. These cells exhibit variety of TLRs like TLR1, -2, -3, -4, -6, -7, and -9, expresses leukocyte recruiting chemokines (CCL2, CCL3, and CCL5) [49]. TLR signaling in association with adaptor proteins myeloid differentiation factor 88 (MyD88), toll/IL-1 receptor domain-containing adaptor protein (TIRAP)/MyD88 adapter-like (Mal), TIR-domain-containing adapter-inducing interferon-beta (TRIF), and transverse rectus abdominis (TRAM) activates NF-κB and mitogen-activated protein kinase (MAPK) pathways. The induction of the NF-κB pathway promotes the expression of antimicrobial peptides (AMPs), IL-6, TNF-α, IL-8, and IL-12, resulting in stimulation of inflammatory response, recruitment of phagocytic cells [50].
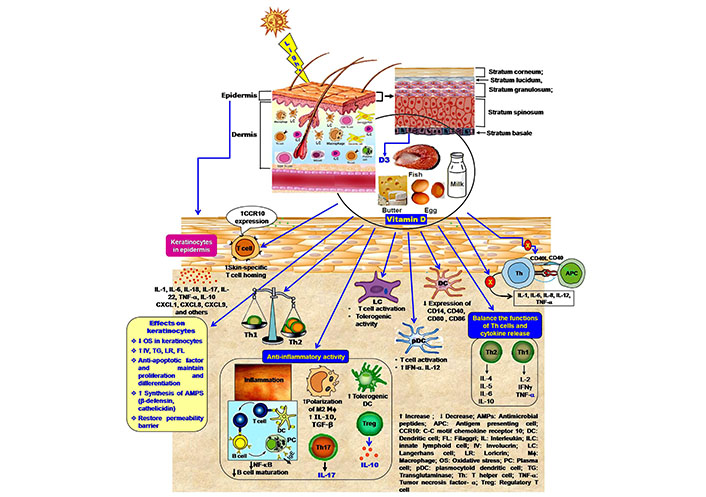
Immunomodulatory effects of vitamin in the cutaneous immune compartment. 1,25(OH)2 vitamin D polarizes the Th2 development and maintains a balance in cytokine secretion. Active vitamin D regulates the inflammatory response by inhibition of Th17 cell development and facilitation of Treg cell generation, formation of tolerogenic DC, the release of IL-10, and suppression of IgE synthesis. Vitamin D also inhibits the expression of the co-stimulatory molecule on DCs and blocks the activation of Th cells, as well as cytokine production. Moreover, vitamin D facilitates the skin-homing capacity of T-cell
Macrophages, monocytes, DCs, LCs are the APCs of the skin. In humans, there are four subtypes of DCs: LCs, dermal dendrocytes (DDs), plasmacytoid DCs (pDCs), and inflammatory DCs (iDCs). LCs and DDs express CD13 and CD33, can activate CD4+ and CD8+ T-cells, secrets IL-12. However, Shklovskaya et al. [51] reported that despite the inflammatory activity LCs induce tolerogenic responses. This effect is mediated by tolerizing CD8+ T-cells and the formation of Treg cells for the production of IL-10 [52, 53]. pDCs release IFN-α, IL-12 and also activate CD4+ and CD8+ T-cells (Figure 2). iDCs express CD206 and immunoglobulin (Ig) fragment crystallizable (Fc)-like receptors for IgE, promote allergic response [6, 10, 54]. The main functions of DCs are capturing the antigens and represent the T-cells with major histocompatibility complex (MHC) class II after processing. Thus, DCs are involved in T CD4+ and Treg activation, and secretion of different cytokines.
The skin has a much greater population of T lymphocytes than that of blood. Clark et al. [55] reported that approximately 20 billion T-cells reside in the skin. This number is approximately double in comparison to the T-cell population of the blood. The epidermal T-cells are located in the suprabasal and stratum basale, nearer to the LCs. Equal numbers of CD4+ and CD8+ lymphocytes are distributed within the skin, particularly to the capillaries and the epidermal-dermal junction [56, 57]. They are mostly memory T-cells [58, 59], express cutaneous lymphocyte-associated antigen and make the first-line defense against pathogenic invaders [60].
Cutaneous T-cells are a heterogeneous population, comprise Th1, Th2, Th3, Th17, and Treg cells [10]. Moreover, other subpopulations of T-cells like Th9, Th22, Th25 are also found in the skin during infection. T-cells secrete different types of cytokines for the activation of B-cells, antibody production, induction of inflammatory response, and regulation of immune response. The cytokine profile of T-cells are: i) Th1: IFN-γ, TNF-α IL-2; ii) Th2: IL-4, IL-5, IL-6, IL-13; iii) Th3: transforming growth factor-β (TGF-β), IL-4, IL-10; iv) Th17: IL-17, IL-22; v) Th22: IL-22, TGF-β, IL-13, TNF-α; vi) Th25: IL-25, IL-4, IL-13; vii) Th9: IL-9, IL-10, IL-22, IL-4, TGF-β; viii) Treg: IL-10, TGF-β [6, 61]. Good numbers of mast cells are present in the skin. They express complement factor C5a (CD88) and IgE receptors [62], involve in the release of vasoactive and pro-inflammatory agents. NK cells give IL-12, IFN-γ, produce granzyme, perforin, and granulysin that can directly destroy the microorganisms. NKT cells release IFN-γ, IL-22, IL-17 and induce the production of AMPs [63]. Very few B-lymphocytes are present in normal skin but antigenic challenges increase their migration [6]. B-lymphocytes move toward the site of the antigenic microenvironment, where APCs start the activation of naive lymphocytes [64]. B-cells are involved in both immunostimulatory and immunosuppressive actions. B lymphocytes play an important role during Staphylococcus aureus skin infections. They produce specific antibodies, induce opsonization and phagocytosis by macrophages and neutrophils [65]. Infiltration of B lymphocytes causes skin lesions AD [64]. Another subpopulation is regulatory B (Breg) lymphocytes. They are capable to release IL-10 as an anti-inflammatory agent [66].
Skin exerts first-line defense against infections. APCs, T-cells, B-cells, NK cells, and others are the resident immune cells in the skin [10, 34]. These cells bear VDRs having a high affinity for effective binding with vitamin D [67]. AMPs are the most important factors in the skin that exert challenges to microbial invaders. Different cells like keratinocytes, sebocytes, eccrine gland cells, mast cells, neutrophils, and NK cells are the contributor of AMPs. They can synthesize more than 20 types of antimicrobial proteins; among these, β-defensin and cathelicidins are most effective [68, 69]. Humans bear a single copy of the cathelicidin gene that produces peptide human cationic antimicrobial protein (hCAP18). Cathelicidin LL-37 appears as the final product from peptide hCAP18 through cleavage [34]. During infection, keratinocytes secret IL-17 and IL-22 that induce the production of AMPs [70].
Several studies have established the role of vitamin D on immunity. The immune cells can synthesize active vitamin D but the process is differentially regulated. The expression of 1-α-hydroxylase is not parathyroid hormone-dependent. 25-OH vitamin D, IFN-γ, IL-1, TNF-α induce the expression of 1-α-hydroxylase in the immune cells. At the immunologic microenvironment, vitamin D acts through the autocrine/paracrine fashion [15, 71, 72]. Vitamin D has an impact on both innate and adaptive immunity. Several in vitro experiments had established the immunomodulatory role of 1,25(OH)2 vitamin D on innate and adaptive immunity. Vitamin D modulates the maturation of macrophages, LCs, B-cells, and T-cells (particularly Th2 cells) (Figure 2) [72]. It advances the development of Treg cells but diminishes differentiation of Th1 and Th17 cells; that favor anti-inflammatory effects (Figure 2) [73]. PAMPs or DAMPs activate immune cells through TLR response. An in vitro study had revealed that vitamin D modulates TLR-induced DC maturation, promotes IL-8, IL-6, and IL-10 production, and inhibits LPS-dependent IL-12 secretion [74]. The VDR polymorphisms delink the vitamin D-mediated immunomodulatory effects [75–77]. Mutant VDR cannot regulate Th1 response and IFN-γ, IL-17A, and IL-22 secretion in the presence of vitamin D [78]. Thus, defective VDR overrides the immunomodulatory effects of calcitriol.
Vitamin D has an effect on hCAP18/LL-37 and β-defensin synthesis [34, 79]. Disruption of cutaneous barrier and skin infection increases the expression of AMPs. Infectious challenges enhance the expression of CYP27B1 in the microenvironment of the cutaneous compartment, which uplifts the local synthesis of active vitamin D. Skin injury increases TLR-2 response that elevates the vitamin D-induced expression of AMPs [80, 81]. Several studies have shown the role of vitamin D on the expression of hCAP18/LL-37 and defensins after supplementation of 1,25(OH)2D3. Lee et al. [82] reported that the application of vitamin D in cultured sebocytes promotes the expression of cathelicidin. An in vivo experiment on human skin had established the inductive effects of vitamin D during wounding. Topical application of vitamin D analog calcipotriol increases the expression of hCAP18/LL-37 to prevent infection. 1,25(OH)2D3 acts through activating protein-1 (AP-1) and p38-induced peroxisome proliferator-activated receptor-γ (PPAR-γ) mediated signaling for the expression of human beta-defensin 3 (HBD-3) and cathelicidin (hCAP18) in human keratinocytes (Figure 2) [83]. At the molecular level, the mechanism of induction of cathelicidin and β-defensin is slightly different. 1,25(OH)2D-VDR complex binds with VDRE at the promoter region of the cathelicidin gene. Alternatively, expression of the β-defensin is mediated by NF-κB and 1,25(OH)2D-VDR complex [81]. Moreover, 1,25(OH)2D3 induces the expression of specific serine proteases kallikrein-related peptidase (KLK)5 and KLK7. KLK5 is the tryptic type enzyme, while KLK7 belongs to chymotryptic type protease. These serine proteases are responsible for the processing of proform cathelicidin, an active form of cathelicidin. Vitamin D-dependent increased KLK expression differentially regulates the processing of cathelicidin precursor protein hCAP18 [84, 85]. Thus, vitamin D influences the expression and antimicrobial activity of cathelicidin and other AMPs [86]. Mice deficient with the serine protease inhibitor lympho-epithelial Kazal-type-related inhibitor (LEKTI) showed significant improvement in antimicrobial activity [84].
The cells of the innate immunity express VDR. 1,25(OH)2D3-VDR-complex modulates innate immunity by controlling the maturation and activation of NKT cells and NK cells, as well as the production of IL-4 and IFN-γ. Vitamin D also elevates IL-1β and IL-8 levels, while restricts the phagocytic activity of neutrophils and macrophages [87]. Vitamin D acts as an anti-inflammatory agent. It favors the polarization of M2 macrophages that potentially enable to producce of anti-inflammatory cytokines IL-10, TGF-β (Figure 2) [88]. M2 macrophages induce collagen synthesis, extracellular matrix formation during tissue repair and healing. Almerighi et al. [89] reported that 1,25(OH)2 vitamin D inhibits the expression of inflammatory cytokines like IL-1, IL-6, IL-8, IL-12, and TNF-α. To potentiate the effectiveness of innate immunity, active vitamin D increases the synthesis of AMPs (cathelicidin and β-defensin) because microbial infection induces CD40 ligand and IFN-γ release to enhance the VDR and CYP27B1-hydroxylase expression [90].
1,25(OH)2 vitamin D increases lymphocyte-homing capacity in the skin. It influences the T-cell migration and their homing beneath the skin surface. The subset of cutaneous memory T-cells having lymphocyte-associated antigen (CLA+) expresses vitamin D-induced CC-chemokine receptor (CCR)10, which binds with the skin-specific chemokine CCL27. By increasing the expression of CCR10, 1,25(OH)2D3 promotes the T-cell-homing in the skin (Figure 2) [13, 91]. Epidermal keratinocytes exclusively synthesize chemokine CCL27 [13]. However, 1,25(OH)2D3 reduces the gut-homing capacity of the T-cells. It inhibits the expression of α4β7 integrins and CCR9 on T-cells this process is upregulated by vitamin A [14].
T-cells B-cells and APCs express VDR. 1,25(OH)2D3-VDR complex modulates IL-2 levels and regulates the maturation of CD4+ lymphocytes. Alternatively, vitamin D influences Foxp3+ Treg cell differentiation [92]. Chirumbolo et al. [30] reported that vitamin D upregulates the expression of TLR2, NOD2, and IL-1β in the steps of DC-mediated Th maturation towards Treg cells. Moreover, vitamin D enhances the number of memory T-cells [93]. The extensive study by Dam et al. [94] on DCs and LCs had revealed that vitamin D regulates the maturation of APCs and antigen presentation to the T-cells. They used 1,25(OH)2D3 and the vitamin D3 analog calcipotriol for this purpose. Topical application of calcipotriol on normal human skin decreases the number of CD1a+ DCs and fewer dendrites per cell with altered dendritic morphology. Administration of both 1,25(OH)2D3 and calcipotriol in LC (isolated from human skin) enriched cell suspensions inhibit the LCs-induced antigen-dependent T-cell proliferation. This study strongly supports the immunosuppressive effects of vitamin D on the skin (Figure 2). Naïve B-cells lower the expression of VDR as vitamin D inhibits B-cell proliferation and maturation. Additionally, vitamin D decreases IgG and IgM by inhibiting the differentiation of plasma cells [95].
Vitamin D maintains the balance in the skin immune response (Figure 2). It acts as an immunosuppressive agent, affects antigen presentation by preventing the functions of LCs and other APCs [94, 96]. Active vitamin D restricts the maturation of APCs [24]. Previously, Penna and Adorini [96] reported that 1,25(OH)2 vitamin D inhibits differentiation, maturation, activation, and survival of DCs. These cells are the major APC, are mostly present in the epidermal and dermal region. The maturation of DCs is crucially important as they represent the antigen to the Th cells (primarily the Th1 subset). Immature DCs can not activate T-cells due to inappropriate surface markers. Vitamin D inhibits the expression of human leukocyte antigen (HLA)-DR (HLA-DR), CD14, CD40, CD80, CD83, and CD86 (Figure 2) as co-stimulatory molecules and MHC II on the surface of DCs at the developmental stage [30, 96]. This effect modulates the activation of T-cells and the secretion of cytokines. Vitamin D induces the maturation of CD4+ CD25+ Treg cells (Figure 2). To control the inflammation, vitamin D suppresses the activity of the effector T-cell, promotes the trafficking of Treg cells at the inflammatory site [97]. Topical application of vitamin D analog calcipotriol promotes the development of Treg cells in the skin [98]. Vitamin D favors the development of tolerogenic DCs as an upliftment factor of anti-inflammatory effects [99]. VDR-induced activation of tolerogenic DCs increases the number of Treg cells and prevents allograft rejection and autoimmune diseases [100]. Gorman et al. [101] reported that dietary vitamin D increases the Treg cell (CD3+ CD4+ CD25+ Foxp3+) population and their migration to the skin-associated lymph nodes. Vitamin D-mediated Treg cell maturation and the suppressive activity of Treg cells in the skin-draining lymph nodes inhibit cutaneous inflammation. Vitamin D analog calcipotriol shows immunosuppressive effects by inhibiting CD8+ T priming and lowering the numbers of LCs in the experimental model of transcutaneous immunization technique. Calcipotriol promotes the Treg cell activity that subsequently inhibits the activity of CD8+ T-cells and release of IFN-γ. These events indicate the anti-inflammatory properties of vitamin D or its analog [98].
Normally, the immune system exerts defensive action against invading pathogens in a precise manner. An inflammatory response is a coordinative action of immune cells, cytokines, and other signaling molecules to exercise the effects. Any dysfunction of the immune system causes fatal diseases. The most common symptoms are allergic response, inflammatory diseases, and autoimmunity. An intricate relationship exists between vitamin D and skin functions. Deficiency of vitamin D increases the susceptibility of different skin diseases, including skin cancer, psoriasis, ichthyosis, autoimmune skin disorders (vitiligo, blistering disorders, scleroderma, and SLE), AD, acne, hair loss, infections, and photodermatosis [24, 102].
Psoriasis is a chronic inflammatory disease of the skin. Dysregulation of the immune cell activity is the vital cause of psoriasis. Different cells and agents like T-cells, pDCs, myeloid DCs (CD11c+ mDCs), neutrophils and NK cells, and high levels of AMPs (like β-defensins, S100 proteins, or LL-37) start the psoriatic lesions [34, 103]. Lande et al. [104] reported that IFN-α from pDCs and host DNA along with AMPs trigger the inflammatory cascade in psoriasis. IFN-α-mediated activation of effector T-cell can advance this autoimmune pathogenic lesion. myeloid DC (mDC) synthesizes IL-20, IL-12, IL-23, and recombinant TNF-α (rTNF-α). These cytokines induce nitric oxide synthesis and activate Th1 and CD8+ cytotoxic T-cells. Th1 cells enhance the secretion of IFN-γ and TNF-α, which are the influencing factors of the inflammatory response, Additionally, Th17, NKT, and NK cells can progress this disease. Th17 cells give IL-17, IL-17F, and IL-22 as pro-inflammatory cytokines. IL-17, IFN-γ, TNF-α, induce the proliferation and activation of keratinocytes, resulting in more secretion of cytokines (IL-1, IL-6, TNF-α), chemokines (CXCL8, CXCL11, CXCL20), and S100 proteins (S100A7-9) for infiltration of neutrophils and other phagocytic cells. Alternatively, diminution of Treg cell activity also the inductive factor in psoriasis. Thus, the multifactorial events are associated with psoriasis [103, 105–107]. Vitamin D deficiency impacts the development of psoriasis [108, 109] because it acts as an anti-inflammatory and anti-angiogenic agent [110].
Application of vitamin D3 or its analogs like calcipotriol, tacalcitol, and maxacalcitol can be used as the treatment agents of psoriasis [34, 111]. Phototherapy by using narrow-band UVB light also resolves the problem of psoriasis [112]. Active vitamin D suppresses the activity of pDCs, proliferation, and activation of T-cells, and release of IFN-γ [113]. The effectiveness of CD8+, Th1, and Th17 cells is suppressed by 1,25 (OH)2D3 or its analog [114, 115]. Moreover, vitamin D inhibits the expression of Il-12, IL-23, IL-1α, IL-1β, TNF-α, psoriasin (S100A7), and koebnerisin (S100A15) [116, 117]. Topical application of calcipotriol significantly reduces defensin-β levels, as well as IL-17, IL-17F, and IL-8 to control the inflammation in psoriasis [118]. Another vitamin D analog 1α,25-dihydroxy vitamin D3–3-bromoacetate induces the expression of IL-22 that helps in the reconstruction of the epidermal layer by repressing the activity of protein kinase B (AKT1), mechanistic target of rapamycin (mTOR) pathway and release of IL-8, regulated upon activation normal T-cell expressed and secreted (RANTES), and psoriasin [119]. Vitamin D upregulates the expression of late cornified envelope (LCE) gene products LCE proteins to prevent psoriasis [120]. Vitamin D restores the normal texture of integrins, intercellular adhesion molecule-1 (ICAM-1), CD26, and HLA-DR that have appeared in an alternative form in psoriatic skin [121]. VDR polymorphisms (A-1012G, FokI, BsmI, ApaI, and TaqI) are associated with many diseases. A-1012G and ApaI polymorphisms increase the risk of psoriasis [122].
AD is a chronic skin disease. Genetic and environmental factors in association with disruption of the epithelial barrier, immunological abnormalities, and IgE-mediated chronic inflammation influence AD. Impaired structural proteins (filaggrin, involucrin, loricrin, keratin K5, and K16, etc), alteration in subcutaneous pH, low levels of skin ceramides are the cause of epithelial barrier disruption [123], leading to increased susceptibility of bacterial, fungal, and viral infection. The Th0 can differentiate into Th1 or Th2 cells [124]. The Th2 cytokines (IL-4, IL-5, and IL-13) are elevated during the acute phase, while Th1 cytokines [IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-12] promote the chronic phase of inflammation. Tokura [125] reported that AD patients showed high levels of serum IgE. Thymic stromal lymphopoietin increases the maturation of naïve Th cells towards Th2 cells that start the release of IL-4, IL-5, IL-13, and TNF-α for atopic lesions [126]. The DCs from myeloid origin promote Th1 polarization for the chronic reaction [127]. Moreover, the improper activity of NK cells, and neutrophils, defective TLR2 response, and reduced secretion of AMPs are also associated with AD [128, 129].
The low level of vitamin D increases the severity of AD. The prevalence of AD is high in the population living in higher geographic latitudes, with lower sun exposure [130]. Meta-analysis had shown that children and adults with AD had low levels of serum vitamin D [131]. VDR (BsmI, ApaI, and TaqI) and CYP24A1 polymorphisms increase the risk of AD [78, 132]. Supplementation of vitamin D restores the normal function of Th1 and Th2 cells [133]. Vitamin D can decrease the IFN-γ and IL-17 in vitamin D-deficient individuals [134]. UVA/UVB phototherapy is also beneficial for AD treatment [112]. Vitamin D decreases the IgE producing B-cells in AD patients [135]. Albenali et al. [136] reported that supplementation of Vitamin D increased LL-37 level in AD patients.
Vitiligo is an autoimmune disorder with a defective pigmentation process due to the destruction of functional melanocytes in the epidermis. The common characteristic of this disease is depigmented patches or macules of different shapes and sizes [137]. There is a genetic association with this disease. Vitiligo patients have genetic variants for the components of both the innate (NLRP1, IFIH1, CASP7, C1QTNF6, TRIF) and adaptive immunity (FOXP3, BACH2, CD80, CCR6, PTPN22, IL2R, alpha GZMB, HLA class I and II) [138]. In vitiligo patients, melanocytes are mostly affected. Autoantibody has been produced against melanin-concentrating hormone receptor 1 (MCHR1), tyrosinase, and cell surface antigen of melanocytes. The melanocyte-specific IgG and IgM are the predominant [139, 140]. In this disease, T-cells exhibit more number of IL-2 receptors and a higher ratio of CD8:CD4. Cytotoxic CD8 T-cells undesirably attack melanocytes for destruction. Keratinocytes exhibit HLA-DR for local T-cell reactivity [141]. Th1 cells produce more IFN-γ and TNF-α in the condition of vitiligo [142]. A significantly high level of IL-17 had observed in vitiliginous subjects [143].
Vitamin D deficiency is a contributing factor in the development of vitiligo. Several studies had indicated that a decreased level of vitamin D is significantly associated with vitiligo [144–146]. Vitamin D protects melanocyte proliferation, and integrity prevents T-cell-mediated damage and maintains the secretion of endothelin-3 (ET-3). Moreover, vitamin D restores the melanocyte viability and maturation by modulating the stem cell factor (SCF)/tyrosine kinase (c-Kit) pathway [147]. Active vitamin D reduces the expression of IL-6, IL-8, TNF-α, and INF-γ, inhibits DC-mediated antigen presentation and immune activation [96]. Finamor et al. [111] reported that treatment with vitamin D3 (35,000 IU once daily) improved repigmentation in vitiligo patients. Birlea et al. [148] observed that vitamin D3 analogs in combination with plus UVA (PUVA), narrowband UVB (NBUVB) are effective for the treatment of vitiligo.
Several etiological factors, including environmental toxicants, UV radiation, metabolic disorders, and chronic inflammatory response can progress cancer. Skin faces non-melanoma skin cancer (NMSC), including basal cell and squamous cell carcinoma, as well as melanoma [149, 150]. Vitamin D decreases the risk of cancer. It protects the keratinocytes from UV radiation-mediated damage [151]. The deficiency of vitamin D increases the risk of melanoma [152]. On the other hand, the expression of VDR in melanocytes continuously declines as the cancers progressed. This effect increases the presence of tumor-infiltrating lymphocytes and the progression of ulceration [153]. Constitutive hedgehog signaling promotes basal cell carcinoma. Vitamin D reduces the expression of glioma-associated oncogene homolog 1 (Gli1) mRNA by inhibiting the hedgehog signaling pathway. Additionally, this inhibition also protects nucleotide excision repair enzymes to suppress the basal cell carcinomas [150, 154]. Uncontrolled cell growth, angiogenesis, and metastasis are the fundamental events of cancerous growth. Vitamin D potentially inhibits the cell cycle, cell survival, angiogenesis, and metastasis to block tumor progression [110, 155]. NF-κB signaling plays a vital role in the inflammatory response. Vitamin D3 inhibits NF-κB translocation to the nucleus and its activity [156, 157]. Melanoma cells express a specific transcription factor runt-related transcription factor 2 (RUNX2), which regulates the activity of focal adhesion kinase (FAK) that maintains the growth and mobility of cancer cells. Vitamin D analog cholecalciferol diminishes the transcriptional activity of RUNX2, followed by inhibition of progression of cancer [158]. Matrix metalloproteinases (MMPs) are essential for metastasis. Calcipotriol reduces the expression of MMPs by regulating the p38 and extracellular signal-regulated kinase (ERK) pathways [159]. FGF-2 is an angiogenic agent. The heparin-binding protein 17/FGF binding protein-1 (HBp17/FGFBP-1) is essential for the activity of FGF-2. Active vitamin D2 decreases the levels of HBp17/FGFBP-1 and the activation of FGF-2 [160].
Pemphigus vulgaris and bullous pemphigoid are autoimmune bullous disorders that are mediated by keratinocyte-specific antibodies. Impaired activation of B-cells increases the production of pathogenic antibodies [24]. Low levels of serum vitamin D increase the risk of pemphigus vulgaris and bullous pemphigoid [161, 162]. Vitamin D is protective against this type of autoimmune disorder. This vitamin modulates Th2 cell differentiation, maturation of Treg cells and, apoptosis B-cells [24].
Acne vulgaris is an inflammatory skin disease. Propionibacterium acnes involves in pathogenesis. This bacterium induces the maturation and activation of Th17, followed by the initiation of inflammatory response. 1,25(OH)2D inhibits Th17 differentiation [163]. Rosacea is a chronic skin disease mediated by high serum levels of vitamin D [164]. Thus, excess vitamin D is detrimental to the skin.
There is a possibility for cutaneous fibrosis during vitamin D deficiency. Vitamin D can be the treatment choice against keloids as this vitamin inhibits skin fibroblasts and keloid fibroblasts [165]. Topical supplementation of vitamin D analogs can be used for the therapeutic purpose in morphoea and lichen sclerosus et atrophicus. Vitamin D analogs have the ability to regulate fibroblast proliferation, collagen synthesis, and endothelial cell function, as well as IL-2 secretion from T lymphocytes [166, 167]. UV light promotes skin damage, typically called photodamage, which is characterized by DNA damage, inflammatory responses, cutaneous cell apoptosis, skin aging, and skin carcinoma. The experimental study had revealed that vitamin D3 exhibits photoprotective effects [168, 169]. Active vitamin D-VDR complex modulates β-catenin-mediated hair follicle differentiation and decreases hair loss [24, 170]. Vitamin D also regulates hedgehog signaling for recycling the hair follicle [171].
Vitamin D shows hormone-like activities through its intracellular nuclear receptor. Fish oil, egg, milk are the common dietary sources of this vitamin. Human skin synthesizes this vitamin from 7-dehydrocholesterol on exposure to sunlight. The active form of vitamin D is directly associated with skin functions. The cutaneous compartment is the greatest harbor of immune cells. Various environmental challenges activate the immune response in the skin. Vitamin D modulates the activity of immune cells. It maintains the cutaneous barrier, functions of keratinocytes, and controls the immune response starting from DC maturation, followed by T-cell activation, cytokine production, etc. This vitamin effectively suppresses the inflammatory response through the polarization of M2 macrophages, Th2 cells, and Treg cells. The deficiency of vitamin D causes calcium imbalance and many other disorders associated with impaired immune functions. Finally, it can be concluded that vitamin D is a potent immune regulator in the skin and exerts protection against several immune-related diseases including autoimmunity. Moreover, vitamin D can potentially be used as a therapeutic agent or may be applied as an adjuvant in the treatment purpose of skin diseases.
AD: atopic dermatitis
AMPs: antimicrobial peptides
APCs: antigen-presenting cells
CCL: CC chemokine ligand
CCR: CC-chemokine receptor
CXCL: C-X-C motif ligand
CYP: cytochrome P450
DBP: D-binding protein
DCs: dendritic cells
FGF: fibroblast growth factor
GlcCer: glucosylceramide
hCAP18: human cationic antimicrobial protein
HLA: human leukocyte antigen
IFN-α: interferon-alpha
IFN-γ: interferon-gamma
Ig: immunoglobulin
IL: interleukin
KLK: kallikrein-related peptidase
LCs: Langerhans cells
LPS: lipopolysaccharides
NF-κB: nuclear factor kappa B
NK: natural killer
NLRs: nucleotide-binding oligomerization domain like receptors
PAMPs: pathogen-associated molecular patterns
pDCs: plasmacytoid dendritic cells
RANK: receptor activators of nuclear factor kappa B
RXR: retinoid X receptor
SRC: steroid receptor co-activator
TGF-β: transforming growth factor-β
Th: T-helper
TLRs: Toll-like receptors
TNF-α: tumor necrosis factor-alpha
Treg: regulatory T
UV: ultraviolet
VDRE: vitamin D response element
VDRs: vitamin D receptors
The author is grateful to Midnapore College, Midnapore, West Bengal, India, for providing all kinds of facilities to prepare this manuscript.
The author contributed solely to the work.
The author has no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Yen Hai Vu ... Gaku Tsuji
Masutaka Furue, Mihoko Furue
Mariko Seishima ... Kuniaki Saito
Ichiro Katayama ... Mari Wataya-Kaneda
Kanami Orihara
Xinhui Ni, Yuping Lai
