Abstract
Mesenchymal stem/stromal cells (MSCs) are known as multipotent cells due to their ability to differentiate into various cell lineages of mesoderm origin. Recent developments in stem cell biology have provided a new ray of hope for the treatment of diseases and disorders that are yet to be treated. These cells have been widely used in animals and clinical trials in humans. To date, there are more than 920 clinical trials on humans related to MSCs as cell-based therapy in various conditions. The purpose of this review is to provide a summary of the characteristics of MSCs, evaluate their immunological properties, activation of MSCs that dictate their soluble factors, possible pathway, and mechanisms involved by MSCs and immune cell interaction, and various application of MSCs in different diseases.
Keywords
Mesenchymal stem cells, immunomodulation, regenerative medicine, animal model, cell-based therapyIntroduction
Stem cells are considered the building blocks of life. They are undifferentiated cells present in all life stages, i.e., embryo, fetus, and adults. These are specialized cells with peculiar characteristics of a) ability to proliferate extensively (self-renewable), b) ability to differentiate into different cell types (potency), and c) clonality. Depending on their potency, they are further categorized into totipotent, pluripotent, multipotent, and unipotent cells. Totipotent cells are mainly found in early developmental stages and they have the ability to form both embryonic and extraembryonic tissue, while embryonic stem cells (ESCs) are pluripotent as they can differentiate into three germ layers—endoderm, ectoderm, and mesoderm. Multipotent cells have limited potency and self-renewable capacity as compared to ESCs. Mesenchymal stem/stromal cells (MSCs) are an example of multipotent stem cells, which can be obtained from different sources and organs in adults like bone marrow (BM), Wharton’s jelly, dental pulp, adipose tissue (AT), peripheral blood, etc. Tissue-specific stem cells can be either oligopotent or unipotent stem cells. Oligopotent stem cells have the capacity to self-renew and differentiate into 2 or more lineages within a specific tissue. While unipotent stem cells have the ability to self-renew and differentiate into a single lineage only. Their role within the tissue is mainly during the time of injury and repair. We present a brief overview of MSC’s potential for immunomodulation and provide a comprehensive overview of future applications of various MSCs in regenerative medicine, as well as the challenges.
MSCs
Amongst the various stem cell types, MSCs are considered to be most widely used clinically as cell-based therapy. Upon isolation, MSCs can be characterized by their ability to grow and expand on plastic plates that are tissue culture treated and have fibroblastic-like morphology, and can differentiate into multiple lineages like osteocytes, chondrocytes, myocytes, adipocytes, and express certain cell surface markers like cluster of differentiation (CD) CD90, CD105, CD73 and are negative for markers like CD45, CD34, CD14, CD11b, CD19. In addition, they express low level of major histocompatibility complex (MHC) class I, CD40, CD80, CD86 and do not express MHC class II molecules, which make these cells immune privileged and an ideal candidate for allogenic transplantation in diseased patients [1, 2]. In the case of ESCs, their major controversy is related to their ethical use, as the ESCs are isolated from embryonic tissue. Even though ESCs have great pluripotency, their use gets limited due to the associated risk of teratoma formation after transplantation [3, 4]. Therefore, MSCs are considered an ideal candidate for cell-based therapies.
BM is considered a gold standard source for isolation of MSCs. MSCs were first identified and isolated from BM aspirate only. After BM, AT, and umbilical cord (UC) are considered accessible sources of MSCs. However, BM-derived MSCs (BM-MSCs) and AT-derived MSCs (AT-MSCs) require invasive and painful procedures while UC-derived MSCs (UC-MSCs) are obtained from birth-associated tissue which is usually considered a medical waste after the delivery of the baby. Thus, placenta, UC tissue, cord blood, and amniotic membrane (AM) are alternate and easy sources of MSC isolation. Recently, some new sources have also been proposed like dental pulp, menstrual blood, skin, tendon, and muscle (Figure 1). However, with all these sources the major factor to consider is the age of the donor. It has been observed younger donor MSCs have high proliferative ability as compared to aged donors [5]. According to International Society for Stem Cell Research (ISSCR), apart from expressing cell surface markers, MSCs must differentiate into trilineage. Currently, several methods are available to drive the cells towards differentiation. For osteogenic differentiation in vitro, culture medium rich in dexamethasone, ascorbic acid, and β-glycerophosphate is used. Osteoblast cells are further characterized using alizarin red which typically stains the calcium deposit in cells. While adipogenic differentiation, uses iso-butylmethyl-xanthine and indomethacin in addition to dexamethasone and oil red O is used to stain the lipid droplets and characterize differentiated adipocytes.
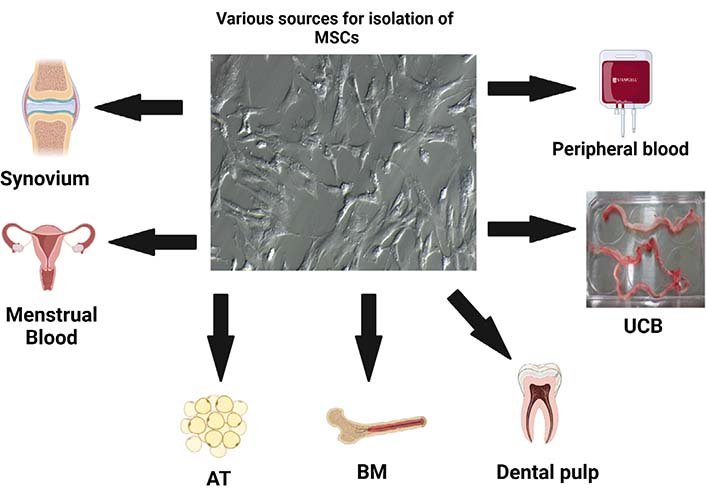
Various sources of MSCs isolation. UCB: UC blood. Figure created with BioRender.com
Immunomodulation by MSC
MSCs affect both the cells of innate and adaptive immunity, thus possessing broad spectrum of immunomodulatory potential. A large number of studies have shown MSCs, irrespective of the source of their isolation, have inhibitory effect on proliferation of T cells which are stimulated via different polyclonal mitogens or any specific antigens [1]. The mechanism underlying its inhibitory action is considered to be either apoptosis or through cell cycle arrest in G0/G1 phase [2]. In addition, MSCs also modulate the cytokine milieu generated by different T cell subsets by decreasing the pro-inflammatory cytokine production and increasing the anti-inflammatory cytokine production [4, 5]. Some cytokines like interferon-γ (IFN-γ), tumor necrosis factors (TNFs)-α, interleukin (IL)-17 are usually reported to be downregulated and IL-10, IL-4 upregulated, thereby indicating a possible MSC-mediated alternation in T helper (Th)1/Th2/Th17 subset balance. Apart from its effect on T cells, it also has inhibitory effect on cytotoxic T lymphocyte (CTL)-mediated cytotoxicity [5, 6]. Some of the published literature, also suggests its effect on B cells, that MSCs inhibit in vitro activated B cell proliferation and production of immunoglobin [6]. Apart from adaptive immunity, MSCs also affect the innate immunity.
Several published data have highlighted that MSCs inhibit the in vitro maturation of monocytes, downregulate the expression of cell surface markers like MHC-II, CD83, CD86, CD11c on mature dendritic cells (DCs), inhibit the maturation of hematopoietic progenitor cells into DCs [7]. MSCs have effect on the cytotoxic properties of natural killer (NK) cells and down-regulate the expression of activation markers like NKp30, NKG2D, and NKp44 on their surface, inhibit their proliferation and suppress the production of IFN-γ [8]. On activated neutrophils, MSCs reduce the in vitro production of hydrogen peroxide thereby suggesting that upon inflammatory stimulation, MSCs can potentially limit the level of respiratory burst [9]. Taken together, the MSCs are found to downregulate the intensity of an immune response by acting on both innate and adaptive immunity.
Factors involved in MSC immunoregulation
MSCs mediate their immunomodulation via release of soluble factors or by cell-cell contact manner (Figure 2). Some key soluble factors are: transforming growth factor (TGF)-β, hepatocyte growth factor (HGF), IL-6, indolamine dioxygenase (IDO), prostaglandin E2 (PGE2), human leukocyte antigen (HLA)-G5, TNF-stimulated gene-6 (TSG-6), IL-1 receptor antagonist (IL-1Ra) and IL-10. TGF-β and HGF were initial molecules to be involved in the immunosuppression of antigen activated lymphocytes [10]. In addition to decreased proliferation, they help in induction of T-regulatory cells and decrease proliferation of NK cells. Another key factor is indoleamine-2,3-dioxygenase. It is an enzyme that catalyses process of conversion of tryptophan to kynurenine [11]. IDO is also involved in decreased T-lymphocyte proliferation because it either leads to the depletion of tryptophan or accumulation of kynurenine. Experimental evidence supporting the two possible mechanisms of IDO are well tested, where in one study indicates exogenous tryptophan led to reestablishment of antigen activated T cell proliferation in the presence of MSCs [12], while in the second study kynurenine was added and it lead to the decreased proliferation of lymphocytes in the absence of MSCs. In addition to the reduced lymphocyte proliferation by MSCs, IDO causes reduced proliferation and cytotoxicity of NK cells [13], decreases the Th17 differentiation with induction of T-regulatory cells and inhibition of the maturation and function of DCs [14].
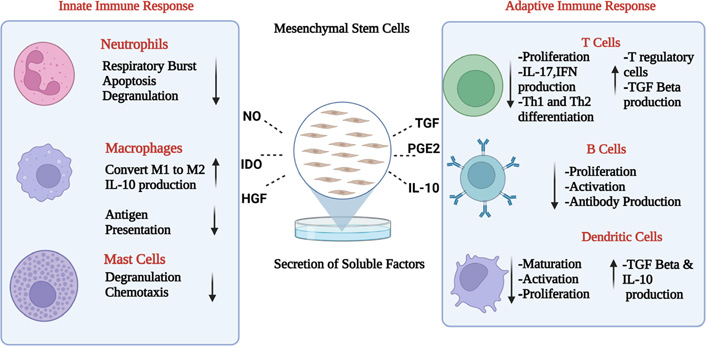
Immunomodulation of MSC. A summary of the range of soluble that may mediate the effects of human MSC on immune responses. NO: nitric oxide. Figure created with BioRender.com
Next important soluble factor produced by MSCs for its immunomodulation is PGE2. PGE2 is produced when arachidonic acid gets converted in prostaglandin (PG) through the enzymatic activity of cyclooxygenases (COX)1 and COX2 enzymes [15]. PGE2 are usually constitutively produced by MSCs and they decrease the proliferation and enhance the production of cytokines like IL-4, IL-10 and induce the generation of forkhead box P3 (FoxP3) positive regulatory cells [16, 17]. Several studies have used PGE2 blockers and shown reversal in the mechanism of proliferation and cytokine secretion but not to the same levels as observed in case of complete absence of MSCs. It has effects on other mechanisms also like decrease NK cell proliferation and function and decrease differentiation of monocytes into DCs [18]. Another important factor is TSG-6. TSG-6 is 35–38 kDa protein produced by MSCs and other immune cells like neutrophils, macrophages, and monocytes. It has been observed, it is produced by MSCs in response to inflammatory signals and it mediates many of MSCs immune-modulatory and repair activities [19]. It plays role in regulating matrix organisation, control association of matrix molecules with cell surface receptors and with signalling factors like chemokines and act as inhibitor of neutrophil migration and suppresses inflammatory signals. A recently published study on AM-derived MSCs has explored the anti-inflammatory role of TSG-6 where it reduces the production of neutrophil extracellular traps (NETs) by BM derived neutrophils [20]. In addition to mediating repair and anti-inflammatory properties, TSG-6 as works in autocrine function to regulate MSCs cellular processes. Loss of TSG-6 impairs the quality and organization of actin filaments, affects cell morphology, proliferation ability and differentiation potential [21]. A large body of studies have accumulated to describe the therapeutic role of another cytokine secreted by MSCs-IL-1Ra which mainly functions by inhibiting IL-1 functioning. Usually IL-1 binds to its receptor and activates a series of pro-inflammatory events and induces the secretion of chemokines to attract other immune cells resulting in tissue inflammation [22]. When IL-Ra binds to IL-1 receptor, no such event is initiated. Protective role of IL-1Ra secreted by MSCs has been demonstrated by inhibiting TNF-α and IL-1α produced by recruiting macrophages, neutrophils and lymphocytes in mice model of lung injury [23]. IL-1Ra has been delineated as mediator for suppression of macrophage-driven inflammation in injured livers. Lee and colleagues [24] showed that MSCs in IL-1Ra dependent manner induced differentiation of macrophages toward immunosuppressive M2 phenotype and higher level of IL-10 which resulted in alleviation of acute liver failure. Similarly, Zheng and co-workers [25] demonstrated therapeutic potential of IL-1Ra-overexpressing amniotic fluid-derived-MSCs (AF-MSCs) in alleviation of fulminant hepatitis.
IL-10 is a cytokine which is thought to be produced both by human MSCs and murine MSCs. IL-10 is also involved in T-regulatory cell induction, decreased maturation and function of DCs and decreasing the production of IL-12 by DCs [26]. Some studies point that MSCs require cell-cell contact between BM-MSCs and activated T-lymphocytes to produce increased concentration of IL-10 [27]. IL-10 has also shown to increase the secretion of HLA-G5, which is another immunomodulatory factor produced by MSCs [28]. HLA-G are non-classical HLA molecules which exist in both forms: membrane bound (HLA-G1, G2, G3 and G4) and soluble form (HLA-G5, G6 and G7). It has been reported that BM-MSCs, express HLA-G1, HLA-G5 and IL-10 promote their expression. Decreased proliferation of effector T cells and induction of T-regulatory cells are some of the immune-suppressive mechanisms which are regulated by these factors [29]. Studies have shown that the use of antibodies against these molecules lead to nearly reversal in the effect of proliferation of antigen-activated peripheral blood mononuclear cells (PBMCs) in presence of MSCs [30]. Another identified soluble factor is galectin, which belongs to family of proteins that bind to β-galactoside [31]. There are around 15 isoforms of galectin. Role of galectin 1, 3, and 9 have been described in MSCs. Mainly their role in inhibiting T and B cell proliferation is known. It has been proven experimentally, that inhibition of galectin-3 expressing MSCs with either small interfering RNA (siRNA) specific for galectin-3 or antibody has shown to reduce the immunosuppressive capacity on alloantigen-activated T lymphocytes [32, 33]. Similarly, for galectin-9, authors observed that in inflammatory conditions, MSCs express more of galectin-9 and blocking of these leads to reversal in MSC-mediated inhibition of proliferation of stimulated T and B lymphocytes. Apart from soluble factors, some membrane bound molecules like program death-1 (PD-1)/program death ligand-1 (PD-L1), Jagged-1, intracellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule (VCAM)-1 are involved in MSC-mediated immune-regulation [34]. In human MSCs, it has been observed that IFN-γ up-regulates the PD-L1 expression and it helps in MSC-mediated immunosuppression of T cell proliferation, which was markedly reversed when monoclonal antibody was used against it [35]. ICAM-1 and VCAM-1 are important adhesion molecules and their role in MSCs-mediated T Cell immunomodulation has been well studied using antibodies against both the adhesion molecules. In a mice model, ICAM-1 and VCAM-1 were blocked and effect of MSCs on anti-CD3/CD28 activated splenocyte proliferation was partially re-established. After the exposure of MSCs to IFN-γ, the expression of ICAM-1 and VCAM-1 usually increase, thereby indicating, MSCs enhance the capacity to recruit inflammatory lymphocytes and then modulates its own functions towards another regulatory phenotype [36].
Moreover, it has been demonstrated that adhesion molecules maybe involved in T cell immune-regulation through induction of CTL-associated antigen-4 (CTLA-4) expression on T cells. MSCs isolated from BM and UCB, in cell-to-cell manner with activated CD3+ lymphocytes, increased the expression of CTLA-4 [37, 38]. Apart from effect of cell-to cell interaction on T cells, it has been shown that cell contact between MSCs and T cells is imperative for immunosuppression of B cells by MSCs. It is observed by a few researchers that effect of MSCs on B cell proliferation is caused due to the interaction between MSCs and T cells which further lead to the secretion of soluble factors which in turn inhibit B cell proliferation [39]. Notch signalling also plays a key role in immunosuppression. A study conducted shows Jagged-1, a notch ligand is expressed on MSCs and when it was blocked by antibodies, MSCs inhibitory effect on alloantigen activated CD4+ lymphocytes was decreased [40]. Thereby, pointing towards the role of Notch signalling in MSC immunomodulation.
Apart from soluble factors, MSCs exert their ability to differentiate into various germ layers like ectoderm, endoderm and mesoderm under specific conditions. MSCs can differentiate into mesoderm lineage cells like adipocytes, osteocytes, which can thereby be used in bone repair and regeneration. Another unique phenomenon shown by MSCs is that they can transfer their own healthy mitochondria into injured cells present in its vicinity and this transfer occurs via formation of gap junctions, nanotubes, cell fusion or by direct uptake of the mitochondria. This property of mitochondrial transfer has been exploited into repair of cardiomyocytes during myocardial infarction (MI) and during damage of corneal epithelium in the eye [41, 42].
Regenerative potential of MSCs
In recent years, MSCs have emerged as a promising cell therapy for many diseases. Increasing advancements in understanding the immune-modulatory mechanisms of MSCs have helped boost its utility in therapeutic and regenerative medicine. Some of the on-going applications of MSCs will be summarized in further sections.
MSCs and cardiac diseases
Vascular injury is the major cause of cardiovascular disease, which is a leading cause of death worldwide. Conventional treatment involves the use of small molecules which have therapeutic potential to restore the blood flow partially. Thus, cell-based therapy using MSCs is being explored in this regard. MSCs being both regenerative and immunomodulatory in nature, are being explored for vascular regeneration either by direct injection of MSCs (systemically or locally), by seeding MSCs on engineered vascular tissue, or by differentiating MSCs in vitro and then injecting in patients to relieve ischemia and restore blood flow [43]. In these cases, the angiogenic potential of MSCs relies on their differentiation capacity to smooth muscle and endothelial lineages, as well as their paracrine effects. Functional improvement such as ischemia alleviation and blood flow restoration are achieved in the end through angiogenesis and modulation of inflammation. MSCs isolated from various sources posed the potential to differentiate toward an endothelial lineage. Human BM-MSCs when cultured in presence of vascular endothelial growth factor (VEGF) could differentiate toward angiogenic endothelial-like cells expressing kinase insert domain receptor (KDR) and von Willebrand factor (vWF) [44]. Using a canine chronic ischemia model, BM-MSCs were shown to differentiate into an endothelial phenotype to facilitate revascularization and improve heart function [36]. UC-MSCs also demonstrate endothelial lineage commitment potential in vitro on 2D or 3D gel scaffolds, as well as in vivo in an ischemic hindlimb mouse model to improve limb perfusion [45].
Another cardiac disease is, MI which causes blockage of blood flow to cardiomyocytes and leads to ischemic cell death. In an effort to enhance cardiac regeneration, cell-based therapy using stem cell can emerge as a promising choice. Stem cells being easily accessible and having differentiation ability are best fit for cell-based therapy. MSCs were shown to differentiate into cardiomyocytes in vivo, representing the basis of harnessing MSCs for cardiac regeneration in which massive cell replenishment is necessary [46] (Figure 3). However, limited engraftment of systematically administered MSCs at the infarct site and a lack of definite evidence demonstrating the differentiation of MSCs into cardiomyocytes has raised the recognition of paracrine mechanisms which contributes to improved cardiac function after MSC treatment.
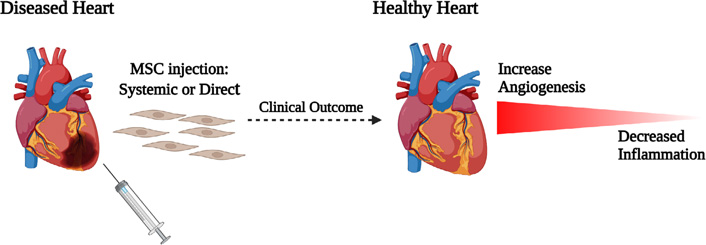
Underlying mechanisms of MSCs in treatment of cardiovascular diseases. Figure created with BioRender.com
MSCs and ocular disorders
Many ocular disorders are debilitating and affect the quality of life in adverse manner. These defects can be related to cornea or retina. Since cornea is directly exposed to the external environmental factors, a variety of clinical disorders have been reported, such as aniridia and Stevens-Johnson syndrome, or chemical, mechanical, and thermal injury induced corneal disorders. Stem cells in the eyes are located in the peripheral region of cornea containing limbal epithelial stem cells (LESCs), which help renew human corneal epithelial cells, but in cases of severe cornea damage, corneal transplant is the only option. Hence, the therapeutic role of LESCs in combination with AM was investigated. In few clinical/pre-clinical studies LESCs cultured on an AM effectively inhibited inflammation and reconstruct the injured corneal surface [47]. Ma et al. [48] transplanted human MSCs grown and expanded on the AM into chemically burned corneas of rats and post 4 weeks, the damaged corneal surface and the vision of the rats were significantly improved. The expression of immune cells (CD45, IL-2) and matrix metalloproteinase-2 (MMP2) which are associated with inflammation and inflammation related angiogenesis was significantly reduced post MSC treatment [48]. In addition, subconjunctival injection of MSCs improved the wound healing of corneas with alkali burns and MSCs were not detected in the injured corneas. It up-regulated the expression levels of the anti-angiogenic factor thrombospondin-1 (TSP-1) and the anti-inflammatory cytokines IL-10, TGF-1, and IL-6 while down-regulated the expression levels of the pro-inflammatory factors IL-2, IFN-γ, macrophage inflammatory protein-1α, and VEGF [49]. In another study by Oh et al. [50], MSCs up-regulated the expression of TSG-6 which is an anti-inflammatory protein that significantly decreased neutrophil infiltration, pro-inflammatory cytokine and chemokine levels in injured corneas. Thus indicating, MSC, help in reconstruction of damaged cornea by inhibiting inflammation and angiogenesis and not by their epithelial differentiation ability [48].
Apart from corneal disease, retinal degeneration is a major medical condition that affects the health and welfare of adults and children. It includes age-related macular degeneration (ARMD), glaucoma, optic neuropathies, and retinal vascular complication. Few studies have shown MSC’s ability to correct defective function of retina photoreceptors, ganglion cells, retinal pigment epithelium (RPE), and optic nerve. A clinical trial (NCT02024269) is on-going for treatment of ARMD patients using i.v. injection of adipose derived MSCs. Adipose derived MSCs have also shown to be neuroprotective in nature against light-induced retinal damage through secretion of several proteins such as the tissue inhibitor of metalloproteinase-1 (TIMP-1) both in vitro and in vivo [51]. A study conducted by Isabel and group [52] have shown, human UCB (hUCB)-derived MSCs contribute towards neural repair through rescue and regeneration of injured neurons. Hence, stem cell-based therapy holds an extraordinary prospective in improving the lives of people who suffer from visual disorders (Figure 4). Research in this area will continue to grow to develop new remedies in treating and preventing the problem of vision loss.
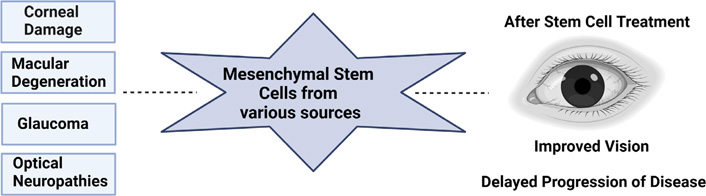
Therapeutic potential of MSCs in treatment of ocular diseases. Figure created with BioRender.com
MSCs and skin regeneration
Skin is the first line of defence against microorganisms and physical damage. Skin is composed of three major layers: epidermis, dermis and hypodermis. Epidermis, the outermost layer, plays a major defensive role and is predominated by the presence of keratinocytes. While the dermis provides structure and is rich in elastin and collagen fibres. The hypodermis is the deepest layer of the skin, being mainly composed of adipocytes. Typically, wound healing is a complex process which comprises of: haemostasis, inflammation, proliferation, epithelization and maturation. It has widely been studied that MSCs migrate to the spot of skin injury, inhibit inflammation, and elevate the proliferation and differentiation potential of fibroblasts, epidermal cells, and endothelial cells and hence enhances the process of wound healing and curtails scarring. MSCs-secrete factors like TSG-6 which help improve wound healing by limiting macrophage activation, inflammation, and fibrosis [53], VEGF secreted by MSCs promote keratinocyte-mediated wound healing [54] and angiotensin II promotes bone-marrow derived stem cells (BMSC) differentiation into keratinocytes through the mitogen-activated protein kinase/c-Jun N-terminal kinase (JNK)/Janus kinase 2 signalling pathways [55]. In vitro studies using BM- and gingival-derived MSCs (G-MSCs) have also indicated the ability of MSCs to skew the polarization of macrophages from M1 to M2 phenotypes, a type of macrophage which is anti-inflammatory in function [56]. Smith et al. [57] revealed that BM-MSCs release soluble signalling factors that increase migration, proliferation, and chemotaxis of dermal fibroblasts. MSCs along with various scaffolds is also being tried in skin regeneration. A large number of scaffolds, such as those involving fibrin hydrogels, 3D-hybridized chitosan and poly (ɛ-caprolactone) and sodium carboxymethyl cellulose have been developed to support MSC-based regeneration of defective skin [58, 59]. Thus, these reports illustrate that MSCs and MSC-derived factors, have wound healing capacity (Figure 5) and these applications can be subjected to clinical trials, and optimized treatment plans and patient types can be decided.
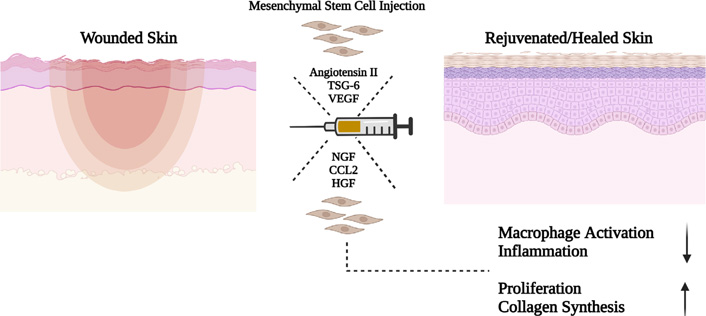
A model for a possible paracrine mechanism of how MSCs promote skin regeneration via the secretion of soluble factors. CCL2: chemokine ligand; NGF: nerve growth factor. Figure created with BioRender.com
MSCs and liver diseases
Liver is an important human organ and failure of its functioning can be rectified by liver transplantation. However, liver transplant becomes difficult as donor organ shortage is the main reason and hence whole organ or hepatocyte transplants cannot be done frequently. So, MSCs potential therapeutic application in liver disease is being explored for a long time now. MSCs potential therapy for liver diseases relies on differentiation into hepatocytes, besides immunomodulation by release of trophic factors affecting function of NK cells and stellate cells [60]. Under specific differentiation conditions, MSCs have been shown to adopt functional features of differentiated hepatocytes and successfully engrafted into mouse liver. In a rat model of liver fibrosis, injected MSCs facilitated recovery from the diseased state by reducing collagen levels in liver extract, elevating HGF levels in portal vein and reducing the overall fibrosis [61]. Taking the work forward, few clinical trials using MSCs have been conducted in liver repair. Phase I and II clinical trials for liver cirrhosis suggested that both differentiated and undifferentiated MSC transplantation improved liver function [62, 63]. Follow-up of patients at 3- and 6-months post-transplant revealed partial improvement of liver function tests with elevation of prothrombin concentration and serum albumin levels, decline of elevated bilirubin and improved model for end-stage liver disease (MELD) score [64]. Although preclinical and clinical studies have given promising results, thorough investigations are required to translate these studies into routine treatment.
MSCs and kidney diseases
MSCs because of their immunomodulatory nature primarily caused by their secretory capabilities, have found potential applications in kidney diseases. MSC can play an important role in the treatment of inflammatory kidney disease, such as primary and secondary glomerulonephritis, or in the prevention of rejection of the transplanted kidney. Previous studies have shown, due to release of trophic factors, mitogenesis of kidney cells is stimulated and they stimulate the resident stem cells of the kidney to repair itself [65]. In one of the initial studies conducted, it was reported that MSCs when injected into acute renal injury mice model, leads to renal repair by trans differentiation into tubular epithelium. However, it was found that very less percentage of injected MSCs (2–2.5%) showed engraftment [66, 67]. In a study contrary to this, with arterial injection of MSCs, there was improved renal function, increased proliferation of mesangial cells and expression of α-smooth muscle actin (α-SMA), yet no traces of MSC incorporation into kidney structures was seen [68]. These beneficial effects of MSC result in maximum conditions from their secretory properties, not replicative-differentiating potential.
The use of MSC in the clinical setting of renal ischemia was the subject of a study conducted by researchers from Minnesota. Fourteen patients with unilateral renal artery stenosis were administered autologous adipose-derived MSC (105 or 2.5 × 105 cells per kg body weight) to the stenotic renal artery. After the subsequent three months, the blood flow increased in both the stenotic and the contralateral kidney, and glomerular filtration was higher by 21% compared to the control group [69].
Inflammatory glomerulopathies and renal transplantation are also some potential areas where applications of the MSCs is being explored. In an animal model of membranoproliferative glomerulonephritis, intravenous infusion of allogeneic foetal MSCs reduced glomerular expression of proinflammatory cytokines, decreased monocyte infiltrates, mesangial hyperplasia, synthesis of connective tissue matrix and proteinuria [70]. Likewise, in the rat model of focal segmental glomerulosclerosis (doxorubicin-induced nephropathy), several intravenous infusions of BM-MSCs increased glomerular VEGF synthesis, which was accompanied by attenuations of: glomerular monocyte infiltration, apoptosis of the podocytes, and the extent of podocyte-parietal epithelial bridging [71]. On the other hand, one of the largest clinical trials has been conducted in China by recruiting 104 renal transplant patients who received autologous dose of MSCs at graft reperfusion and again after two weeks in place of anti-IL-2 receptor antibodies. Such induction of immunosuppression was associated with faster organ regeneration in the first month after transplantation, as well as a lower rate of cellular rejection (7.6% vs. 21.6% in the control group) and its milder course in the six-month follow-up [72]. Thus, there are enough reports of MSCs repopulating the damaged kidney with varying degrees of significance. If properly standardized, MSC based therapy can be a breakthrough in treating kidney disorders worldwide.
MSCs and bone regeneration
The most common disorders of the skeletal system include osteoporosis, bone fractures, osteogenesis imperfecta (OI). For each of the diseases, because of lack of proper medication, side effects, lack of an adequate supply of autologous bone grafts and the unsuitability of allografts, there has been some impetus to use MSCs to encourage repair and treat bone disorders. Studies in animal models have revealed that both allogeneic and autologous BM-MSCs transplantation are applicable for the treatment of osteoporosis. In an animal model of glucocorticoid induced osteoporosis in mice, allogenic BM-MSCs were injected and it showed osteoblastgenesis and promoted new bone formation [73]. MSCs from other tissue sources like human UC (hUC), hUCB, amnion, and chorion, have attracted special attention for osteoporosis improvement and preventing bone loss by decreasing the number of osteoclasts, showed higher bone mass, collagen content, and osteoblasts number [74].
Bone fracture, is a major problem amongst children and elderly people. Few clinical trials are undergoing in different phases using MSCs from BM, AT, periosteum-derived and UC either via direct injection or after seeding them onto an osteogenic matrix. The required number of cells needed for fracture repair depends on the specific fracture characteristics, cell source, stimulation method, differentiation state, and using biomaterials. A comparison between three main sources of stem cells used to repair bone fractures suggested that isolation efficiency was higher from AT compared to other sources with respect to cell yield and feasibility. Although the ability for osteogenic differentiation seems to be higher in periosteum-derived MSCs (PD-MSCs) [75], the most widely used cell source is yet allocated to BM for bone fracture repair strategies in recent clinical trials.
MSCs and rheumatic diseases
Osteoarthritis (OA) is one of the most common arthritis-related chronic disorders characterized by articular cartilage degeneration, thickening of subchondral bone, and osteophyte formation. The current treatment regime for OA includes physical therapy, drug therapy, and surgery, which manage the pain, stiffness, and swelling but are not effective to prevent the OA progression. Hence, novel therapies in form of cell therapy may be considered. The MSCs having immunomodulatory and differentiation ability makes it the most suitable choice (Figure 6). It has been shown that i.a. injection of BM-MSCs in focal cartilage defects in immunocompetent transgenic rat can lead to collagen and matrix formation [76]. Similar results were observed when adipose derived and UC-MSCs were used for the disease treatment. In a phase I/II trial BM-MSCs were injected into knee OA patients and it was observed their cartilage biomarker expression, reduced synovial inflammation, pain, and symptom mitigation, and no serious adverse events [77].
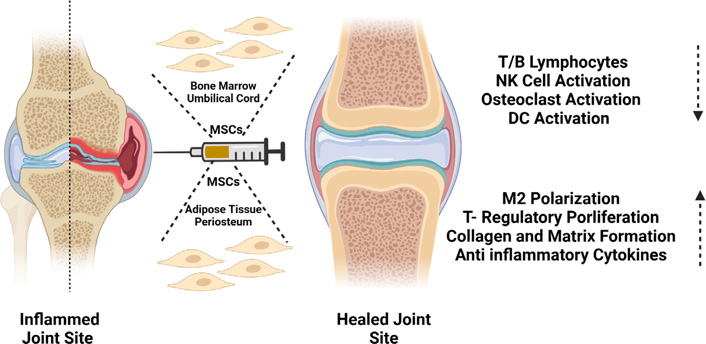
Role of MSCs isolated from different sources in repair of the inflamed synovial joint. Figure created with BioRender.com
Rheumatoid arthritis (RA) is a chronic autoimmune, inflammatory rheumatologic disorder characterized by hypertrophy, hyperplasia and angiogenesis of the synovial tissue which contributes to destruction and disability of the joints. Current treatment regime targets to reduce or suppress the inflammation using disease modifying drugs and biologics. However, approximately 25–30% patients do not respond to the treatment and underlie problem of bone and cartilage damage continues to progress without being resolved. Hence, new treatment needs to be searched and use of MSCs in RA is being greatly explored. Our group has also shown that the MSCs from Warton’s jelly of hUC decreased the tissue injury in the synovial joint by suppressing the auto-immune effector T cell functions when injected intraperitoneally into collagen-induced arthritis (CIA) model in rats. The data indicated that the hUC-derived MSC (hUC-MSC) injection seems to be reinstating the broken self-tolerance by inducing T-reg proliferation and skewing the cytokine repertoire towards anti-inflammatory type [78]. Similar studies have shown MSCs playing an important role in inducing apoptosis of activated T cells via Fas ligand (FasL)/Fas signalling pathway in arthritis disease [79]. An experimental study using different MSC sources have been tried in RA and human G-MSCs were also found to inhibit the process of osteoclast formation (osteoclastogenesis) in vitro as well as in vivo using CIA model, partially via CD39-CD73-adenosine signals [80]. In the first human trial using human cord blood MSCs in patients with RA observed no major toxicity, no serious adverse events, or major abnormalities in serum chemicals or hematologic profiles, both during and after the treatment [81]. Thus, MSC-based therapy is being introduced as a promising treatment strategy with potential ameliorating effects on disease progression in bone disorders.
MSCs and cartilage repair
Cartilage tissue is made of chondrocyte embedded within an extracellular matrix (ECM) which mainly consists of collagen and proteoglycan. It has avascular nature and a limited capacity for self-repair. In case of cartilage defect, cartilage repair mechanism is carried out using non-surgical methods like physiotherapy and i.a. injection and surgical alternatives like soft tissue grafting and autologous chondrocyte transplantation. Currently, there is considerable research interest in using MSCs for cell-based therapy of cartilage since these cells proliferate extensively in culture while maintaining their characteristics and are capable of differentiating into a chondrogenic phenotype [82, 83]. In case of cartilage repair, MSCs harvested and expanded are either mixed with gel glue (sealant-based) or seeded on biocompatible scaffolds (scaffold-based) for cell delivery towards the site of defect [84, 85]. Numerous pre-clinical trials for testing the utility of scaffold-based MSC implantation in treatment of cartilage defects has been done, for examples, poly-L-glutamic acid [PLGA, a copolymer of polylactic acid and poly glycolic acid which is approved by the United States Food and Drug Administration (FDA) for clinical use] scaffold seeded with cultured MSC has been used for cartilage repair of large defects in rabbit knees and the results demonstrated successful induction of in vivo chondrogenesis [86]. In 2007, a clinical trial was registered by Kuroda et al. [87], where they used autologous MSCs with collagen polymer scaffold for cartilage repair and found a full thickness repair of articular cartilage defect in the medial femoral condyle of an athlete. Hence, many preclinical and clinical studies have demonstrated the feasibility of using MSC for the treatment but there are some challenges which still need to be addressed.
Challenges of using MSCs in regenerative medicine
Although the MSCs are being used widely in pre-clinical and some limited clinical trials because of their regenerative and immunomodulatory characteristics, despite this many challenges are faced while using them in regenerative therapy. The first challenge is deciding the appropriate source of MSC isolation. Many studies use MSCs from different sources with different isolation and expansion protocols to treat a particular disease. So, standardizing this process of MSC isolation and processing needs to be addressed. Proper Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) compliance related regulations need to be followed along with other microbial safety measures while manufacturing MSCs. Secondly, the dosage of MSCs injected for effective treatment is also critical. In clinical trials, the dose of MSCs per person is based on the body weight and is roughly calculated as mean of 6.64 × 106 cells per kg body weight. Setting proper dose calculation protocols for treatment of the diseases needs to be laid down. Third, several approaches have been tried while administering MSCs. In general, they are either injected locally, systemically or delivered on a prefabricated scaffold either alone or with other types of cells. In systemic delivery comes the route of i.v., i.a., i.p., while in local delivery mode comes i.m. or direct injection at the site of injury like i.c., intralesional, and intrathecal. The delivery system through each route has its own advantages and disadvantages. Like in case of i.v. injection, it has been seen that entrapment of the cells in lung and formation of micro-emboli, a phenomenon called “pulmonary first pass” effect is observed which can be overcome by giving i.a. instead of i.v. [88]. While, local administration of MSCs is a more controlled delivery approach, making it easier to access the disease site that often results in better therapeutic responses. In case of ischemic stroke, MSCs were administered locally at the damaged site and it showed better response in terms of improved neurological score as compared to MSCs that were administered i.a. and i.v. [89]. However, there are still clinical challenges associated with local administration that hinder therapeutic efficacy, primarily due to insufficient retention and survival of transplanted MSCs at the site of administration. The lack of retention following local administration has been attributed to multiple issues after administration, including cell death due to the hostile environment encountered at the disease site and poor engraftment into the tissue [90]. Although highly dependent on the clinical application, some cases have shown that less than 5% of administered cells remain at the site of injection in the hours following transplantation. To improve retention and other properties of administered MSCs, multiple strategies are being investigated and among these strategies, priming MSCs in vitro seems a simpler approach [91].
Pre-conditioning of MSCs to improve their regenerative potential
Immunosuppressive nature of MSCs has been widely reported in the literature, but the differences occur in the microenvironment of in vitro or in vivo setup. Firstly, MSCs which are grown under culture conditions in vitro, grow in conditions that range from 20–21% O2 concentration, which is much higher than the actual micro environment in which they reside (1–5% O2). These differences in O2 concentration influence the outcome of the MSCs in terms of their ability to proliferate, differentiate, and immunomodulate [92]. Secondly, these cells are exposed to immune cells of the host and their soluble mediators (cytokine and chemokines), which can also greatly influence the functional outcome of MSCs. The cytokine milieu of the diseased patient might change at different stages of the disease, thus impacting the efficiency of MSC therapy in preclinical and clinical study. Moreover, the donor age seems to play a critical role in MSCs regenerative potential. When the regenerative potential of adult and young MSCs was compared, it was observed that elderly MSCs are greatly affected by age related changes. The key properties of proliferation, differentiation and yield were significantly adversely affected by age of the donor. Thus, attempts are being made by adopting different methodologies to improve the compromised stem cell function. Preconditioning, genetic modification and optimizing culture conditions have greatly helped to improve the MSC functioning both in vitro and in vivo.
Pre-treatment or pre-conditioning MSCs with hypoxia, growth factors, cytokines, chemical agents, physical factors and gene modification has impacted there biological and functional properties that can improve their regenerative potential before clinical use [93, 94]. Various studies have shown that hypoxia-inducible factor (HIF) pathway gets activated under hypoxic condition and HIF-1α and HIF-2α help the MSCs to survive under hypoxic condition [95]. More genetic stability and lower level of apoptosis is seen in MSCs grown in hypoxic condition as compared to normoxic condition where they can damage the DNA and protein with increased reactive oxygen species (ROS) production. In a rat model of diabetic cardiomyopathy, when hypoxic conditioned MSCs were injected, upregulation of Bcl-2/Bax ratio and decreased the activation of caspase 3 was observed. Similarly, upregulation of soluble factors like IDO, TGF-β, TSG-6, NO, PGs, chemokine, cell adhesion molecules was seen when cells were pretreated with pro-inflammatory cytokines like IFN-γ and TNF-α [96]. Thus, pre-treatment of MSCs with inflammatory cytokines enhance their immunomodulatory ability, which can prove helpful in future potential cell-based therapy [36, 97]. Apart from cytokines, certain growth factors like stromal-derived factor (SDF) 1 alpha (SDF-1) also aid in preconditioning of MSCs. In a study, it has been demonstrated that BM-MSCs when pretreated with SDF-1 leads to greater cell survival, decreased apoptosis in vitro and in vivo in a rat model of left anterior descending artery ligation causing improvement in myocardial functions via SDF/chemokine receptor type 4 (CXCR4) signaling activation [98].
Recently few papers have reported role of toll-like receptors (TLRs) in MSCs where it suggests that the MSCs become polarized either to pro-inflammatory or anti-inflammatory phenotype, depending on the type of TLR activation downstream [99]. TLRs play a critical role in the human immune system, where a specific ligand can lead to the activation of downstream signaling cascade by binding to a specific TLR, which ultimately leads to release of cytokines and soluble mediators that influence the outcome of MSCs. Few studies have highlighted the role of TLR3, 4, and 9 in MSCs where TLR3 priming induces anti-inflammatory phenotype and TLR4 priming induces pro-inflammatory phenotype while TLR9 priming increases the immunomodulatory ability of MSCs by increasing TGF-β level and decreasing TNF-α levels [100]. But there is a need to extensively study the effects of pre-activation of TLRs in the regenerative potential of MSCs.
Conclusions
The broad applications of MSCs are mainly due to their regenerative potentials as well as their immunomodulatory characteristics. Several clinical studies as quoted in the review have demonstrated that the cell-based therapy with adult MSCs from various sources has the ability of immune-modulation and repair of injured tissues through facilitated regeneration. Despite these properties, there are many challenges which need to be addressed before the broad-spectrum use of these cells. A combination of various strategies as discussed in this review could be interesting in promoting the perception of MSCs potentials, functions and clinical perspectives.
Abbreviations
| AM: | amniotic membrane |
| AT: | adipose tissue |
| BM: | bone marrow |
| BM-MSCs: | bone marrow-derived mesenchymal stem/stromal cells |
| CD: | cluster of differentiation |
| DCs: | dendritic cells |
| ESCs: | embryonic stem cells |
| HGF: | hepatocyte growth factor |
| HIF: | hypoxia-inducible factor |
| HLA: | human leukocyte antigen |
| hUC: | human umbilical cord |
| ICAM: | intracellular adhesion molecule |
| IDO: | indolamine dioxygenase |
| IFN-γ: | interferon γ |
| IL: | interleukin |
| IL-1Ra: | interleukin-1 receptor antagonist |
| LESCs: | limbal epithelial stem cells |
| MHC: | major histocompatibility complex |
| MSCs: | mesenchymal stem/stromal cells |
| NK: | natural killer |
| OA: | osteoarthritis |
| PGE2: | prostaglandin E2 |
| RA: | rheumatoid arthritis |
| SDF: | stromal-derived factor |
| TGF: | transforming growth factor |
| Th: | T helper |
| TLRs: | toll-like receptors |
| TNFs: | tumor necrosis factors |
| TSG-6: | tumor necrosis factor-stimulated gene-6 |
| UC: | umbilical cord |
| UCB: | umbilical cord blood |
| UC-MSCs: | umbilical cord-derived mesenchymal stem/stromal cells |
| VCAM: | vascular cell adhesion molecule |
| VEGF: | vascular endothelial growth factor |
Declarations
Author contributions
MV: Writing—original draft, Data curation, Methodology, Investigation, Software, Formal analysis, Validation. SKA: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The data is available with authors and can be shared on request.
Funding
Not applicable.
Copyright
© The Author(s) 2023.