Affiliation:
1Department of Reproductive Biology, Pan African University of Life and Earth Sciences (including Health and Agriculture), University of Ibadan, Ibadan 200005, Nigeria
Email: tannibih@gmail.com
ORCID: https://orcid.org/0000-0003-4667-7415
Affiliation:
2Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Yaoundé, Cameroon
3Fertility Laboratory, Gynecological Endoscopic Surgery and Human Reproductive Teaching Hospital, Yaoundé, Cameroon
Affiliation:
4Obstetrics and gynecology, University College Hospital, University of Ibadan, Ibadan 200005, Nigeria
Affiliation:
4Obstetrics and gynecology, University College Hospital, University of Ibadan, Ibadan 200005, Nigeria
Explor Med. 2022;3:443–450 DOI: https://doi.org/10.37349/emed.2022.00105
Received: June 21, 2022 Accepted: August 15, 2022 Published: October 27, 2022
Academic Editor: Yingyong Zhao, Northwest University, China
Aim: Given the male infertility’s pluri-etiological nature, thorough examinations are needed for its evaluation. Fructose and citric acid are simple biomolecules, easy to assay, which provide reliable information on the seminal vesicles and prostate, respectively. This study aimed to compare the seminal fructose and citric acid levels in men undergoing fertility evaluation and determine the relation between these markers and sperm parameters.
Methods: A prospective cross-sectional study was conducted on consenting male participants. Following 2010 seminal fluid analysis (SFA) manual of World Health Organization (WHO), semen samples were analyzed for several sperm parameters, seminal fructose and citric acid. Statistical analyses were performed using IBM SPSS 24.0 software. Significant statistical difference was considered at P < 0.05.
Results: There is no significant difference between seminal fructose and citric acid levels amongst men with normal and abnormal sperm parameters as median seminal fructose and citric acid levels were 11.1 (7.4–17.1) mg/mL and 11.4 (7.3–15.2) mg/mL respectively (P ≥ 0.05). However, a high level of fructose was observed in the two groups according to the reference value. The study revealed a significant positive correlation between seminal fructose levels and semen volume (coefficient rho = 0.663; P = 0.001) and between seminal citric acid levels and semen volume (coefficient rho = 0.319; P = 0.004).
Conclusions: These biomarkers secretions can serve as markers of the state of their respective secreting glands and hence play a vital role in the investigation of male infertility.
Infertility is characterized by the failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse [1]. Recent studies showed that, 14–30% of couples of reproductive ages suffer from infertility, and nearly 50% of the cases are due to male factors [2]. Male infertility is considered complicated due to its pluri-etiological nature which affects the physiology of the reproductive organs of affected men and leads to lower sperm count, reduced sperm motility, and anomalous sperm morphology, which are the key attributes of male infertility [2, 3]. The accessory sex glands secrete a variety of substances, composed of organic and inorganic elements, which may not necessarily play a pivotal role for fertilization, yet provide valuable information about the functioning of the spermatozoa and the state of the male reproductive system [2].
Fructose is the principal source of energy for spermatozoa and is essential for spermatozoa metabolism, progressive spermatozoa motility and viscosity [4, 5]. Determination of seminal fructose concentration has been used in evaluating obstructive azoospermia and inflammation of male accessory glands [2]. The fructose levels in seminal plasma, therefore, are an indicator of the status of seminal vesicles, endocrine anomalies, and ejaculatory duct obstruction. Citric acid, on the other hand, is an organic substance secreted by the prostate primarily to maintain pH, and to convert protein, fat, and sugar into carbon dioxide. Determination of the levels of this vital biochemical constituent provides information on the condition of the prostate, coagulation and liquefaction of semen in men [2]. Hence, it plays a vital part in sperm motility and hyaluronidase activity. As such, these biochemical secretions serve as a marker of the state of their respective glands. This study aimed to compare the seminal fructose and citric acid levels in men undergoing fertility evaluation and determine the relation between these markers and sperm parameters.
A prospective cross-sectional study was performed on consenting male participants who met the inclusion criteria. This study was conducted in the semiology service at Laboratoire d’Excellence (LABEX) of Yaoundé, Cameroon. LABEX is a privately owned laboratory, certified, accredited and equipped receiving many referrals from district hospitals, health centers, other laboratories, or voluntary patients.
Study participants were recruited among male adult attendants (21 years and above) presented for semen fluid analysis at LABEX, Yaoundé. Most of these men were referred from different laboratories, hospitals, urology and gynecology clinics within and outside Yaoundé. Non-compliance to sample collection conditions and hypospermia (< 1.5 mL) were the exclusion criteria.
The fifth edition of the 2010 World Health Organization (WHO) laboratory manual for the examination and processing of Human Semen stipulates a minimum of 2 days and a maximum of 7 days of sexual abstinence prior to semen collection. Additionally, subjects were instructed to wash their hands with soap, urinate and wash the glans penis and coronal sulcus with soap and water. Samples were collected in a private room near the laboratory, by masturbation directly into a 100mL sterile container, conveyed to the laboratory and analyzed within one hour after ejaculation. The samples were labelled, indicating the date, time of collection and patient code. Analysis was done immediately after semen liquefaction (37°C or room temperature) following WHO recommendations. Samples were assessed for several parameters such as volume, pH, sperm motility (progressive and non-progressive), vitality, morphology, concentration, and count, and were stored at –20°C not more than two hours later for subsequent assay of fructose and citric acid. Abnormal sperms are counted and classified according to WHO manual recommendation.
After determination of parameters as described above, the participants were divided into two groups following the fifth edition of the WHO manual, published in 2010 (Tables 1 and 2).
2010 WHO reference values for normal sperm parameters
| Parameter | Lower reference limit 2010 |
|---|---|
| Semen volume | 1.5 mL |
| Sperm concentration | 15 × 106 spermatozoa/mL |
| Total sperm number | 39 × 106 spermatozoa/ejaculate |
| Progressive motility | 32% |
| Total motility | 40% |
| Vitality (live sperms) | 58% |
| Sperm morphology | 4% |
| pH | ≥ 7.2 |
| Leucocyte | < 1 × 106 leucocytes/mL |
| Mixed antiglobulin reaction (MAR)/immunobead test | < 50% |
Distribution of characteristics in the study population
| Characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (years old) | ||
| (25–35) | 13 | 16.5 |
| (35–45) | 34 | 43.0 |
| (45–55) | 24 | 30.4 |
| (55–65) | 7 | 8.9 |
| ≥ 75 | 1 | 1.3 |
| Educational level | ||
| Primary | 3 | 3.8 |
| Secondary | 10 | 12.7 |
| Tertiary | 66 | 83.5 |
| Occupation | ||
| Professional | 51 | 64.6 |
| Unskilled | 20 | 25.3 |
| Unemployed | 8 | 10.1 |
| Marital status | ||
| Single | 5 | 6.4 |
| Married | 40 | 50.6 |
| Cohabitation | 34 | 43.0 |
| Infertility | ||
| Yes | 35 | 44.3 |
| No | 44 | 55.7 |
| Oligospermia | ||
| Severe | 18 | 22.8 |
| Moderate | 6 | 7.6 |
| Mild | 7 | 8.9 |
| No | 48 | 60.8 |
| Asthenospermia | ||
| Yes | 22 | 27.8 |
| No | 57 | 72.1 |
| Necrozoospermia | ||
| Yes | 26 | 32.9 |
| No | 53 | 67.1 |
| Viral infections | ||
| Hepatitis B | 5 | 6.3 |
| Hepatitis C | 2 | 2.5 |
| HIV | 3 | 3.8 |
Seminal fructose levels were detected using the Fructose-Sperm 360® kit (Fertipro, Beernem, Belgium). The principle of the fructose test was that fructose reacts, in the presence of hydrochloric acid (HCl) under heat, with indole and produces a colored complex which can be measured at a wavelength of 450–492 nm. Kit reference value for fructose is 2.4 mg/ejaculate or more and 13 μmol/ejaculate or more.
Seminal citric acid levels will be detected using the CitricScreen® kit (Scopescreen Co, Tamil Nadu, India). The citric acid test works in two steps: firstly, spermatozoa and particles are removed by addition of isopropanol; next, after centrifugation, ferric chloride is added to the supernatant. The Fe3+ ions and citrate form a complex that turns the solution to a pale green color. The intensity of the color is directly related to the amount of citrate and can be measured in a photometer or plate reader. Kit reference value for citric acid is 10 mg or more per ejaculate.
Statistical analysis of the findings was performed using IBM SPSS 24.0 software. Data was expressed in mean (standard deviation, SD), median (interquartile, IQ), and frequency. Descriptive statistics were used; comparison between variables with the Mann–Whitney test and relation test was done using the Spearman correlation. Significant statistical differences between median sperm parameters of both groups of men and biomarker levels were considered at P < 0.05.
Ethical approval was obtained from the local institutional review board: University of Ibadan/University College Hospital Ethical Committee (UI/UCH EC) and Cameroon National Ethical Committee of Research for Human Health (NECRHH). Signed consent forms were collected upon acceptance to participate in the study. Access to any data was restricted only to the members of the study team who stored the data in a secure database. Confidentiality was respected. The results of the study have been used for scientific purposes only.
During this study period, a total of 103 patients visited the laboratory for seminal fluid analysis (SFA). Of all these 103 patients, 24 were excluded; 10 refused to participate in the study and 14 had hypospermia.
The study revealed that the ages of the participants varied from 26–71 years old with the age group (35–45 years old) representing 43.0% (34) of the sample. The tertiary educational level (66; 83.5%) and the professional occupation (51; 64.6%) were the most represented. Most participants were married (40; 50.6%) or cohabiting (34; 43.0%) with only (5; 6.4%) being single. Nearly half of the study population had an infertility (35; 44.3%). Severe oligospermia, asthenospermia and necrozoospermia were seen in different proportions in our study population, 22.8% (18), 27.8% (22) and 32.9% (26) respectively. The prevalence of hepatitis B, C and human immune deficiency viruses was 6.3% (5), 2.5% (2) and 3.8% (3) respectively (Table 2).
The prevalence of men with abnormal sperm parameters amongst the study population was 44% (Figure 1).
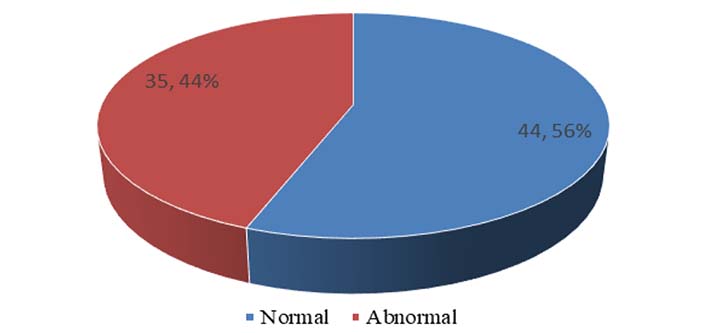
Prevalence of men with normal and abnormal sperm parameters amongst study population
No significant difference was observed in the seminal fructose and citric acid levels amongst men with normal and abnormal sperm parameters as median seminal fructose and citric acid levels were 11.1 (7.4–17.1) mg/mL and 11.4 (7.3–15.2) mg/mL respectively (P ≥ 0.05). However, a high level of seminal fructose was observed in the two groups according to the reference value (Table 3).
Evaluation of seminal fructose and citric acid levels amongst men with normal and abnormal sperm parameters for study population
| Parameter | Normal parameters (mg/mL) | Abnormal parameters (mg/mL) | P |
|---|---|---|---|
| Median (Q1–Q3) | |||
| Fructose (mg/mL) | 11.6 (7.3–17.3) | 11.1 (7.4–15.7) | 0.493 |
| Citric acid (mg/mL) | 10.0 (6.7–15.2) | 11.8 (7.5–14.9) | 0.741 |
Q1: lower quartile; Q3: upper quartile
The study revealed a significant positive correlation between seminal fructose levels and semen volume (coefficient rho = 0.663; P = 0.001) and between seminal citric acid levels and semen volume (coefficient rho = 0.319; P =0.004) (Table 4).
Evaluation of seminal fructose and citric acid levels relative to sperm parameters
| Parameters | Fructose coefficient rho | P | Citric acid coefficient rho | P |
|---|---|---|---|---|
| Concentration | –0.044 | 0.703 | 0.022 | 0.848 |
| Count | 0.102 | 0.371 | 0.057 | 0.621 |
| Motility | –0.114 | 0.325 | 0.064 | 0.585 |
| Morphology | 0.025 | 0.839 | –0.045 | 0.707 |
| Vitality | –0.034 | 0.770 | 0.105 | 0.364 |
| Volume | 0.663 | 0.001 | 0.319 | 0.004 |
The current study demonstrated that the prevalence of men with abnormal sperm parameters amongst the study population was 44%. Amongst these were primary and secondary infertile men with prevalence of 40% and 60% respectively. Benksim et al. [6] in 2018 conducted a study in Morocco and had similar prevalence values (32.63% and 67.37% respectively). However, Gudeloglu et al. [7] in 2015 highlighted contrasting results. These discrepancies could be due to the different study locations.
The results indicated that there was no significant difference between the median fructose and citric acid levels in men with normal and abnormal sperm parameters. These results were contrasting with those from Toragall’s study in 2019 [2].
The study highlighted marked differences of the biomarker levels amongst the different abnormal sperm conditions when compared to those of normospermic men. Notably, relatively lower fructose levels were observed amongst the asthenozoospermic and higher levels amongst the oligoasthenozoospermic and asthenocryptozoospermic men. This confirms that fructose is important for proper sperm motility and how its underutilization can be observed in oligospermic samples respectively. Similar results were observed by Said et al. [8] in 2009 and AL-Khazali et al. [9] in 2020. Also, there was no marked difference in citric acid levels amongst the different infertility conditions except for cryptozoospermic men who represented just 2.9%. This could be due to chronic prostatitis or obstruction of the prostatic ducts due to inflammation for that single patient, (acute or chronic bacterial prostatovesiculitis) as demonstrated in other studies [9]. Similar results were observed by Toragall’s [2] except for asthenozoospermia, asthenoteratozoospermia and azoospermia. These results answer the problem of determining the differences in the levels of the seminal biomarkers fructose and citric acid in men with fertility problems.
The results obtained also provided the relationship between the biomarker levels and sperm parameters. They indicated that there exists a significant positive relationship between fructose levels, citric acid levels and volume. The accessory glands contribute up to 70–80% of total seminal fluid, hence the significant positive relationship. Also, there exists a positive relationship between sperm count, volume, and morphology while an inverse relation between fructose levels, motility, and sperm concentration. As such, the lower the sperm concentration in a sample, the higher the fructose levels and vice versa. This explains why participants who were oligoasthenozoospermic and cryptoasthenozoospermic had relatively higher fructose levels and poor motility.
Additionally, a positive correlation was observed between sperm count, concentration, motility, volume, and citric acid concentrations while an inverse relation exists between citric acid levels and morphology. Given that the prostate contributes to almost 50% of the seminal volume, it is normal that there exists a direct relation between volume, concentration, count, and citric acid concentrations.
Citric acid plays a vital role in the liquefaction of seminal fluid and thus has an indirect effect on the motility of the sperm and eventual fertility of the male. It has also been demonstrated that prostate gland secretion plays a role in balancing osmotic equilibrium of semen, which affects the membrane activity and morphology of the spermatozoa. Trang Thi Nguyen [10] also determined a similar correlation between fructose and semen parameters. Shemshaki et al. [11] determined a similar relation between these biomarkers, sperm parameters, and body mass index (BMI).
The results from this study support existing theories on the one hand, as they highlight the role of the biomarkers, and implicitly, the accessory glands for male fertility. Conversely, they challenge other existing theories on the notion of having significant clear-cut lower biomarker concentrations among men with normal and abnormal sperm parameters.
These discrepancies could be due to study design employed, smaller sample size, reagents and equipment used and analysis. Given that total fructose calculation is determined after including volume, this factor could potentially cause discrepancies. Also, accessory gland function could be altered by microorganisms altering their epithelia without affecting sperm density, motility, or morphology. This could cause sperm parameters to be inconsistent with biomarker levels. Practically, this implies that further studies should take into consideration bacteriological testing of seminal fluid samples, manipulation of samples and equipment. Also, to ascertain male fertility, at least two to three sperm samples within a 3-month period should be analyzed [5, 12]. Fructose and citric acid levels are known to be affected by other factors such as nutrition, and especially body mass index as indicated in the study conducted in 2017 [11, 13]. These factors were not considered for the current study.
The evaluation of seminal biomarkers and sperm parameters is quite helpful in understanding the functionality of accessory glands and investigating causes of male infertility. It should, however, be associated with other diagnostic tests to determine specific cause of infertility.
LABEX: Laboratoire d’Excellence
WHO: World Health Organization
The research team sincerely appreciates Laboratoire d’Excellence (LABEX) for their cooperation and support to carry out this study. Sincere gratitude also goes to all participants of the study.
BT, AO and COA contributed conception and design of the study; BT organized the database; EVV and BT performed statistical analysis; BT wrote the first draft of the manuscript; AO, COA and EVV corrected sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
Ethical approval was obtained from the local institutional review board: University of Ibadan/University College Hospital Ethical Committee (UI/UCH EC) and Cameroon National Ethical Committee of Research for Human Health (NECRHH).
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Access to any data was restricted only to the members of the study team who stored the data in a secure database to respect the confidentiality. The results of the study have been used for scientific purposes only.
Funding was received from the African Union in the form of scholarship. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
