Abstract
Aim:
Extensive research is carried out throughout the world in healthy persons with obesity phenotype in concern with prevalence, metabolic profiling, etc. To the best of the authors’ knowledge, not many studies have investigated the status of adiponectin, specific inflammatory changes, oxidative damage in healthy adolescents and young adults with obesity. Present study was undertaken in adolescents and young adults of urban population in a district of North Karnataka, India, in a view to understand relationship between hormone adiponectin, oxidative stress markers like C3, C4, high sensitivity C-reactive protein (hs-CRP) in non-hypertensive, non-diabetic, euthyroid individuals with and without obesity.
Methods:
Participant selection was done using cluster sampling technique. Participating adolescents and young adults, each with and without obesity were included in the study. Screening of participants for diabetes, hypertension, and thyroid disorders was done, their serum level of adiponectin, hs-CRP, C3, C4, ceruloplasmin (Cp), thiobarbituric acid reactive substances (TBARS), and total antioxidant capacity (TAC) were estimated using standardized methods in National Accreditation Board for Testing and Calibration Laboratories (NABL) laboratory.
Results:
Adiponectin (young adults lower than adolescents, P = 0.01) levels were low, while hs-CRP and Cp (young adults higher than adolescents, P = 0.01) levels were high with increasing age in non-obese. While in persons having obesity, aging adiponectin levels were low while hs-CRP, C3, Cp levels were high significantly. Females without obesity had significantly higher values of C3 than males. Adiponectin showed higher levels in females than males, however, statistical significance could not be achieved (P = 0.308). While females with obesity, exhibited statistically lower levels of adiponectin, and higher levels of C3 and C4.
Conclusions:
Being non-diabetic and non-hypertensive yet obese, tagged by one time of assay, does not suffice to be categorized as healthy. Healthy young adults with obesity are exhibiting lower levels of adiponectin and higher levels of inflammatory and oxidative stress markers compared to adolescents with obesity. This implies, the so categorized “healthy obese” participants are in a phase of transition towards an unhealthy state.
Keywords
Obesity, adiponectin, healthy non-obese, inflammatory status in obesityIntroduction
For centuries, the world witnessed scarcity of food and people had low body mass index (BMI), but with industrial revolution, especially in developed countries, deficiency of food became a myth and people started gaining weight. In around the 19th century, overweight individuals outnumbered the underweight, but the scenario in developing countries was still the same, malnutrition was prevailing. By 2008, World Health Organization (WHO) estimated that more than 1.4 billion adults were overweight. These figures compelled researchers to find the causes and effects of obesity, the outcome of their extensive research was that, obesity was found to be multifactorial in origin, with delicate imbalance between genetic and environmental factors.
Obesity has become pandemic owing to an obesogenic environment. While research was mainly focused on understanding obesity and its path physiology, some new findings were observed that, not all individuals with obesity seemed to display metabolic abnormalities. Such individuals in the due course of time were named as healthy obese or metabolically healthy obese (MHO). These healthy individuals with obesity are characterized by high BMI or body fat with normal blood pressure (BP), lipid profile, and sugar levels [1]. Most of the studies have defined healthy individuals with obesity based on the above said parameters. However, far more studies have included proportion of visceral fat, less liver fat, 0 or less than 2 metabolic syndrome criteria based on National Cholesterol Education Program, Adult Treatment Panel III, and inflammatory profile. However, the fact is that, till date, no uniform definition has been framed. As per the Indian statistics, 13.3% of south Indians are MHO [2]. The proposed hypotheses for favorable health in obese yet healthy individuals, may be genetic, less visceral adipose tissue (VAT), ectopic fat deposition, healthy lifestyle, etc., however, detailed research in these phenotypes is needed to endorse these hypotheses.
Adiponectin is well characterized and studied with relevance to metabolic disorders associated with obesity. Many studies have shown high levels of adiponectin in healthy adults with obesity when compared to metabolically unhealthy persons with obesity.
C-reactive protein (CRP) is an acute phase protein. However, the lower limit of detection of CRP assays is about 2 mg/L. Hence, improvements were required, to detect lower levels, lower than 2 mg/L. Recent improvements in assay development have resulted in a new generation of high sensitive assay that can detect CRP at levels 100 folds lower than earlier.
Complement system constitutes many proinflammatory proteins. Among those, C3 is very important. It is critical for activation of the complement system. While C4 is the main protein of the classical pathway. C3 and C4 levels are considered as acute phase response protein assays.
Ceruloplasmin (Cp) is a member of inflammation-sensitive plasma proteins (ISPs) family that is used in clinical practice to measure the degree of inflammation. Its secretion and expression are high in obesity, and Cp found in adipose tissue is a major contributor to the circulating Cp level.
This study was done to look for the metabolic status in healthy individuals with and without obesity of different age groups and gender, which will help us in understanding the biochemical changes. We have investigated the levels of hormone adiponectin, the real adipocyte hormone, well characterized and studied with relevance to metabolic disorders associated with obesity. Secondly, we studied inflammatory markers as inflammation is the local protective response to tissue injury [3] along with systemic reactions known as acute phase response, this response is due to elevated levels of CRP, complement factors, Cp (acute phase proteins) [4], etc. CRP activates and modulates complement system. It binds to the first component of the complement cascade, and initiates the classical pathway, thereby increases local inflammation. It is also known that CRP inhibits the alternate and lectin pathways of complement through the recruitment of factor H. Thus, CRP plays a dual pro and anti-inflammatory role in its regulation of the complement system. Keeping in view obesity associated inflammation, caused by activated innate immunity responders, we consider innate immunity effectors as markers of inflammations like high sensitivity C-reactive protein (hs-CRP), C3, C4, and Cp. Thirdly, we studied oxidative stress markers, as inflammation and oxidative stress go hand in hand. It’s a vicious cycle, inflammation causes oxidative stress and vice versa. Adipose tissue is not an inert organ, and especially hyperplastic adipose tissue is active in releasing cytokines, which leads to consequences of the oxidative stress and damage to the lipid, proteins, and nucleic acids, leading to lipid, protein, and DNA peroxidation [5]. Common indicators of oxidative stress, particularly lipid peroxidation markers thiobarbituric acid reactive substances (TBARS) and total antioxidant capacity (TAC), even though nonspecific, are commonly used as indicators of lipid peroxidation and oxidative stress [6]. Previous studies have quoted that high BMI exerts increased oxidative stress.
Hence, we wanted to study levels of adiponectin and inflammatory markers like hs-CRP, C3, C4, and Cp, oxidative stress markers like TBARS and TAC in healthy adolescents (14–18) and young adults (19–25) with and without obesity of both the genders. The reason to choose this population was that, these individuals are firstly at increased risk of gaining weight due to physiological processes and at the same time have reduced physical activity as burdened by academics. Secondly, inflammatory process is still in the primitive state, as inflammation increases with aging. All these points were convincing for us to take up this study as to what happens as we age that is transition phase from adolescence to young adulthood in healthy males and females with and without obesity.
What is known
Studies in western population [7–11] concluded that healthy individuals with obesity are exhibiting favorable inflammatory status, indicated by high adiponectin, and low inflammatory markers as compared to metabolically unhealthy obese (MUO) individuals.
Gap in the knowledge
Limited data is available in concern with biochemical alterations occurring with age during transition from adolescents to young adults in males and females, at cellular level in relation to inflammatory and oxidative stress status in Asian Indian population.
Methodology
This cross-sectional study was conducted in 16 schools and 23 pre-university colleges, belonging to 4 different quadrants of Belagavi city of north Karnataka in south India, Asia. Adolescents aged 14–18 and young adults aged 19–25 were selected as defined by WHO [12].
A pilot study was conducted on 5 healthy young adults and 5 adolescents with obesity, considering adiponectin as a main parameter and ensuring 5% error and 95% power of the test.
Details of sample size calculation:
n: sample size
Z: the number of standard deviations (SDs) an observation is from the mean for a set of data
S: SD
D: the expected difference between the means, divided by the expected SD
α: probability of making a Type I error, measure of the strength of the evidence that must be present in your sample before you will reject the null hypothesis and conclude that the effect is statistically significant
β: probability of making a Type II error (accepting the null hypothesis when the null hypothesis is false)
Adiponectin was estimated, and following results were derived:
SD of adiponectin in young adults = 2.0547 = S1
SD of adiponectin in adolescents = 2.987 = S2
Mean difference between young adults and adolescents = 1.1019
The following observations were put up in the formula:
Accordingly, a final sample size of 280 was calculated, of which 140 adolescents and 140 young adults were enrolled. Sampling technique: cluster sampling technique was used, according to Belagavi district report, there are 46 private schools and 31 pre-university colleges in urban Belagavi city. Belagavi area was divided into 4 quadrants and from each quadrant schools and pre-university colleges were selected proportionately. Of these, only 16 schools and 23 pre-university college deans gave permission for data collection.
Study protocol: once the ethical committee (JNMC Institutional Ethics Committee on Human Subjects Research) clearance was obtained (Ref No: KLEU/D 6596-6599; date: June 29, 2010) then, permission was obtained from the selected heads of the institution prior to examining the students of schools and pre-university colleges.
Adolescents: school health records were accessed to look for height, weight, then BMI was calculated, and students with and without obesity were identified.
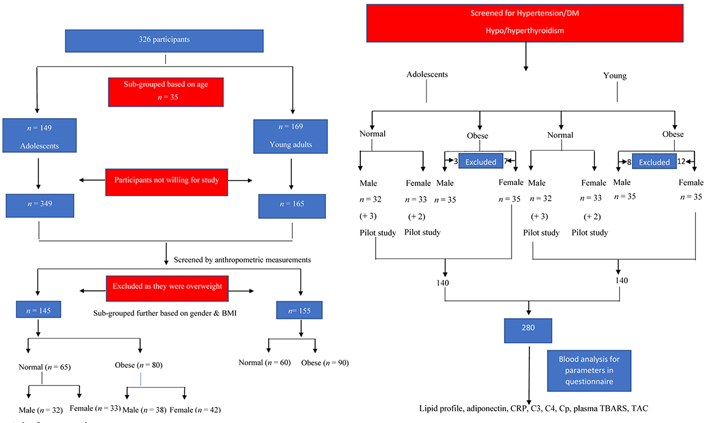
Young adults: while in colleges, most of the participants were aware about their BMI, hence individuals approximately in the range of normal and obese BMI were identified (n = 326) and further screened (details mentioned in Figure 1).
Identified participants were explained about the study in detail and requested to fill in the written consent form if aged more than 18 and assent from the participants with consent of the parent or the guardian if aged less than 18. A total of 300 individuals were enrolled, of which 145 were adolescents and 155 were young adults. Details of the enrollment, inclusion, and exclusion of the participant are given as in Figure 1.
Materials and methods
Anthropometric measurements
Height, weight, waist circumference, hip circumference, and waist-hip ratio (WHR) were obtained using standardized techniques by two trained study personnel. Interobserver and intraobserver coefficients of variation were less than 5%. Anthropometric measurements are discussed briefly here, as details are mentioned in an earlier article [14].
Height (cm)—a non-elastic tape was used to measure height (nearest to 1 cm).
Weight (kg)—portable balance (Krups) was used, it was kept on a firm horizontal surface and readings were noted.
BMI (kg/m2)—Quetelet’s index was used for calculation:
BMI (kg/m2) = weight (kg)/height (m2) [15]
Waist circumference (cm): non-elastic measuring tape was used and the smallest circumference of the natural waist at minimal respiration was measured [16].
Hip circumference (cm): non-elastic measuring tape was used and the widest part of the buttocks area was measured to the nearest 0.1 cm [16].
Metabolic screening: including BP measurement, estimation of thyroid-stimulating hormone (TSH) levels and fasting blood sugar levels.
A total of 145 adolescents and 155 young adults who fall into the criteria of being obese and with normal BMI were screened for BP, fasting blood sugar and TSH.
BP measurement (mmHg)
BP was measured for the normal and obese participants, by standardized mercury sphygmomanometer (Diamond BPMR120 Deluxe Conventional Mercurial Type BP Instrument; Diamond Deluxe apparatus, Pune, India). In case of young adults if the BP was more than 130/85 [17], and in case of adolescents, BP equal to or more than 120/80 was considered hypertensive. To rule out hypertension in adolescents, a standard BP chart based on gender, age, and height percentile as designed by National Institutes of Health (NIH) was used [18].
Details of blood collection and analysis
Under aseptic precautions 7 mL of blood was collected, of this 7 mL, 3 mL was collected in ethylenediaminetetraacetic acid (EDTA) tubes for the estimation of whole blood TBARS, the rest 4 mL was allowed to clot for 1 h. Samples were centrifuged at 3,000 rpm for 10 min to separate serum and were stored at 4°C before analysis. All samples were analyzed within two days of blood collection. Firstly, blood glucose was estimated and then TSH was estimated and if found within normal limits, then the samples were further analyzed for adiponectin, hs-CRP, C3, C4, Cp, TBARS, and TAC.
(1). Estimation of blood glucose was done by glucose oxidase-peroxidase (GOD-POD) method.
(2). Estimation of TSH was done by immunoenzymometric technique.
(3). Estimation of adiponectin was done by enzyme-linked immunosorbent assay (ELISA) [19].
(4). Estimation of hs-CRP by particle enhanced turbidimetric immunoassay (PETIA) technique [20].
(5). Estimation of C3 by turbidimetric method [21].
(6). Estimation of C4 by turbidimetric method [21].
(7). Estimation of Cp by copper oxidase method [22].
(8). Estimation of TBARS/total malondialdehyde (MDA) by TBARS method [23].
(9). Estimation of serum total antioxidant activity by Koracevic et al. [24] method.
Statistical analysis
Data was entered into the Excel sheet. Data analysis was done by using statistical package for social sciences trial (SPSS software, version 16). All the numerical values were summarized as mean ± SD, with the P level of less than 0.05 as significant.
Results
Two hundred and eighty individuals aged 14–25 years meeting the eligibility criteria were enrolled in the study. Of these 280, 140 were adolescents and the rest 140 were young adults. Further, equal number of non-obese and obese were enrolled. Details of study participants are provided in Table 1.
Details of study participants
| Total participants (n = 280) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Adolescents (n = 140) | Young adults (n = 140) | ||||||
| According to BMI | Non-obese (n = 70) | Obese (n = 70) | Non-obese (n = 70) | Obese (n = 70) | ||||
| Genderwise | Female (n = 35) | Male (n = 35) | Female (n = 35) | Male (n = 35) | Female (n = 35) | Male (n = 35) | Female (n = 35) | Male (n = 35) |
Impact of age on adiponectin: adiponectin levels were low with increase in age, in participants of both categories, non-obese (P = 0.01) and obese (P = 0.01, Table 2).
Comparative result showing impact of age on various markers
| Variables | Adolescents (n = 140) | Young adults (n = 140) | P value | ||
|---|---|---|---|---|---|
| Non-obese (n = 70) | Obese (n = 70) | Non-obese (n = 70) | Obese (n = 70) | ||
| Adiponectin (μg/mL) | 8.7 ± 1.79 | 7.1 ± 1.79 | 5.9 ± 1.94 | 4.1 ± 1.43 | |
| hs-CRP (mg/L) | 1.9 ± 0.85 | 1.9 ± 0.83 | 2.1 ± 0.77 | 2.2 ± 0.98 | |
| C3 (mg/dL) | 150.7 ± 30.93 | 153.5 ± 32.23 | 156.9 ± 14.68 | 172.6 ± 17.8 | |
| C4 (mg/dL) | 30.7 ± 11.41 | 38.5 ± 15.92 | 33 ± 8.20 | 36.3 ± 7.28 | |
| Cp (IU/L) | 68.2 ± 20.05 | 72 ± 20.17 | 78.07 ± 20.12 | 96.3 ± 16.22 | |
| TBARS (nmol/mL) | 5.1 ± 0.77 | 5.1 ± 1.31 | 4.7 ± 1.31 | 6.7 ± 0.01 | |
| TAC (mmol/L) | 1.6 ± 0.56 | 1.7 ± 0.56 | 1.6 ± 0.34 | 0.7 ± 0.48 | |
Results presented as geometric mean ± SD. $ unpaired t test; * non-obese adolescents vs. non-obese young adults; # obese adolescents vs. obese young adults. Intergroup analysis: 2 way analysis of variance (ANOVA)
Impact of age on inflammatory markers: hs-CRP levels were high with increasing age in both categories, non-obese (P = 0.04) and obese (P = 0.01, Table 1). C3 and C4 levels did not vary with age in participants with obesity (C3: P = 0.51, C4: P = 0.681), while C3 levels were higher with increasing age, in participants with obesity (C3: P = 0.001, whereas C4 did not change: P = 0.38). Cp levels were higher with increasing age, in participants without obesity (P = 0.01) and participants with obesity (P = 0.01, Table 2).
Impact of age on oxidative stress markers: there was no significant difference in the levels of TBARS (P = 0.289) and TAC (P = 1.0), with increasing age in the non-obese. While in the obese, TBARS (P = 0.01) levels were higher and TAC (P = 0.01) levels were low (Table 2).
Impact of gender on adiponectin levels: there was no difference in adiponectin levels amongst non-obese females and males (P = 0.308), while obese females had lower levels than males (P = 0.01, Table 3).
Adiponectin in non-obese and obese females and males
| Variables | Females (n = 140) | Males (n = 140) | P value |
|---|---|---|---|
| Non-obese | 7.54 ± 2.14 | 7.14 ± 2.46 | 0.308 |
| Obese | 5.17 ± 2.35 | 6.10 ± 1.93 | 0.01 |
unpaired t test
Impact of gender on inflammatory markers: there was no significant difference in the levels of hs-CRP among females and males belonging to non-obese and obese categories [non-obese (P = 0.628), obese (P value = 0.913)]. Whereas there were significantly higher levels of C3 in females, than males in both non-obese and obese categories [non-obese (P value = 0.01), obese (P value = 0.01)]. No change was observed in the levels of C4 amongst non-obese females and males (P = 0.08), while it was higher in obese females than males (P = 0.01, Table 4). Levels of C3 and C4 were higher in females than in males, except for hs-CRP & Cp.
Inflammatory markers in females and males (non-obese and obese)
| Variables | Females (n = 140) | Males (n = 140) | P value |
|---|---|---|---|
| hs-CRP (mg/L) | |||
| Non-obese | 1.98 ± 0.78 | 2.05 ± 0.857 | 0.628 |
| Obese | 2.11 ± 0.89 | 2.09 ± 0.94 | 0.913 |
| C3 (mg/dL) | |||
| Non-obese | 159.39 ± 25.74 | 148.26 ± 21.6 | 0.00 |
| Obese | 171.16 ± 22.9 | 154.0 ± 29.21 | 0.00 |
| C4 (mg/dL) | |||
| Non-obese | 33.35 ± 9.97 | 30.39 ± 9.81 | 0.08 |
| Obese | 40.55 ± 13.51 | 34.54 ± 5.65 | 0.00 |
| Cp (IU/L) | |||
| Non-obese | 73.72 ± 19.76 | 76.37 ± 20.89 | 0.443 |
| Obese | 81.54 ± 23.09 | 82.94 ± 23.06 | 0.718 |
students unpaired t test
Oxidative stress markers
Impact of gender on oxidative stress markers: there was no difference in the levels of TBARS females and males of obese and non-obese categories [non-obese (P value = 0.971), obese (P value = 0.94)]. There was no difference in the levels of TAC in females and males who were not obese (P = 0.22) whereas females with obesity exhibited significantly lower levels than males (P = 0.01, Table 5).
Oxidative stress markers (TBARS and TAC) in non-obese and obese females and males
| Variables | Females | Males | P value |
|---|---|---|---|
| TBARS (nmol/mL) | |||
| Non-obese | 4.9 ± 15.6 | 4.9 ± 1.58 | 0.971 |
| Obese | 5.94 ± 1.53 | 5.9 ± 1.27 | 0.94 |
| TAC (mmol/L) | |||
| Non-obese | 1.63 ± 0.45 | 1.72 ± 0.4 | 0.229 |
| Obese | 1.03 ± 0.69 | 1.34 ± 0.711 | 0.01 |
students unpaired t test
Summary of results
Impact of age: with increasing age, adiponectin levels were low while inflammatory markers, and oxidative stress marker levels were high, mainly among participants with obesity, while no change was observed among participants without obesity, except for hs-CRP and Cp.
Impact of gender: among participants without obesity, C3 was statistically on the higher range in females than males, though adiponectin was on higher range, in females statistical significance could not be achieved. No change was seen in hs-CRP, C4, Cp, TBARS, and TAC.
Among individuals with obesity, adiponectin and TAC were in the lower range in females than males, while C3, and C4 were in the higher range in females, than in males. No changes were seen in hs-CRP and Cp.
Discussion
With increase in age, adiponectin levels were low in healthy individuals with and without obesity. Our results are in line with a study conducted by Vilarrasa et al. [25] in 2005, while most of the studies [26–28] were contrary to our findings as no changes in levels of adiponectin with age were documented [29]. With aging, percent fat mass increases in terms of VAT, hence decline in adiponectin levels is a physiological process. Also, age-related decline in testosterone and estrogen levels has been shown to inhibit adiponectin production [30].
In our study, there was a notable trend of high CRP values with increasing age (adolescents have lower values than young adults) in non-obese and obese categories. The same results have been documented previously [31, 32]. Inflammatory changes are a common phenomenon of aging.
Age had no impact on the levels of C3 and C4 in persons without obesity, as the levels in adolescents and young adults did not vary. However, in persons with obesity, C3 levels were higher in young adults when compared to adolescents. With aging adiposity increases, consequence of which is increased expression of complement factors, especially when aging is associated with obesity. According to our knowledge, there were no studies documented that could support or contradict our results in relation to C3 and C4.
Cp levels were in higher range in healthy young adults than adolescents, among obese participants. Previous studies have shown that Cp increases with age [33]. However, these studies have been done in adults without adolescents into consideration.
Among participants without obesity, as age increased, TBARS levels were higher and TAC levels were lower. Age-related oxidative stress has been documented previously [34, 35] while a study contradicted our results where higher levels of TAC were observed with aging [36].
Previous studies [37, 38] have convincingly shown that females have significantly higher levels of adiponectin than males. We, too, have documented similar trend in healthy males and females without obesity. However, statistical significance could not be achieved. In males, testosterone selectively reduces adiponectin formation by inhibiting its secretion from adipocytes [39], thereby extending sexual dimorphism to adipose tissue. In our study, females exhibited significantly lower adiponectin compared to males. No studies support our finding. However, higher level of adiponectin in western female population was documented [40–42]. Notably, women in our study had a higher WHR which could be more associated with obesity-related complications leading to low levels of anti-inflammatory, anti-diabetic hormone adiponectin.
In our study, there was no gender difference in hs-CRP levels. Previous studies have shown that women have higher hs-CRP levels than men [43–45]. The quantity and distribution of body fat appear to influence the hs-CRP levels to a greater extent in women than men [46].
In our study, gender had no impact on Cp levels. Previous studies have shown that Cp concentrations are higher in females [47].
From the above discussion, commendable points are that there is a statistically significant link between adiponectin, inflammation, and oxidative stress. In healthy adolescents with obesity, adiponectin levels were low, while inflammatory markers C3, C4, and Cp levels were high when compared to individuals without obesity. The inflammatory marker levels were high and adiponectin and TAC levels were low in young adults.
Reduced levels of anti-inflammatory adipokines, adiponectin, and increase in inflammatory markers, the so classified “healthy obese” are at risk of inflammation or progressing towards metabolically unhealthy obesity, as they progress from adolescent age group to young adulthood [48]. This phase of transition is crucial, hence dietary & lifestyle modifications at this junction may help the individuals to stay healthy.
Furthermore, with the discussion, it looks like participants with obesity may be having higher visceral adiposity and visceral adipocytes tend to be smaller than subcutaneous adipocytes but have greater potential to secrete cytokines [49]. Hence, we had to find the correlation between adipocytokines and obesity.
These points clearly show that transition from adolescence to adulthood leads to increase in visceral adiposity, causing inflammatory and oxidative stress changes in the adipose tissue, particularly in females even though there was higher physical activity status. This transition from adolescence to adulthood is a crucial transition phase in life of human beings. Lower levels of adiponectin, TAC, and higher levels of C3, predicted that participants with obesity are at a state of inflammatory and oxidative stress changes, though they could be at a subclinical state. Hence, participants with obesity, though found to be non-diabetic and non-hypertensive, had to be cautiously screened and treated. Screening of adolescents with obesity, with WHR would predict visceral fat better than just BMI. Decrease in adiponectin and TAC and increase in inflammation emphasizes that adiponectin may have a protective function which prevents the establishment of co-morbidities associated with obesity at an early age of adolescence and young adulthood. Intervention has to be planned considering anti-oxidants in the treatment regime, as treatment with antioxidants could also restore the regulation of adipokines like adiponectin [50]. Improving the expression of adiponectin with its receptor can prove to be useful in the treatment of obesity and obesity related diseases [51].
Conclusion
Healthy young adults with obesity are exhibiting lower levels of adiponectin, higher levels of inflammatory, and oxidative stress markers, compared to adolescents with obesity. The so categorized “healthy obese” participants could be in a phase of transition towards an unhealthy state, accentuated by aging in females when compared to males. Taken together, there is an altered secretory profile of adipocytokines and increase in the involvement of inflammatory and oxidative stress reactions. Categorizing obese persons as “healthy” just because they exhibited normal fasting blood sugar, TSH, and BP by one time of assay does not suffice. Our research aptly pointed that among individuals who were healthy but obese, low grade chronic inflammation sets in with aging and it is more in females compared to males and this reflects that the participants may end up with comorbidities, hence necessary action needs to be taken up during this transition phase of adolescence to young adulthood by health care workers immaterial of BMI status.
Limitations
The followings are some of the limitations of this study:
(1). As it’s a cross-sectional study, cause and effect conclusions could not be done.
(2). We estimated total adiponectin than high molecular weight (HMW) adiponectin. While HMW isoform of adiponectin is very important in the metabolism and inflammatory conditions.
(3). Adiponectin was the only hormone measured, hence we analyzed only the association of this hormone with obesity, while other adipokines, which modify the pathogenesis of obesity, were not estimated. All the hormones influence each other’s expression and production.
Scope for further studies
Longitudinal studies with longer follow-up of the participants are needed in order to assess the relation between the subclinical phenotype, and potent risk factors identified in order to accurately determine the metabolic status.
Abbreviations
| BMI: | body mass index |
| BP: | blood pressure |
| Cp: | ceruloplasmin |
| CRP: | C-reactive protein |
| hs-CRP: | high sensitivity C-reactive protein |
| SD: | standard deviation |
| TAC: | total antioxidant capacity |
| TBARS: | thiobarbituric acid reactive substances |
| TSH: | thyroid-stimulating hormone |
| WHR: | waist-hip ratio |
Declarations
Acknowledgments
The authors thank all participants in the study and the institution without whose support the work would not have been possible. We thank the institution for supporting us with the infrastructure and equipments for the conduct of the study. We also thank the technical staff for providing help with the analysis.
Author contributions
SSS: Conceptualization, Data curation, Methodology, Investigation, Writing—review & editing. VAK: Supervision, Writing—review & editing. RDC: Writing—original draft, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
JNMC Institutional Ethics Committee on Human Subjects Research ethical committee clearance was obtained (Ref No: KLEU/D 6596-6599). This study complies with the Declaration of Helsinki.
Consent to participate
Written consent form if aged more than 18 and assent from the participants with consent of the parent or the guardian if aged less than 18.
Consent to publication
The informed consent to publication was obtained from the parent of the patient.
Availability of data and materials
All data and materials are maintained.
Funding
Not applicable.
Copyright
© The Author(s) 2023.