Affiliation:
1Centre of Excellence in Unani Medicine (Pharmacognosy & Pharmacology), Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India
2Department of Pharmaceutics, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-3687-5506
Affiliation:
1Centre of Excellence in Unani Medicine (Pharmacognosy & Pharmacology), Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India
ORCID: https://orcid.org/0000-0001-8822-1104
Affiliation:
3Shaheed Bhagat Singh College of Pharmacy, Patti, Punjab 143416, India
†These authors contributed equally to this work.
Affiliation:
3Shaheed Bhagat Singh College of Pharmacy, Patti, Punjab 143416, India
ORCID: https://orcid.org/0000-0001-7941-8845
Affiliation:
1Centre of Excellence in Unani Medicine (Pharmacognosy & Pharmacology), Bioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India
Email: sahmadjh@yahoo.co.in
ORCID: https://orcid.org/0000-0003-1573-152X
Explor Med. 2023;4:176–188 DOI: https://doi.org/10.37349/emed.2023.00132
Received: September 12, 2022 Accepted: December 23, 2022 Published: April 25, 2023
Academic Editor: Haijun Yu, Chinese Academy of Sciences, China
Aim: The main objective of the study was to formulate, evaluate and perform an optimization study of chaulmoogra oil-loaded solid lipid nanoparticles (SLNs) based gel.
Methods: The study involves isolation, identification, and quantification of hydnocarpic acid (HA), using high-performance thin-layer chromatography (HPTLC) and characterization using ultraviolet (UV), nuclear magnetic resonance (NMR), and mass spectroscopy (MS), and differential scanning calorimetry (DSC). Different concentration of assorted solid lipids and surfactants was used for the preparation of SLN gel with the improved transdermal application. Size distribution, entrapping efficiency, transmission electron microscopy (TEM), and percent yield were tested for the prepared SLN and the characterization of SLN gel was evaluated on the basis of in vitro diffusion study, stability studies, homogeneity, and skin irritancy test.
Results: The amount of HA quantified in the oil sample was found to be 54.84% w/w. The percent yield and entrapment efficiency (EE) of HA SLNs were 96.176 ± 1.338% and 90.2 ± 0.5% respectively. The in vitro percent cumulative drug release was 80.89% for the developed SLN, the homogeneity test showed no grittiness, and the prepared gel was found to be effective as it shows no signs of erythema post-treatment of 10 days. The in vitro dissolution studies showed better results for SLN gel when compared to SLN suspension.
Conclusions: The nano-gel could be a better option for the topical delivery of herbal drugs with improved bioavailability providing several benefits over conventional formulation.
Chaulmoogra oil (CMO) is a fixed oil derived from the seeds of the Hydnocarpus whightana, H. anthelmintica, and Taractogenose kurzii belonging to the family Flacourtiaceae [1]. The oil contains naturally occurring cyclopentenyl fatty acid as hydnocarpic acid (HA), the bioactive principle acting as an anti-leprotic agent [2]. Several lines of scientific evidence have demonstrated the remarkable potency of CMO, harvested in countries like India, Sri Lanka, or Africa, and is used intravenously or intramuscularly to treat leprosy [3].
The key problem of today’s age scientists in the case of dermatological illnesses is the development of adequate drug delivery technology to overcome low solubility, low bioavailability, and stability concerns. To overcome this problem, fixed oils are incorporated into a nanocarrier mediated gel for efficacious drug targeting [4, 5]. Solid lipid nanoparticles (SLNs) gels have gained a lot of attention from researchers since they have a lot of potential for the development of regulated and site-specific drug delivery systems [6, 7]. The solvent injection technique is a revolutionary approach to preparing SLN gel using lipid precipitation from dissolved lipid in solution and offers several advantages over previous production methods, including the use of a non-toxic organic solvent, simple handling, and a high production rate without the use of technologically advanced equipment [8, 9].
Only one gas-liquid chromatographic method has been reported so far to determine multiple fatty acids such as chaulmoogric acid, HA, and gorlic acid from the Hydnocarpus species [10]. However, no study including the quantification of HA from CMO entrapped in nano-formulation for leprosy patients has been reported. Thus, in the current study HA from CMO, is isolated, chromatographically separated, identified using a standard compound, and quantified via a simple, rapid, sensitive, and specific high-performance thin-layer chromatography (HPTLC) method, followed by characterization using various analytical techniques such as ultraviolet (UV), nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), and mass spectroscopy (MS). As CMO is used for the treatment of leprosy and has poor bioavailability, therefore, it was thought to develop SLNs of CMO as lipid-based formulation by using oil as an oily phase as well as the active constituent of the formulation. The purpose of the study was to formulate and optimize a CMO-loaded SLN gel for efficient drug delivery in leprosy treatment. In addition, for morphology confirmation of nanoparticles, particle size distribution by transmission electron microscopy (TEM) was performed along with in vitro diffusion study, stability studies, entrapment efficiency (EE), homogeneity, and skin irritancy tests were also performed to determine the therapeutic efficacy of the optimized formulation.
All the chemicals and reagents were used of analytical grade. Seven lipids viz. Dynasan 118 (Gattefosse, Germany), Dynasan 114 (Gattefosse, Germany), cetyl alcohol (Merck, India), stearic acid (Merck, India), glyceryl mono stearate (Merck, India), compritol ATO 888 (ATO, Gattefosse, Germany) and gelucire 50/13 (Gattefosse, Germany).
One gm of sodium hydroxide pellets was dissolved in 9 mL of distilled water followed by the addition of 10 mL CMO. Heated on the mantle for 1 h to separate the precipitate, 10% hydrochloric acid (HCl) was added followed by heating. Separation was achieved with chloroform, thrice, and repeated chloroform layer was evaporated to dryness and the volume was adjusted to 10 mL with chloroform.
One mg per mL of stock sample was prepared and absorbance was measured between 200–400 nm range to get the λmax using UV-visible spectrophotometry [11].
The NMR of the isolated constituent was taken by dissolving it in dimethyl sulfoxide (DMSO). 1H-NMR and 13C-NMR showed different peaks for different types of protons and 13C present in the structure of HA [12].
DSC thermogram was obtained in the temperature range of 40°–400°C at 15°C/min rate on the Pyris 6 DSC instrument and the scan was obtained [13].
One mg per mL of stock sample was prepared and 5 μL of this solution was injected in ultra-performance liquid chromatography (UPLC)-MS/MS and the mass spectra were recorded in positive and negative modes [14].
The CMO (1 mL) was dissolved in 4 mL of petroleum ether followed by the addition of 5 mL distilled water, and then two layers were formed. Non-polar oil (1 mL) was re-dissolved into 4 mL of petroleum ether, which was used for the sample application. Hexane:ethyl acetate (8:1, v/v) was used as a mobile phase for separation. Densitometric analysis of HA was carried out at 645 nm [13].
The reference methodology was followed with a few modifications for HPTLC (CAMAG, Muttenz, Switzerland) fitted with winCATS 1.2.3 software for fingerprinting and quantitative analysis [14]. A stock solution (1.0 mg/mL) of the sample was prepared in UPLC-MS grade methanol and spotted in duplicate on a thin layer chromatography (TLC) plate in different concentrations such as 0.2, 0.4, 0.5, 1.0, and 2.0 μL, respectively. The mobile phase consisted of hexane:ethyl acetate (8:1, v/v). After development, the TLC plates were air dried and sprayed with anisaldehyde sulphuric acid reagent and then heated for visualization with the help of an oven at 110°C. Densitometric scanning was performed on CAMAG® TLC Scanner 3 in the absorbance mode at 645 nm. The source of radiation utilized was deuterium and tungsten lamp.
The solubility of CMO was checked in various solvents such as chloroform, methanol, ethanol, dichloromethane (DCM), acetone, petroleum ether, and isopropanol. The 2 mL of oil was dissolved in 2 mL of organic solvent and the solution was sonicated for 10 min and observation was made on the visual basis [15]. The best solvent was chosen for the preparation of SLNs.
Seven lipids (Dynasan 118, Dynasan 114, cetyl alcohol, stearic acid, glyceryl monostearate, compritol ATO 888, and gelucire 50/13) were chosen and the best lipid was selected on the basis of solubility studies in the solvents. The lipid (250 mg) was added in different solvents in increasing quantities starting from 10 mg and solubility was checked visually. The solution was sonicated for 10 min and observation was made on a visual basis [15].
A solvent injection technique was adopted to prepare SLNs as per the reported method with some modifications [5, 16]. CMO (2 mL) and selected lipid (200 mg) were mixed and dissolved in DCM (2 mL) followed by sonication at room temperature till dissolution occurs (organic phase). Further, 0.2% Tween 80 (Sigma-Aldrich, Bangalore, India, 9005-65-6) in distilled water was used as a surfactant with 0.2% polyvinyl alcohol (PVA) dissolved in distilled H2O as a stabilizer. Both solutions were mixed in equal quantities and sonicated at room temperature till the aqueous phase is achieved. The organic phase was rapidly injected into the aqueous phase with continuous stirring on a magnetic stirrer at 2,000 rpm for 5 min. It was further stirred for 1 h to remove the traces of organic solvent.
The EE of HA SLNs was calculated by quantification of free drug content in supernatant obtained after centrifugation of suspension at a high speed of 16,000 rpm at 30°C for 30 min. The following formula was used to calculate % EE:
Where Ws is the amount of HA loaded in the SLNs and Wtotal is the total HA amount added in SLNs dispersion [5].
The droplet size distribution of the developed formulation was determined by photon correlation spectroscopy that analyzes the fluctuations in light scattering due to the brownian motion of the particles, using a Zetasizer (1000 HS, Malvern instruments, UK). The polydispersity index (PDI) denotes the ratio of standard deviation to the mean particle size and indicates the uniformity of particle size within the formulation. The lower the polydispersity value (low particle size distribution), the higher the uniformity in the size of the particles. Droplet size distribution and polydispersity studies were performed as per the reference method [8].
The morphology of the SLNs was studied using TEM [Morgagni 268D solid electrolyte interface (SEI), USA] operating at 200 KV and of a 0.18 nm capable of point-to-point resolution. A combination of bright field imaging at increasing magnification and diffraction mode was used to reveal the form and size of the nanoformulation. The sample preparation was done as per [17].
The yield was calculated gravimetrically after drying 10 mL of suspension at 30°C in an oven until a constant weight was obtained. It is expressed as the percentage ratio of the lipid amount in the SLN dispersion to the theoretical amount.
After one hour of SLN preparation, 1% w/v Carbopol 934 as gelling agent (Sigma Aldrich, Italy) was mixed into the formulation, and pH was measured by pH meter as per the reported method [18]. The prepared SLN gel was then subjected to characterization using different parameters. Different concentrations of the gelling agent were taken and the consistency and viscosity of the SLN gel were observed visually.
The SLN gel formulation (1 g) was taken and dissolved in methanol, filtered, and 0.1 mL of the filtrate was pipetted out and diluted up to 10 mL with methanol. The content of active constituents was estimated spectrophotometrically by using a standard curve plotted at 203 nm (λmax of HA) [19].
The pH of the gel was measured using a digital pH meter by dipping the glass electrode completely into the gel system to cover the electrode. The measurement was carried out in triplicate and the average of the three readings was recorded [19].
The physical appearance in terms of color and odor of the prepared SLN gel was evaluated by visual observation.
A small quantity of prepared gel was pressed between the thumb and the index finger, and the homogeneity of the gel was noticed (whether homogeneous/consistent or not) and also observed for any coarse particles that appeared or detached on the finger.
A skin irritancy study was performed using 8 male Wistar rats, weighing 200–250 g. The developed SLN gel was applied to the left ear of the rat and the right was considered as a control. The development of erythematic was monitored for 10 days using the method as reported by [20–22], according to Table 1.
Erythema and edema scores were used to determine the primary irritation index
| Observation | Values |
|---|---|
| Erythema | |
| No erythema | 0 |
| Very slight erythema (barely perceptible) | 1 |
| Well-defined erythema | 2 |
| Moderate to severe erythema | 3 |
| Severe erythema (beef redness) | 4 |
| Oedema | |
| No oedema | 0 |
| Very slight oedema (barely perceptible) | 1 |
| Slight oedema (edges of area well-defined by definite raising) | 2 |
| Moderate oedema (raised approximately 1 mm) | 3 |
| Severe oedema (extending beyond the area of exposure) | 4 |
In vitro release study of CMO and HA SLNs was carried out by dialysis membrane method. In vitro diffusion study of CMO SLN gel and CMO gel was carried out in Franz diffusion cell as per the reported method [23].
The formulation SLNs was injected into 10 mL ampules and sealed for storage at 4°C and room temperature (25°C) for three months. The color, odor, homogeneity, pH, and net content of the topical herbal SLN gel formulation were determined at different time intervals of one, two, and three months.
Isolation of HA was done using the saponification method via sodium hydroxide followed by extraction with chloroform. The applied method resulted in a good separation of the targeted compound.
UV spectrum of HA was taken at 203 nm (Figure S1). The obtained UV spectra of HA were similar to CMO and the results obtained were equivalent to other reported studies showing λmax in the range of 200–400 nm [24].
1H-NMR and 13C-NMR spectra of HA are given in Figures S2 and S3.
DSC thermogram was obtained in the temperature range of 40°–400°C at 15°C/min rate on Pyris 6 DSC instrument and the scan was obtained as shown in Figure S4.
The results incurred a prominent peak at m/z 253.1523 which indicates the presence of HA (molecular weight of HA 252.39) as shown in Figure S5.
Initially, hexane and ethyl acetate in the varying ratio were tried and the (8:1, v/v) ratio gave good resolution for the separation of HA and its degradation products. Two μL of samples were applied in triplicate on a TLC plate. Densitometric analysis was carried out at 645 nm. The chromatogram showed the separation of a prominent green-colored band at retention factor (Rf) 0.45, for the targeted compound in all samples as shown in Figure 1. The present investigation has reported a new TLC method development and validation for the estimation of HA.
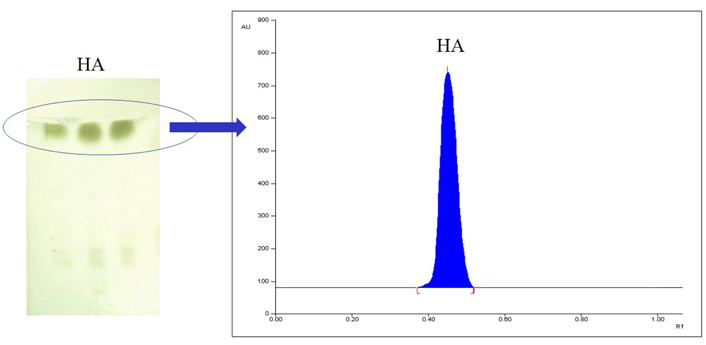
The above figure represents (A) TLC plate with HA, and (B) chromatogram of isolated HA
In the study proposed, a new HPTLC method was developed for the quantification of HA on the basis of chromatographic separation and retention. The linear regression analysis data for the calibration plots (n = 3) in the concentration range of 200–2,000 ng per spot showed a good linear relationship (r2 = 0.9930 ± 0.0005) with respect to the peak area. The mean values of slope and intercept were 8.281 ± 0.6 and 986.533 ± 1.84, respectively. The regression equation thus obtained from calibration plots, Y = 986.533 + 8.281x (where Y = area obtained, x = concentration in ng/spot), was used for quantitative estimation of HA in different samples, all details are shown in Table 2.
Validation parameters of developed HPTLC method of HA (n = 6)
| Linearity parameters | Observation |
|---|---|
| Marker quantified | HA |
| Solvent system | Hexane:ethyl acetate (8:1, v/v) |
| Wavelength | 645 nm |
| Rf obtained | 0.45 |
| Linearity range (ng/spot) | 200–2,000 |
| Regression equation | Y = 986.533 + 8.281x |
| Correlation coefficient ± SD | 0.9930 ± 0.0005 |
| Slope ± SD | 8.281 ± 0.6 |
| Intercept ± SD | 986.533 ± 1.84 |
| Accuracy (recovery) | 97–103% |
| Limit of detection (ng) | 10 |
| Limit of quantitation (ng) | 25 |
| Precision (%RSD) | 0.64–1.82 |
SD: standard deviation; RSD: relative standard deviation
Different solvents were selected and observed visually for solubility of CMO in which petroleum ether and acetone showed solubility after sonication for 1 min, chloroform showed slight solubility after sonication for the 30 s, ethanol, and methanol showed solubility after sonication for 3 s, and in DCM and isopropanol clear solution was observed within 1 s and 2 s, respectively, therefore DCM was found to be highly soluble among all solvents. Seven lipids were chosen for the formulation of SLN. The solubility of lipids was observed visually in selected solvents as shown in Table 3. The lipids were added in increasing quantities starting from 10 mg to each solvent and solubility was checked visually. The results showed that gelucire 50/30 showed maximum solubility in all solvents at all given concentrations, and thus was selected as the “lipid of choice” for the preparation of SLN.
Particles sizes of SLNs
| Formulation | Tween 80 (%) | PVA (%) | Ratio of Tween 80:PVA | Volume of aqueous phase (mL) | Particle size | Average particle size | PDI |
|---|---|---|---|---|---|---|---|
| CN 1 | 0.1 | - | - | 10 | 139.2 | 333.53 | 1.0 |
| CN 2 | 0.1 | - | - | 25 | 618.4 | 0.772 | |
| CN 3 | 0.1 | - | - | 50 | 243 | 0.566 | |
| CN 4 | 0.5 | - | - | 10 | 942.4 | 554.2 | 1.0 |
| CN 5 | 0.5 | - | - | 25 | 433.4 | 0.721 | |
| CN 6 | 0.5 | - | - | 50 | 287 | 0.981 | |
| CN 7 | 1 | - | - | 10 | 476 | 409.6 | 0.530 |
| CN 8 | 1 | - | - | 25 | 390.5 | 0.546 | |
| CN 9 | 1 | - | - | 50 | 362.5 | 0.764 | |
| CN 10 | 0.1 | 0.1 | 1:1 | 10 | 368.9 | 362.43 | 0.676 |
| CN 11 | 0.1 | 0.1 | 1:1 | 25 | 467.1 | 0.671 | |
| CN 12 | 0.1 | 0.1 | 1:1 | 50 | 251.3 | 0.023 | |
| CN 13 | 0.5 | 0.5 | 1:1 | 10 | 697.6 | 405.43 | 0.912 |
| CN 14 | 0.5 | 0.5 | 1:1 | 25 | 207.1 | 0.633 | |
| CN 15 | 0.5 | 0.5 | 1:1 | 50 | 311.6 | 0.613 | |
| CN 16 | 1 | 1 | 1:1 | 10 | 462.5 | 369.83 | 0.5 |
| CN 17 | 1 | 1 | 1:1 | 25 | 474 | 0.6 | |
| CN 18 | 1 | 1 | 1:1 | 50 | 173 | 0.8 | |
| CN 19 | 0.1 | 0.1 | 10:1 | 10 | 690 | 581.13 | 0.9 |
| CN 20 | 0.1 | 0.1 | 10:1 | 25 | 647.5 | 0.6 | |
| CN 21 | 0.1 | 0.1 | 10:1 | 50 | 405.9 | 0.5 | |
| CN 22 | 0.5 | 0.5 | 10:1 | 10 | 591.7 | 415.7 | 0.65 |
| CN 23 | 0.5 | 0.5 | 10:1 | 25 | 528.1 | 0.392 | |
| CN 24 | 0.5 | 0.5 | 10:1 | 50 | 127.3 | 0.492 | |
| CN 25 | 1 | 1 | 10:1 | 10 | 494.7 | 748.36 | 0.718 |
| CN 26 | 1 | 1 | 10:1 | 25 | 725.4 | 0.801 | |
| CN 27 | 1 | 1 | 10:1 | 50 | 1,025 | 1.0 |
CN: cetyl alcohol nanoformulation; -: no data
SLN was prepared using a solvent injection technique with slight modifications using gelucire as lipid carrier, Tween 80 as a surfactant, and PVA as a stabilizer.
The concentration of HA was determined spectrophotometrically at 203 nm, and the blank sample showed no absorbance at this wavelength. The average percent EE of HA SLNs was 90.2 ± 0.5%.
The percentage ratio of Tween 80:PVA, and volume in mL of the best selected formulations were found to be CN 3 (0, 10), CN 8 (0, 25), CN 10 (1:1, 10), CN 12 (1:1, 50), CN 14 (1:1, 25), CN 17 (1:1, 25), CN 23 (1:1, 25) and CN 24 (10:1, 25). In Table 3, the average of three readings of particle size at each concentration (0.1, 0.5, and 1% w/v) was incorporated.
The morphology of the prepared SLN was studied using TEM (Morgagni 268D SEI, USA) operating at 200 KV and of a 0.18 nm capable of point-to-point resolution. A combination of bright field imaging at increasing magnification and diffraction modes was used to reveal the form and size of the nano-emulsion as shown in Figure 2. The particles were found to be round, smooth, and uniform in shape.
The yield refers to the amount of SLNs recovered from the preparation method and it is calculated as the percentage ratio of lipid amount in SLN suspension to the theoretical amount of lipid. The yield was found to be 96.176 ± 1.338.
The HA was quantified by winCATS software using a regression equation obtained from the calibration curve and the mean of triplicate samples was calculated with respect to height and area separately. No interference was observed in samples with immediate constituents, and the resolution between the peaks was also good. The compound at Rf 0.45 at 645 nm (HA) showed the highest concentration of 54.839% w/w using the developed method. The Rf value, peak area, and corresponding concentrations of HA in all samples applied on the TLC plate were recorded. Furthermore, the area for sample oil, formulation C, and formulation D was obtained to be 7,676.44 ± 44.80, 4,591.68 ± 72.10, and 1,968.28 ± 25.29, respectively. TLC of isolated HA and its formulation is depicted in Figure 3.
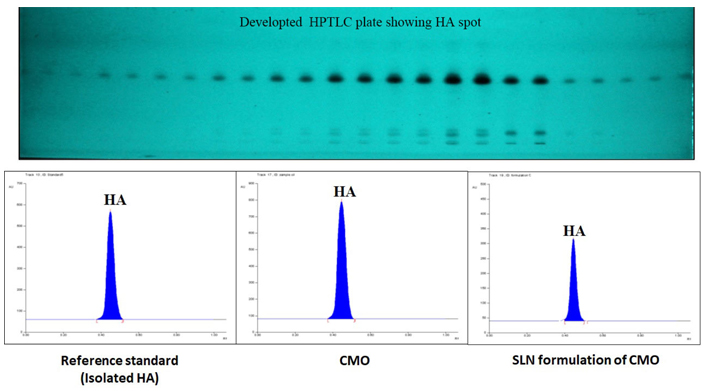
Developed TLC plate of HA at different concentrations and chromatogram of standard, CMO, and developed SLNs
The gel formulation is the most preferred topical dosage form among semi solid dosage forms because of the long residence time on the skin surface, moisturizing effect, high viscosity, bio-adhesiveness, ease of application, better release profile, less irritation, etc. Carbopol 934 was used as a gelling agent because of its bio-adhesiveness, biodegradability, biocompatibility, non-irritant, and not absorbed in the systemic circulation. The concentration of gelling agent Carbopol 934 was optimized on the basis of the consistency of the SLN gel. Propylene glycol is used as a permeation enhancer and it was selected on the basis of the reported literature. Disodium edetate and triethanolamine were used to adjust the pH of the SLN gel formulation [18].
Prepared SLN gel was found to be homogenous and good in appearance and consistency. The pH was adjusted in the neutral range and was found to be 7.3 ± 0.4. Because of the neutral pH, it didn’t cause any skin irritation.
A small quantity of gel was pressed between the thumb and the index finger and the consistency and homogeneity of the gel were noticed. The gel was homogenous, and no gritty and coarse particles were appeared or detached on the finger.
Last but not least, we investigated the erythema forming potential of CMO-loaded SLN gel in vitro. The results incurred from the skin irritancy study, performed using eight rats were worthy enough. The formulation was applied to the left ear of the rat and the right was considered as a control. The development of erythema was monitored for 10 days using the method as reported with slight modification [25]. Erythema was not observed after 10 days of application on the left ear as compared to the right ear indicating that no irritation was produced after the application of formulated SLN gel. The results are given in Table 4.
Primary skin irritation test for CMO gel formulation on six rats (mean ± SD, n = 6)
| Skin condition | Time duration | Control | Treatment |
|---|---|---|---|
| Erythema | 1 h | 0 | 0 |
| Oedema | 0 | 0 | |
| Erythema | 24 h | 0 | 0 |
| Oedema | 0 | 0 | |
| Erythema | 72 h | 0 | 0 |
| Oedema | 0 | 0 | |
| Erythema | 5 days | 0 | 0 |
| Oedema | 0 | 0 | |
| Erythema | 7 days | 0 | 0 |
| Oedema | 0 | 0 | |
| Erythema | 10 days | 0 | 0 |
| Oedema | 0 | 0 |
In vitro release profile of SLN suspension of CMO formulation was compared with the conventional CMO using the dialysis membrane method and in vitro diffusion study of SLN gel was compared with CMO gel using Franz diffusion cell (Figure 4). Phosphate buffer saline pH 7.4 was used for the in vitro release studies of SLN suspension and gel formulation. The highest percent cumulative release was 80.89% for developed SLN, whereas below 20% for CMO. The results of the diffusion study of gel showed the maximum release of drug after 12 h whereas, conventional gel released the maximum drug in 4 h showing the sustained release pattern of drug loaded SLN gel. The prolonged release of the drug from SLN gel made it suitable for sustained release and for better patient compliance.
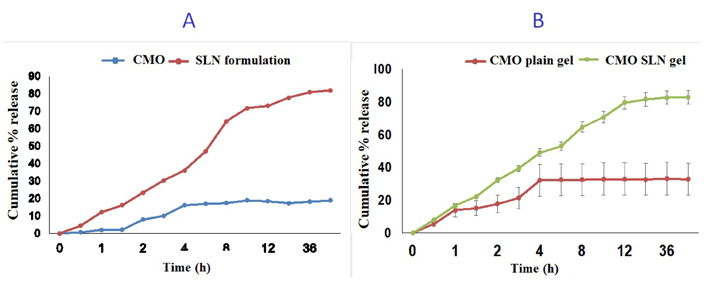
The figure represents (A) comparison of in vitro drug release profile of SLN formulation and CMO; (B) comparison of in vitro drug release profile of plain gel of CMO and SLN gel (SLN formulation)
To ensure the quality, safety, and efficacy of the developed gel formulation throughout the shelf life, stability studies are important. Stability studies were performed and found that there was no drastic or significant change observed during the three months period of stability studies. There was no change in color, odor, homogeneity, pH, and net content of the topical SLN gel formulation after 0, 1, 2, and 3 months of stability testing.
The study involved a developed and validated HPTLC method for the identification and quantification of HA on the basis of their chromatographic separation and isolation from CMO followed by characterization using high edge analytical techniques such as UV, NMR, DSC, and MS. CMO constitutes more than 50% of HA, among all the cyclopentyl fatty acids as per a reported study [24]. The amount of HA quantified in the sample was found to be 54.839% w/w. The identification and characterization of HA via the UV method showed similar UV spectra to that of CMO showing λmax in the range of 200–400 nm [13]. The peak obtained in 1H-NMR and 13C-NMR spectra of isolated HA showed similar results as per the previously reported studies [26]. The molecular weight of chaulmoogric acid and HA is different but the structure of both are somewhat similar except for one functional group. Therefore the mass spectra is showing two fragmentation patterns in negative and positive modes as shown in Figure S5. The result obtained is in compliance with the reported mass spectrum of HA wherein the peak of the molecular ion was obtained at m/z 252 [M]+ [14].
The developed method offers an excellent analytical tool for the routine analysis of the formulation. The chromatogram showed the separation of a prominent green-colored band at Rf 0.45, for the targeted compound in all samples as shown in Figure 1.
SLN colloidal drug carrier combines the advantage of polymeric nanoparticles, due to various advantages, including the feasibility of incorporation of lipophilic and hydrophilic drugs. The preparation of SLN formulation was done using the solvent injection method which offers several advantages like the use of pharmaceutically acceptable organic solvents, easy handling, avoidance of high-pressure homogenization, and less time-consuming technique without the use of sophisticated equipment/instruments. This method is based on lipid precipitation from a dissolved lipid in a solution [5]. Optimisation and characterisation of the prepared formulation was done using percent EE and the average percent EE HA SLNs was found to be 90.2 ± 0.5%. Different batches of SLNs were prepared with varying droplet sizes and PDI as shown in Table 3 and the best formulation was selected for further studies. The average particle size was smaller with lower concentrations of Tween 80 supported by the recently reported literature [27]. Prepared SLN gel was found to be homogenous and good in appearance and consistency. The pH was adjusted in the neutral range and was found to be 7.3 ± 0.4. Because of the neutral pH, it didn’t cause any skin irritation.
Erythema forming potential of CMO-loaded SLN gel was tested in vitro. Erythema was not observed after 10 days of application on the left ear as compared to the right ear indicating that no irritation was produced after the application of formulated SLN gel.
The site specific and sustained release effect of the drug was better achieved by CMO loaded SLN based topical gel and compromised bioavailability was also improved with the prepared formulation. They are relatively novel drug delivery systems, having received primary attention from the early 1990s, and holds great future promise for its systematic investigation and exploitation.
CMO: chaulmoogra oil
CN: cetyl alcohol nanoformulation
DCM: dichloromethane
DSC: differential scanning calorimetry
EE: entrapment efficiency
HA: hydnocarpic acid
HPTLC: high-performance thin-layer chromatography
MS: mass spectroscopy
NMR: nuclear magnetic resonance
PDI: polydispersity index
PVA: polyvinyl alcohol
Rf: retention factor
SD: standard deviation
SLNs: solid lipid nanoparticles
TEM: transmission electron microscopy
TLC: thin layer chromatography
UV: ultraviolet
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001132_sup_1.pdf.
RP, Nafis, MS and SA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Validation, Supervision. HMM: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The animals were procured from the Central Animal House of Jamia Hamdard for skin irritation study approved by the Institutional Animal Ethics Committee (IAEC), Jamia Hamdard, New Delhi (registration no. and date of registration 173/GO/RE/S/2000/CPCSEA and 28th January, 2000). The committee follows the guidelines of the Indian CPCSEA.
Not applicable.
Not applicable.
All datasets [GENERATED/ANALYZED] for this study are included in the manuscript and the supplementary files.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
