Affiliation:
1Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO 80309, USA
Email: gregory.giordano@colorado.edu
ORCID: https://orcid.org/0000-0002-5971-8397
Affiliation:
1Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO 80309, USA
ORCID: https://orcid.org/0000-0001-8940-8587
Affiliation:
1Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO 80309, USA
ORCID: https://orcid.org/0000-0002-6684-2126
Affiliation:
2Department of Medicine, Division of Medical Oncology, University of Colorado School of Medicine, Aurora, CO 80045, USA
†These authors contributed equally to this work.
Affiliation:
2Department of Medicine, Division of Medical Oncology, University of Colorado School of Medicine, Aurora, CO 80045, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-1406-0963
Affiliation:
3Department of Psychiatry, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-4805-9277
Affiliation:
1Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO 80309, USA
ORCID: https://orcid.org/0000-0002-6019-3380
Explor Med. 2023;4:254–271 DOI: https://doi.org/10.37349/emed.2023.00138
Received: October 15, 2022 Accepted: January 13, 2023 Published: April 26, 2023
Academic Editor: M. Kathryn Dahlgren, Harvard Medical School, USA
The article belongs to the special issue Beyond Weed: Clinical Applications of Cannabis and Cannabinoids
Aim: Given the myriad of negative sequalae associated with cancer and its treatment, the palliative use of cannabis by cancer patients is increasingly of special interest. This research sought to explore associations of acute and sustained use of legal market edible cannabis products on pain, cognition, and quality of life in a group of cancer patients.
Methods: In this observational study, cancer patients completed a baseline appointment, a two-week ad libitum cannabis use period, and an acute administration appointment that included assessments before cannabis use, one-hour post-use, and two-hour post-use. Participants completed self-report questionnaires related to the primary outcomes and the Stroop task as a measure of objective cognitive function.
Results: Twenty-five participants [mean (standard deviation, SD) age = 54.3 years (15.6); 13 females (52.0%)] completed all study appointments and were included in the analysis. Sustained cannabis use was associated with improvements in pain intensity, pain interference, sleep quality, subjective cognitive function, and reaction times in the Stroop task, but no change in general quality of life was observed. High levels of cannabidiol (CBD) use during the two-week ad libitum use period was associated with steeper improvements in pain intensity and sleep quality. Participants reported improvements in pain intensity and increased feelings of subjective high after acute use. High levels of Δ9-tetrahydrocannabinol (THC) use during the acute administration appointment was associated with steeper increases in feelings of subjective high. Improvements in pain were associated with improvements in subjective cognitive function.
Conclusions: This observational study is among the first of its kind to examine associations between legal market, palliative cannabis use, and subjective and objective outcomes among cancer patients. These early findings concerning pain intensity, sleep quality, and cognitive function can help to inform future, fully powered studies of this important topic (ClinicalTrials.gov identifier: NCT03617692).
The World Health Organization estimates that one in five people will develop cancer in their lifetime [1]. With more advanced treatments, rates of cure and extension of life are higher than ever before [2]. However, many cancer treatments, such as chemotherapy or radiation, as well as the experience of cancer itself, come with a range of debilitating cognitive, emotional, and physical symptoms. Given the myriad of negative sequalae associated with cancer and its treatment, palliative care is of special interest in cancer research [3].
Among the forms of palliative care used today, one of the most popular options in recent years has been the use of cannabis products. In fact, cancer is an indication for medical cannabis use in most U.S. states with comprehensive medical cannabis programs, and surveys in the U.S., Israel, and Canada suggest that as many as 40% of cancer patients use cannabis [4–6]. In one study of 926 cancer patients, 66% had previously tried cannabis and 24% were current regular users [7]. A survey of oncologists showed general support for the use of cannabis in both adults and children with cancer, but only 30% felt “sufficiently informed” to provide recommendations to their patients about cannabis use [8]. Thus, while cancer patients want to use and are using cannabis, they often do so without any guidance from their clinicians about what to take, how much to take, and how often to take it [9].
Cannabis research is complicated by its classification as a Schedule 1 substance under the Controlled Substances Act, limiting the ability of researchers to study its effects in a controlled laboratory environment [10]. Existing clinical research on cannabis use among cancer patients mostly examines the effects of synthetic cannabinoids such as nabilone or dronabinol [11]. These studies of synthetic cannabinoids lack ecological validity as most cancer patients use state-legal market cannabis products [12]. Compared to pharmaceutical synthetic formulations, legal market products range widely in their formulation (e.g., edible, flower, concentrate) and cannabinoid composition [e.g., the potency of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD)]. As such, studies that examine the effects of state-legal market cannabis products are critically needed, especially in the context of palliative care among cancer patients.
Cancer diagnoses and the treatment plans that come with them are inherently stressful, and many cancer patients experience elevated stress and anxiety [13–15], as well as high levels of pain and/or sleep disruption [16–18]. Surveys suggest prevalence rates of pain near 53% among cancer survivors [19], 59% for those in active treatment [19], and rates of sleep problems ranging from 30% to 87% [20]. Among the many barriers to effective symptom management in cancer palliative care [21], there is some research to suggest that cancer patients will deliberately choose to forego additional medication (e.g., opioids) due to fears around addiction, mental fog, or other side effects [22–24].
Cancer patients increasingly report turning to legal market cannabis for pain, stress, and sleep problems, with the majority reporting at least moderate benefits [7]. Recent literature suggests that there is evidence that cannabis use can alleviate anxiety, reduce pain, and improve sleep in cancer patients [12, 25–28], and that its use is largely safe, effective, and well-tolerated [29]. One study among Israeli cancer patients before and six to eight weeks after they received a medical cannabis license found reductions in self-reported mood disorders, sleep disorders, and pain [30]. Likewise, among a sample of patients with a current or previous diagnosis of cancer, medical cannabis resulted in improvements in pain, depression, anxiety, and sleep problems, though these did not reach statistical significance [26]. Though existing research is equivocal as to whether cannabis is an appropriate treatment for pain [31, 32] or sleep [33], the literature to date suggests that cannabis has potential and should be further explored as a contribution to palliative cancer care.
It is also important to consider the potential negative effects of cannabis use on cognition. Acute use of cannabis can temporarily compromise cognitive function [34], while evidence regarding the long-term effect of cannabis on cognitive function is mixed. For instance, although one study found no evidence of long-term deficits in working memory and selective attention [35], a recent review suggested that decision-making, concept formation, and planning were harmed by long-term cannabis use but basic attentional and working memory abilities can be recovered upon cessation of use [36]. This discrepancy may be due to differences in cannabinoid content as studies suggest that while THC is associated with memory and other cognitive impairment [34, 36–38], CBD may attenuate THC’s negative effects [39, 40].
Cancer patients may experience reduced cognitive functioning in the context of treatment (e.g., chemotherapy) [41–44], and report that one of their greatest concerns are changes to their cognitive abilities [45–47]. Cancer patients are also faced with both self-regulatory challenges (i.e., adhering to complicated drug regimens, healthy eating, and exercising to reduce fatigue) and cognitively taxing decisions (e.g., weighing the pros and cons of treatment options) that rely on high-level working memory and executive function processes [48]. Finally, subjective patient reports of cognitive difficulties are often discrepant from their performance on objective measures of cognition [49], making it essential to examine the impact of cannabis use on both objective and subjective cognitive function in this population.
Overall, cannabis use in the context of cancer-related palliative care is rapidly increasing, but the field lags in providing empirical data to inform patient and provider decisions as to the risks and benefits of cannabis use. Thus, the objectives of this study were to (1) explore the feasibility of an observational study of cancer patients who wish to use legal market edible cannabis products to treat symptoms associated with their cancer or its treatment; (2) evaluate the acute associations of legal market edible cannabis products with cancer patients’ pain, anxiety, and cognition; and (3) explore the relationship of sustained (two-week) use of legal market edible cannabis products with cancer patients’ pain, anxiety, sleep quality, and objective and subjective cognitive function. It was hypothesized that cannabis use would be associated with decreased pain and anxiety, as well as acutely worse objective cognitive functioning. Tests of the relationship of cannabis use to sleep quality, subjective cognitive function, as well as the potentially differential effects of THC and CBD were considered exploratory.
The study was initially approved by the Colorado Multiple Institutional Review Board (COMIRB Panel D IRB number: IRB00002760) in August 2018 and registered as an observational study on www.clinicaltrials.gov (NCT03617692). Participants were recruited with advertisements placed in local oncology clinics, mailed advertisements, and posts on social media. Participants were compensated with grocery store gift cards for completion of the baseline ($60) and acute administration ($100) appointments.
Trained research staff screened participants via telephone for the following inclusion criteria: (1) stated willingness to comply with all study procedures and be available for the duration of the study; (2) aged 21 years or older; (3) have a diagnosis of any solid tumor type and has undergone or is undergoing either curative or palliative treatment; (4) have the intent to use cannabis to treat their symptoms; (5) no use of other non-prescription drugs of abuse (e.g., cocaine, heroin, or methamphetamine) in the past 60 days; (6) not actively seeking or in treatment for any substance use disorder; (7) no acute illness other than cancer that could affect cognition or compliance per the decision of the study physician; (8) not pregnant or trying to become pregnant; and (9) score better than 20 (above the moderately to the severely impaired range) on the Telephone Interview for Cognitive Status [50]. Participants did not need to be naive to cannabis to participate in the study. Participants were subject to polypharmacy while participating in the study and were not given instructions about prescription medication use from research staff.
Changes to the eligibility criteria were made after the study’s commencement to enhance recruitment. Specifically, the allowed cancer diagnoses were expanded from including only lung cancer patients to including any solid tumor type. As a result, only edible cannabis products were allowed in the present study because the original design did not allow the use of inhaled cannabis products in the lung cancer patient population.
Due to the spread of coronavirus disease 2019 (COVID-19), the study transitioned from an in-person to a remote modality to reduce the risk of exposure. Remote participants (n = 17) interacted with research staff via Zoom or over the phone during study appointments and completed all the same procedures and measures as in-person participants (n = 11) with the exception that remote participants did not have blood collected. All study data were collected and managed using Research Electronic Data Capture (REDCap) electronic capture tools hosted at the Colorado Clinical and Translational Sciences Institute [51].
In-person participants provided written informed consent at the University of Colorado Boulder. Remote participants provided electronic informed consent via REDCap. Female participants of child-bearing age completed a urine pregnancy test to verify eligibility (research staff delivered a urine receptacle to remote participants). All participants completed self-report questionnaires and a measure of objective cognitive function. Participants were then provided with basic information about edible cannabis products, including examples of commonly found products, their various cannabinoid profiles, safety guidelines for use, approximate prices, and locations where these products can be purchased. Research staff also conducted a medical record review for demographic information and cancer diagnosis.
Following the baseline appointment, participants purchased the edible cannabis product of their choice from the local medical or recreational dispensary of their choice and used the product ad libitum for two weeks without any instruction from research staff about dosing or frequency. Participants were asked to not use any other cannabis products during this two-week period. Participants submitted photos of their cannabis product and its state-required label that details the cannabinoid content of the product via an online REDCap survey. A member of the research staff not responsible for data collection managed the product photo surveys to keep the research staff responsible for data collection blind to the products selected by participants.
Participants were instructed to not use any cannabis on the day of the acute administration appointment before it was scheduled to begin. The acute administration appointment included three rounds of assessments: (1) pre-use; (2) one-hour post-cannabis use; and (3) two-hour post-cannabis use, as previous research supports circulating cannabinoids peaking approximately two hours following oral cannabis ingestion [52–54]. In-person participants ingested their edible cannabis products in their homes but completed all assessments in a federally compliant, university-approved mobile pharmacology laboratory, as described in a previous publication [55]. Remote participants completed the entirety of the acute administration appointment in their homes, communicating with the research staff via Zoom or over the phone during assessments.
The pre-use assessment included self-report questionnaires and a measure of objective cognitive function. In-person participants also completed a blood draw for circulating cannabinoids (i.e., THC and CBD). Participants then ingested their desired amount of cannabis, recorded the amount of product ingested in a blinded fashion (e.g., “one gummy”, “one mL”), and waited one hour before resuming assessments. A member of the research staff not responsible for data collection translated the amount of product ingested into milligrams of THC and CBD consumed using the product photo surveys.
The one-hour and two-hour post-cannabis use assessments involved self-report questionnaires, a measure of objective cognitive function, and blood draws (for in-person participants only).
Sex, age, race, ethnicity, cancer diagnosis, and current treatment were collected from the medical record review at baseline. Participants self-reported their education level, height, and weight at baseline.
A 30-day Timeline Followback (TLFB) [56] administered via an online interactive calendar [57] was completed at the baseline appointment to collect information about the use of alcohol, cannabis, and tobacco. A 14-day online TLFB was completed during the pre-use assessment of the acute administration appointment.
The Beck Depression Inventory®-II (BDI-II) [58] and Beck Anxiety Inventory® (BAI) [59] were completed at baseline and at the pre-use assessment of the acute administration appointment. The BDI-II is a 21-item measure of depression severity (Cronbach α = 0.86), and the BAI is a 21-item measure of common symptoms of anxiety (α = 0.84). The 20-item state anxiety component of the State-Trait Anxiety Inventory (STAI, α = 0.88) [60] was completed pre-use, one-hour post-use, and two-hour post-use at the acute administration appointment.
A single item from the Pittsburgh Sleep Quality Index (PSQI) [61] modified to ask about the last two weeks was completed at baseline and the pre-use assessment of the acute administration appointments. Participants were asked to rate their sleep quality on a scale from 0 (“very good”) to 3 (“very bad”).
Participants were asked to rate their pain on average over the past seven days at baseline and the pre-use assessment of the acute administration appointment. Additionally, participants were asked to rate their pain “right now” at the pre-use, one-hour post-use, and two-hour post-use assessments of the acute administration appointment. Response options ranged from 0 (“no pain”) to 10 (“worst pain imaginable”). Further, the Patient-Reported Outcomes Measurement Information Systems (PROMIS) bank version 1.1 pain interference computer adaptive test [62] was administered at baseline and pre-use and asked participants about how pain interfered with or affected their enjoyment of various daily activities in the past seven days. These measures of pain do not differentiate between the different types of pain (e.g., neuropathic, nociceptive).
The Functional Assessment of Cancer Therapy-General version 4 (FACT-G, α = 0.80) [63] was administered at baseline and the pre-use assessment of the acute administration appointment. The FACT-G is a measure of health-related quality of life that asks participants to rate 27 statements (e.g., “I am content with the quality of my life right now”) on a scale from 0 (“not at all”) to 4 (“very much”) as it applies to the last seven days. Higher scores reflect a better quality of life.
The Functional Assessment of Cancer Therapy-Cognitive Function version 3 (FACT-Cog) [64] is a measure of subjective cognitive function that includes two components: 18 items assessing perceived cognitive impairments (PCI, α = 0.96) and 7 items assessing perceived cognitive abilities (PCA, α = 0.89). For the PCI component, participants rated statements (e.g., “I have had trouble forming thoughts”) as they apply to the last seven days from 0 (“not at all”) to 4 (“several times a day”) with items being reverse scored. For the PCA component, participants rated statements (e.g., “my mind is as sharp as it has always been”) as they apply to the last seven days from 0 (“not at all”) to 4 (“very much”). Higher scores reflect better quality of life. The FACT-Cog was completed at the baseline and the pre-use assessment of the acute administration appointments.
Participants rated subjective high from a single question [55] [“I feel high (as in ‘drug high’) right now”] from 0 (“not at all”) to 10 (“strongest feeling possible”) at the pre-use, one-hour post-use, and two-hour post-use assessments at the acute administration appointment.
The Stroop task [65] was administered by research staff using Tatool Web [66]. In-person participants completed the task on a tablet using the touchscreen to input responses. Remote participants completed the task on the device of their choice (smartphone, tablet, computer), using a touchscreen or keyboard to input responses, and were asked to use the same device at all time points. The Stroop task presented one to four characters on the screen at a time, asking participants to count the number of characters on the screen while ignoring the magnitude of the characters on the screen. The task included congruent trials, where the number of characters matched the magnitude of the characters (e.g., 333), neutral trials where the letter “X” was were presented instead of digits (e.g., XXXX), and incongruent trials where the number of characters did not match the magnitude of characters presented (e.g., 44). Participants completed 12 practice trials followed by 144 test trials (48 congruent; 48 neutral; 48 incongruent). The Stroop effect on reaction time was calculated as the difference in mean response time between incongruent and congruent trials for correct responses. The Stroop effect on error rate was calculated as the difference in error rate between incongruent and congruent trials. Consistent with prior research [67, 68], trials with reaction times less than or equal to 200 ms were removed from analyses. One participant’s baseline data was removed from the analysis because they had no correct responses recorded due to a failure of their input device. The Stroop task was administered at baseline and at the pre-use, one-hour post-use, and two-hour post-use assessments of the acute administration appointment.
A phlebotomist collected 7 mL of blood from in-person participants at the pre-use, one-hour post-use, and two-hour post-use assessments of the acute administration appointment. Plasma was separated from erythrocytes by centrifugation (1,000 g for 10 min), transferred to a fresh microcentrifuge tube, and stored at –80°C. Plasma samples were shipped to the iC42 Lab at the Anschutz Medical Campus for quantification of THC and CBD via validated high-performance liquid chromatography/mass spectroscopy. The lower limit of quantification for circulating THC and CBD was 0.39 ng/mL, thus values below this threshold were replaced with 0.39 ng/mL for analyses.
To examine the sustained effects of cannabis use on cancer-related outcomes, analyses of subjective and cognitive effects from the baseline appointment to the pre-use assessment of the acute administration appointment were conducted separately using a series of mixed-effects models estimating random intercepts for each participant. In each model, fixed effects included (1) linear change over time (baseline to pre-use); (2) total milligrams of CBD ingested during the two-week ad libitum use period as reported in the online TLFB (mean-centered); and (3) total milligrams of THC ingested during the two-week ad libitum period as reported in the online TLFB (mean-centered). Interaction effects tested whether the linear change over time varied by total CBD ingested, total THC ingested, and their interaction.
To examine the acute effects of cannabis on cancer-related outcomes, analyses of subjective and cognitive effects across the three acute administration time points (pre-use, one-hour post-use, and two-hour post-use), were conducted separately using a series of mixed-effects models estimating random intercepts for each participant. In each model, fixed effects included (1) linear change over time; (2) quadratic change over time; (3) CBD ingested during the acute administration appointment (mean-centered); and (4) THC ingested during the acute administration appointment (mean-centered). Interaction effects tested whether the linear and quadratic change over time varied by THC ingested, CBD ingested, and their interaction. In models where both the linear and quadratic effects were significant, we focused on the stronger effect (in all cases, the linear effect was stronger).
All analyses were conducted all analyses in R version 4.1.2 (www.rstudio.com). Mixed effects models were conducted using the lme4 package version 1.1–25 [69], which implements maximum likelihood (ML) estimation.
The progress of participants through the study is presented in Figure 1. Twenty-eight participants were recruited for the study. Twenty-five participants completed both the baseline and the acute use appointments and were included in the analyses. Descriptive information about study participants is provided in Table 1, and Table 2 provides information about participant cancer status and current treatment. Seventeen (68%) of participants reported no cannabis use in the seven days prior to the baseline appointment, and eight (32%) of participants reported no cannabis use in the 30 days prior to the baseline appointment. Participants reported no adverse events related to cannabis use during the two-week ad libitum use period, and no adverse events were observed following acute cannabis administration.
Baseline characteristics of participants (n = 25)
| Characteristic | n | % | Mean | SD | |
|---|---|---|---|---|---|
| Demographics | Age (year) | - | - | 54.3 | 15.6 |
| Sex (female) | 13 | 52.0 | - | - | |
| Height (cm) | - | - | 167.7 | 10.9 | |
| Weight (kg) | - | - | 72.2 | 17.3 | |
| Body mass index (BMI, kg/m2) | - | - | 25.8 | 6.1 | |
| Education (Bachelor’s degree or higher) | 16 | 64.0 | - | - | |
| Race (white) | 20 | 80.0 | - | - | |
| Ethnicity (Hispanic or Latino) | 2 | 8.0 | - | - | |
| Substance use history (last 30 days) | Days of cannabis use | - | - | 12.5 | 11.7 |
| Days of alcohol use | - | - | 4.4 | 5.9 | |
| Days of tobacco use | - | - | 2.2 | 7.8 | |
SD: standard deviation; -: not applicable
Participant cancer and treatment information (n = 25). Chemotherapy and immunotherapy treatment counts are not mutually exclusive
| Characteristic of cancer diagnosis and treatment | n (%) | |
|---|---|---|
| Cancer stage | Stage 1 | 1 (4.0%) |
| Stage 2 | 3 (12.0%) | |
| Stage 3 | 5 (20.0%) | |
| Stage 4 | 11 (44.0%) | |
| Unknown | 5 (20.0%) | |
| Cancer type | Lung | 7 (28.0%) |
| Colon | 3 (12.0%) | |
| Breast | 3 (12.0%) | |
| Adenoid | 2 (8.0%) | |
| Prostate | 2 (8.0%) | |
| Rectal | 2 (8.0%) | |
| Thyroid | 1 (4.0%) | |
| Germ cell | 1 (4.0%) | |
| Brain | 1 (4.0%) | |
| Ewing’s sarcoma | 1 (4.0%) | |
| Kidney | 1 (4.0%) | |
| Skin | 1 (4.0%) | |
| Treatment | Observation | 11 (44.0%) |
| Chemotherapy | 12 (48.0%) | |
| Immunotherapy | 4 (16.0%) | |
On average, participants used an edible cannabis product for 8.1 days (SD = 5.1, range = 0–14) and ingested a total of 116.4 mg (SD = 178.0, range = 0–625) of THC and 162.7 mg (SD = 420.3, range = 0–2,100) of CBD, or 8.3 mg of THC and 11.6 mg of CBD per day, during the two-week ad libitum use period. Participants reported using eighteen different brands of cannabis products. Fourteen participants used an edible cannabis product in the form of candies (e.g., gummies, chocolate), seven used tinctures, two used pill forms, one used beverages, and one used baked goods (stroopwafels). Milligrams of THC and CBD ingested at the acute administration appointment are presented in Figure 2. All participants confirmed using their purchased edible cannabis product at the acute administration appointment. Mean circulating THC at the acute administration appointment was 5.58 ng/mL (SD = 15.48, range = 0.39–46.87) at pre-use (n = 9), 10.43 ng/mL (SD = 26.72, range = 0.39–81.50) at one-hour post-use (n = 9), and 7.59 ng/mL (SD = 18.96, range = 0.39–54.50) at two-hour post-use (n = 8) for in-person participants with available blood data (participant agreed to blood collection and blood was successfully collected). Mean circulating CBD at the acute administration appointment was 3.57 ng/mL (SD = 3.42, range = 0.39–10.78) at pre-use (n = 9), 10.81 ng/mL (SD = 15.02, range = 0.47–43.70) at one-hour post-use (n = 9), and 9.67 ng/mL (SD = 8.40, range = 2.28–24.96) at two-hour post-use (n = 8) for in-person participants with available blood data. Circulating cannabinoid levels are also presented in Figure S1. Significant positive correlations were observed between THC ingested and circulating THC (r = 0.98, P < 0.001) and CBD ingested and circulating CBD (r = 0.85, P = 0.003) at one-hour post-use during the acute administration appointment for in-person participants with blood data (n = 9). The positive correlation between THC ingested and circulating THC was reduced (r = 0.65, P = 0.079) when one outlier (60.0 mg of THC ingested) was removed.
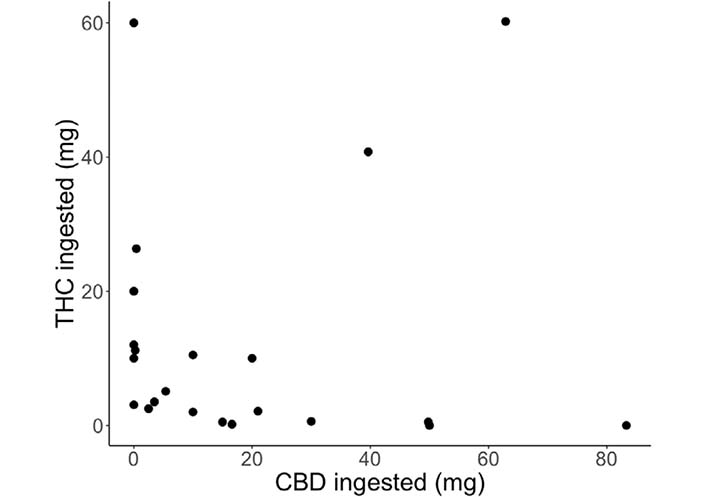
Cannabinoids (mg) ingested at the acute administration appointments for each participant [THC (mg): mean = 12.14, SD = 17.49, range = 0–60.2; CBD (mg): mean = 18.91, SD = 23.72, range = 0–83.3]
A significant effect of time on sleep quality was observed (baseline mean = 1.2; pre-use at acute administration mean = 0.87; B = −0.43, standard error (SE) = 0.17, P = 0.02), such that sleep quality improved from baseline to the acute administration appointment. The time X CBD interaction term was significant (B = 0.01, SE = 0.0005, P = 0.02), such that participants who reported higher CBD consumption showed steeper improvements in sleep quality (see Figure 3A). No other significant interactions were observed (Ps > 0.10), and there was no significant effect of CBD consumption on baseline sleep quality (P > 0.11).
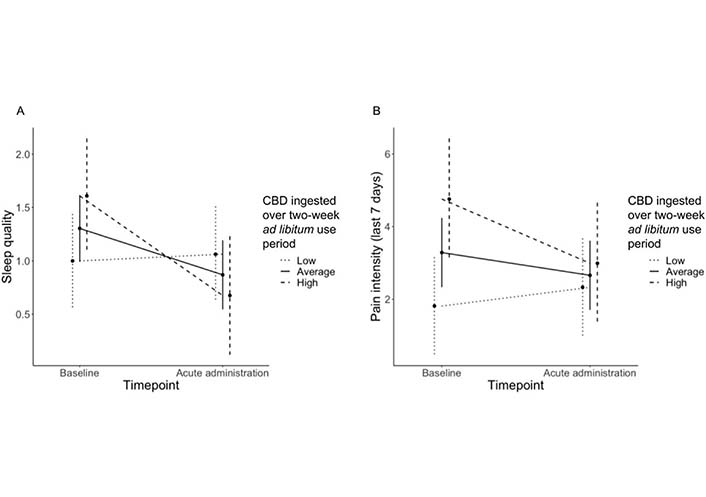
Time X CBD ingested over two-week ad libitum use period interactions for (A) sleep quality and (B) pain intensity over the last seven days. Sleep quality was rated on a scale from 0 (“very good”) to 3 (“very bad”), such that lower values reflect higher ratings of sleep quality. Pain intensity was rated on a scale from 0 (“no pain”) to 10 (“worst pain imaginable”), such that lower values reflect lower ratings of pain intensity
A significant effect of time on pain intensity was observed (baseline mean = 3.08; pre-use at acute administration mean = 2.48; B = −0.63, SE = 0.26, P = 0.02), such that pain intensity improved from baseline to the acute administration appointment. A significant time X CBD interaction was also observed (B = −0.003, SE = 0.008, P = 0.002), such that participants reporting higher CBD use showed steeper reductions in pain intensity (see Figure 3B). No other significant interactions were observed for pain intensity (Ps > 0.22). A significant effect of CBD consumption on baseline pain intensity (B = 0.03, SE = 0.01, P = 0.02) was observed, such that higher baseline pain intensity was associated with higher levels of CBD use. Controlling for total THC and CBD ingested during the two-week ad libitum use period, a significant effect of time on pain interference was observed (baseline mean = 53.43; pre-use at acute administration mean = 50.15; B = −3.99, SE = 1.44, P = 0.01), such that pain interference improved from baseline to the acute administration appointment. No significant interactions were observed for pain interference (Ps > 0.15).
A marginal effect of time on anxiety (baseline mean = 9.40; pre-use at acute administration mean = 7.44; B = −1.81, SE = 0.95, P = 0.07) and depression (baseline mean = 10.20; pre-use at acute administration mean = 9.20; B = −1.52, SE = 0.79, P = 0.07) scores were observed, such that participants reported fewer anxiety and depressive symptoms at the acute administration appointment relative to baseline. There were no significant interactions with a level of cannabinoids ingested for anxiety (Ps > 0.22) or depression (Ps > 0.06) scores.
There was no significant effect of time on the overall quality of life (baseline mean = 70.48; acute administration mean = 73.44; B = 3.19, SE = 3.25, P = 0.34), and no significant interactions with level of cannabinoids ingested (Ps > 0.58).
No significant effect of time on FACT-Cog PCI was observed (baseline mean = 55.39; pre-use at acute administration mean = 58.56; B = 3.75, SE = 2.24, P = 0.11), but a significant effect of time on FACT-Cog PCA was observed (baseline mean = 18.40; pre-use at acute administration mean = 20.71; B = 3.03, SE = 1.03, P = 0.008), such that PCA increased in the two weeks between the baseline and the acute administration appointment. No significant interactions with ingested cannabinoid levels were observed for FACT-Cog PCI (Ps > 0.46) or FACT-Cog PCA (Ps > 0.05).
Given meta-analytic evidence of impaired cognitive performance among individuals experiencing chronic pain [70], an exploratory analysis was conducted to test the association between subjective cognitive functioning (FACT-Cog PCA) and pain interference after two weeks of cannabis use. As can be seen in Figure 4, there was a negative association, such that as pain interference increased, PCA decreased, although this association did not achieve statistical significance.
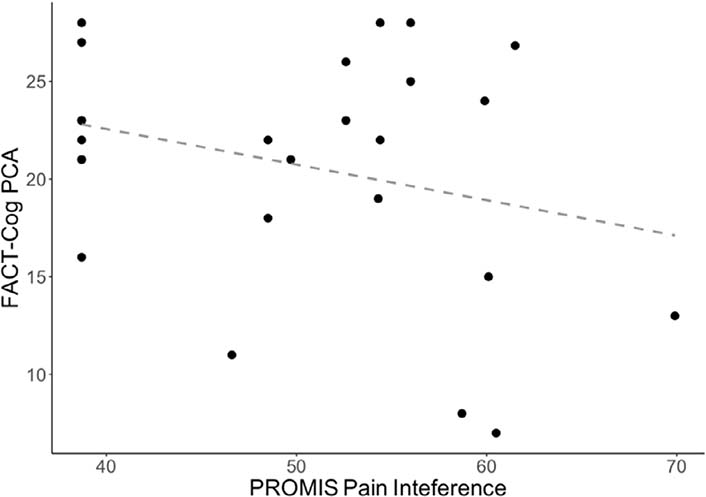
Scatterplot of FACT-Cog PCA versus PROMIS pain interference at the acute administration appointment (r = −0.28, P = 0.17). Higher values on the FACT-Cog PCA reflect better quality of life. Higher values on the PROMIS pain interference reflect greater consequences of pain on one’s life
There was no significant effect of time on the Stroop effect on reaction time (ms, baseline mean = 84.93; pre-use at acute administration mean = 68.61; B = −23.46, SE = 14.54, P = 0.12), and no significant interactions were observed based on ingested cannabinoids for the Stroop effect on reaction time (Ps > 0.17). A significant effect of time was observed for congruent reaction time (ms, baseline mean = 1,091.99; pre-use at acute administration mean = 1,013.94; B = −71.72, SE = 29.57, P = 0.02) and incongruent reaction time (ms, baseline mean = 1,176.93; pre-use at acute administration mean =1,082.55; B = −93.98, SE = 25.43, P = 0.001), such that both reaction times decreased from baseline to the acute administration appointment. No significant interactions with ingested levels of cannabinoids were observed for Stroop congruent reaction time (Ps > 0.48) or Stroop incongruent reaction time (Ps > 0.27).
There was no significant effect of time on the Stroop effect on error rate (baseline mean = 0.04; pre-use at acute administration mean = 0.03; B = −0.01, SE = 0.01, P = 0.53), the error rate of congruent trials (baseline mean = 0.06; pre-use at acute administration mean =0.04; B = −0.02, SE = 0.02, P = 0.43), or the error rate of incongruent trials (baseline mean = 0.10; pre-use at acute administration mean = 0.07; B = −0.02, SE = 0.02, P = 0.22). No significant interactions with amount of cannabinoids ingested were observed for Stroop effect on error rate (Ps > 0.09), congruent error rate (Ps > 0.66), or incongruent error rate (Ps > 0.34).
Descriptive statistics for changes in acute administration outcomes are presented in Table 3.
Acute administration outcomes across the three assessment time points (n = 25). Values are mean (SD)
| Outcome | Pre-use | One-hour post-use | Two-hour post-use |
|---|---|---|---|
| Subjective high* | 0.04 (0.20) | 3.16 (3.01) | 3.84 (3.26) |
| Pain intensity* | 1.88 (2.11) | 0.88 (1.30) | 0.96 (1.51) |
| Anxiety | 30.32 (11.31) | 28.16 (8.56) | 29.56 (9.98) |
| Stroop | |||
| Effect on reaction time* | 68.61 (36.00) | 84.46 (58.43) | 94.31 (72.58) |
| Congruent reaction time* | 1,013.94 (164.76) | 965.54 (174.19) | 962.66 (176.66) |
| Incongruent reaction time | 1,082.55 (164.39) | 1,050.00 (187.42) | 1,056.96 (198.94) |
| Effect on error rate | 0.03 (0.05) | 0.04 (0.05) | 0.04 (0.05) |
| Congruent error rate | 0.04 (0.10) | 0.05 (0.16) | 0.06 (0.15) |
| Incongruent error rate* | 0.07 (0.15) | 0.09 (0.16) | 0.10 (0.15) |
* Significant linear effect of time (P < 0.05)
There was a significant linear effect of time on subjective high, such that participants reported feeling higher over time (B = 1.90, SE = 0.25, P < 0.001). Both the time X THC (B = 0.05, SE = 0.02, P = 0.003) and time X CBD (B = −0.02, SE = 0.01, P = 0.05) interaction terms were significant, with participants who ingested more THC exhibiting a steeper increase in high over time and participants who ingested more CBD exhibiting a smaller increase in high over time (see Figure 5). The time X CBD X THC interaction term was not significant (B = −0.0003, SE = 0.0005, P = 0.52).
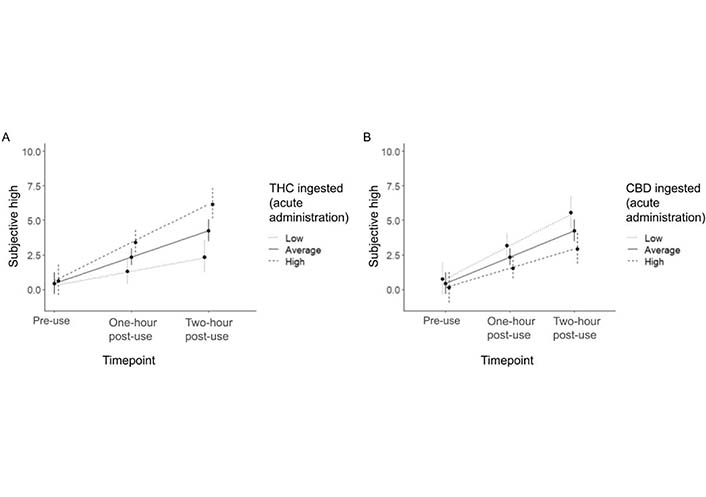
Time X THC ingested (A) and time X CBD ingested (B) during the acute administration appointment for subjective high
There was a significant linear effect of time (B = −0.46, SE = 0.14, P = 0.002) on pain intensity (“right now”), such that participants reported reduced pain intensity over the course of the acute administration session. None of the time X cannabinoid interaction terms were significant (Ps > 0.08).
Anxiety levels did not change over the course of the acute administration session (B = −0.38, SE = 0.74, P = 0.61) and none of the time X cannabinoid interaction terms were significant (Ps > 0.47).
The Stroop effect on reaction time exhibited a significant linear effect of time (B = 12.93, SE = 6.09, P = 0.04), such that there was a significant increase in the Stroop effect over the course of the acute administration appointment (i.e., performance worsened). None of the time X cannabinoid interaction terms were significant (Ps > 0.26). Specifically, while reaction time on congruent trials decreased over time (B = −25.75, SE = 8.44, P = 0.004), reaction time on incongruent trials did not significantly change (B = −12.82, SE = 8.04, P = 0.12).
The Stroop effect on error did not change over the course of the acute administration appointment (B = 0.004, SE = 0.007, P = 0.59), and none of the time X cannabinoid interaction terms were significant (Ps > 0.36). Specifically, while the error rate on incongruent trials increased over time (B = 0.01, SE = 0.007, P = 0.05), the error rate on congruent trials did not significantly change (B = 0.01, SE = 0.007, P = 0.14).
This study is among the first to explore associations of acute and sustained legal market cannabis edible use, including the use of both THC and CBD, with changes in pain, cognition, and quality of life in a group of cancer patients. Patients reported improvements in pain, sleep quality, and subjective cognitive function following a two-week ad libitum cannabis use period, as well as reductions in pain intensity, increases in feelings of subjective high, and some decreases in cognitive function following acute cannabis use. The observational nature of the study also allowed a window into the kinds of products cancer patients select, how frequently they choose to use their products, and what doses of THC and CBD they choose to take. Participants in the present study used a variety of edible forms of cannabis and a wide range of doses of both THC and CBD. This is perhaps somewhat unexpected given the rapidly growing interest in CBD over the past five years [71]. A significant effect of CBD use on baseline pain intensity was observed, such that greater CBD use over the two-week period was associated with greater pain intensity at baseline, suggesting that individuals with higher pain intensity at baseline may have intentionally selected products higher in CBD or consumed more CBD over the two weeks to cope with these heightened pain levels. A greater understanding of how cancer patients come to make decisions around cannabis use and how their expectancies may vary by cannabinoid content will be critical in order to develop guidance for both clinicians and patients who wish to use cannabis for palliative cancer care.
Improvements in pain intensity and pain interference were observed following sustained cannabis use, as well as improvements in pain intensity following acute use. Interestingly, a time X CBD ingested interaction emerged in the two-week models of pain intensity, with greater CBD use during the two-week ad libitum period being associated with steeper improvements in pain intensity. Notably, no moderating effect of CBD use was observed during the acute use appointment. Two early clinical trials in cancer patients that utilized THC oil capsules demonstrated a trend toward progressive pain relief with increasing doses of THC over a six-hour period [72], as well as significant improvements in pain following 20 mg of THC compared to placebo over a seven-hour period [73], although adverse reactions to this dose were observed in this group of predominantly inexperienced cannabis users [73]. In addition, a two-week trial of oromucosal sprays showed improvements in pain compared to placebo following the use of a combination of CBD and THC spray, but not with a THC-only spray [74]. More recent trials have yielded mixed results on the effectiveness of cannabinoids for pain [75, 76], but the picture that emerges from across the literature suggests that THC and CBD may influence pain relief over different time courses. Pharmacokinetic research will be key to addressing these potentially differential effects of THC and CBD depending on the time course of sustained versus acute use.
Improvements in sleep quality following sustained cannabis use were observed, with higher CBD use during the two-week ad libitum use period being associated with steeper improvements in sleep quality. A recent meta-analysis that included five randomized controlled trials of oromucosal sprays containing cannabinoids found a very small improvement in sleep disturbance compared to placebo in individuals living with chronic cancer pain [77]. Objective measures of sleep in the context of carefully controlled sleep studies would be ideal for better understanding the differential effects of THC and CBD across the full range of measures of sleep quality.
Two weeks of ad libitum use in the present study was associated with patient reports of improvements in subjective cognitive function. Some objective measures of cognitive function also improved, including reaction times for both congruent and incongruent trials on the Stroop task. Consistent with the literature on pain and cognitive function, an exploratory analysis supported a negative association, such that increases in pain were associated with subjectively worse cognitive function. Given widespread concern—by both clinicians and patients themselves—about decrements in cognitive function associated with cancer treatment, a potential indirect role of cannabis use in improving subjective cognitive function is an intriguing finding to pursue.
Also consistent with past research, some impairments following acute administration on objective cognitive performance were also observed, such that the Stroop effect on reaction time and error rate for incongruent trials both increased (i.e., got worse).
Limitations of the present study include a small sample size which limited statistical power to investigate demographic or other moderators in the analyses. From an internal validity perspective, the lack of random assignment to product condition and the lack of a placebo-control condition are clearly problematic for any causal conclusions regarding the influence of cannabis on any of the outcomes tested in this study. In addition, the study was interrupted by the COVID-19 pandemic, requiring the study to transition to a remote modality to continue data collection. The generalizability of the conclusions of the present study is limited by the largely white sample and by the restriction on the use of cannabis edibles. There is evidence that cancer patients also use inhaled cannabis products [7, 78]. The short two-week timeframe may have limited the capacity of the present study to observe changes following sustained cannabis use. The inclusion of participants with different cannabis use histories and undergoing different treatments may have influenced participant response to acute and sustained cannabis use.
Despite its limitations, this study benefits from increased external validity via its naturalistic design, which allowed participants to select and use legal market edible cannabis products in a manner and environment that reflected their own preferences, needs, and typical use behaviors. The study’s inclusion of multiple cancer types also allowed for a greater age range of participants and an equal representation of sex, which may not be feasible when restricting eligibility to certain cancer types (e.g., breast, prostate).
In this study of legal market edible cannabis products in cancer patients, two weeks of ad libitum cannabis use was associated with improvements in pain intensity and interference, sleep quality and subjective cognitive functioning. It is particularly of note that high CBD, not THC, use during this two-week period was associated with steeper improvements in pain intensity and sleep quality. Acute cannabis use decreased pain intensity, decreased some measures of objective cognitive function, and increased feelings of subjective high. The findings suggest that studies conducted over longer timeframes with larger samples that allow the capacity to test for moderating effects of product type (e.g., inhaled versus edible), cancer type/stage, and demographic characteristics are an important next step in understanding the potential benefits and harms of cannabis use for palliative care in cancer patients.
CBD: cannabidiol
COVID-19: coronavirus disease 2019
FACT-Cog: Functional Assessment of Cancer Therapy-Cognitive Function version 3
PCA: perceived cognitive abilities
PCI: perceived cognitive impairments
PROMIS: Patient-Reported Outcomes Measurement Information Systems
REDCap: Research Electronic Data Capture
SD: standard deviation
SE: standard error
THC: Δ9-tetrahydrocannabinol
TLFB: Timeline Followback
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001138_sup_1.pdf.
ADB: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing. DRC, DWB, and KEH: Conceptualization, Funding acquisition, Methodology, Writing—review & editing. GG: Investigation, Data curation, Formal analysis, Visualization, Writing—original draft, Writing—review & editing. LPG: Data curation, Formal analysis, Visualization, Writing—original draft, Writing—review & editing. RMW: Conceptualization, Funding acquisition, Methodology, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The study was approved by the Colorado Multiple Institutional Review Board (COMIRB Panel D IRB number: IRB00002760). Initial approval was obtained in August 2018.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The deidentified raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Funding for this study was provided by the University of Colorado Cancer Center. LPG is supported by a National Science Foundation Graduate Research Fellowship [DGE 1650115]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Lucile Rapin ... Michael Dworkind
Elizabeth N. R. Schjelderup ... Alasdair M. Barr
Hannah Thurgur ... David J. Nutt
Amanda Stueber, Carrie Cuttler
Cassandra L. Taylor, Schuyler A. Pruyn
Gerhard Nahler
Trevor R. Norman
