Abstract
Aim:
Polycystic ovarian syndrome (PCOS) is the most common endocrine condition, affecting 5–7% of reproductive-age women worldwide. It is associated with low-grade chronic inflammation, insulin resistance, and metabolic syndrome. Studies have shown ceruloplasmin (Cp) as an independent risk factor for metabolic syndrome and magnesium (Mg), which is required for proper glucose utilization. This study aimed to compare the serum Mg and Cp in PCOS and healthy women and correlate their levels with changes in biochemical, hormonal, and gynaecological aspects of PCOS.
Methods:
The study comprised 98 women diagnosed with PCOS using the Rotterdam criteria and 75 age-matched healthy control subjects. The level of serum Cp and Mg were determined using Somani Ambade colorimetric method and methylthymol blue method respectively.
Results:
Serum Cp was higher and Mg levels were lower significantly in PCOS patients in comparison with controls. Mg was inversely correlated with fasting blood glucose and directly correlated with follicle-stimulating hormone (FSH). Cp was inversely correlated with prolactin and thyroid-stimulating hormone. Multiple regression analysis revealed that Cp correlates with both the level of luteinizing hormone (LH) and LH/FSH ratio, whereas serum Mg did not have a significant correlation with any of the clinical variables. Logistic regression analysis revealed elevated Cp, antral follicle count (AFC), body mass index (BMI), weight, and irregular menses increase the risk of developing PCOS, whereas Mg was not a risk factor. However, high LH and LH/FSH ratios were risk factors for hypomagnesemia. In conclusion, serum Cp levels in PCOS may be evaluated as an additional risk factor in association with AFC, BMI, weight, and irregular menses.
Conclusions:
Mg deficiency and high Cp play an important etiological role in PCOS pathogenesis. Thus, research evaluating dietary interventions and supplementation is warranted.
Keywords
Polycystic ovary syndrome, magnesium, ceruloplasminIntroduction
Polycystic ovarian syndrome (PCOS) is a heterogeneous disease condition in women defined by a combination of signs and symptoms of androgen excess and ovarian dysfunction [1]. It affects women in their reproductive age, with an estimated prevalence of 4–20% worldwide [2]. The disorder has a heterogeneous presentation ranging from infertility, irregular menstrual cycles, amenorrhea, hirsutism, alopecia, and insulin resistance (IR) [3]. Although the pathogenesis of the disease is complex and multifactorial, IR and hyperinsulinemia are implicated as major risk factors for PCOS development. It has become a serious health issue as major changes in physical appearance, obesity, and monthly irregular period have all been identified as major contributors to psychological distress, as well as a risk of suicidal tendency [4].
Magnesium (Mg) is an essential cofactor for enzymes involved in carbohydrate metabolism and glucose and insulin regulation [5]. Studies suggest that consumption of extra Mg reduces the risk of diabetes [6], dyslipidemia [7], cardiovascular risk [8], and systemic inflammation [9]. Mg is involved in the phosphorylation of tyrosine kinase (TK) and insulin receptor which modulates insulin sensitivity and glucose uptake by the cell [10]. Its deficiency can manifest IR and thereby hyperinsulinemia. PCOS is linked to the aforementioned problems and metabolic abnormalities, which are usually treated with insulin-lowering medications, anti-androgen therapy, and lifestyle modification. However, the involvement of Mg in the aetiology of PCOS has not been studied and required to explore because of its role in regulation of insulin sensitivity.
Ceruloplasmin (Cp) is a copper-containing glycoprotein mainly produced in the liver and serves a variety of functions, including antioxidant, ferroxidase, and copper transport [10–12]. In addition, Cp is an acute-phase protein, and its serum level rises as a result of enhanced messenger RNA (mRNA) transcribed in hepatocytes mediated by inflammatory cytokines during inflammation, infection, and trauma [13]. Ability of Cp to scavenge superoxide anion radicals helps in the reduction of oxidative stress and studies have demonstrated that Cp promotes neutrophil priming in obese individuals with localised severe periodontitis [14]. Accumulating data also showed that Cp is a useful biomarker for a variety of cardiovascular disorders and inflammatory diseases [15–17]. Previous meta-analyses have shown that patients with PCOS have a 2-fold higher risk of cardiovascular disease (CVD) than women without PCOS [18]. Following studies indicate the markers of CVD and IR are related to a higher body mass index (BMI) in patients with PCOS, raising the possibility that elevated levels of these factors are linked to an increased CVD risk in PCOS women [19]. Furthermore, a study by Kriseman et al. [20] indicated that BMI appeared to be substantially and inversely related with anti-Mullerian hormone (AMH) level in PCOS, contrary to a previous study by Cengiz et al. [21] that reported no variations in serum AMH levels between obese and non-obese teenage PCOS patients. This shows that the relationship between BMI & AMH in PCOS reproductive-aged women is unclear.
In this way the idea that obesity triggers a chronic, low-grade systemic inflammatory response in PCOS has gotten a lot of attention [22, 23]. There are no such studies on PCOS available and there is a lack of reports about the relationship between Cp and PCOS complication. Few studies have looked at the link between serum Cp level and obesity in diabetes mellitus [24, 25], obesity in CVD [26, 27], and metabolic syndrome [28, 29]. With this background, we hypothesised that there might be a role of Mg and Cp in the pathogenesis of PCOS as it is closely associated with IR, obesity, inflammation, and the risk for CVD. Thus, the study aimed to compare the serum Mg and Cp in PCOS and healthy women and correlate their levels with changes in clinical, biochemical, hormonal, and gynaecological aspects of PCOS.
Materials and methods
Subjects in the study group were recruited prospectively from an Assisted Reproductive Technology (ART) centre, Command Hospital Southern Command (CHSC), Pune, between 2019 and 2021, and were between the ages of 18 years old and 40 years old. PCOS was defined according to the Rotterdam criteria in 2003 [30]. Specifically, each woman with PCOS met at least two of the following criteria: 1) hirsutism, also known as hyperandrogenemia, 2) oligomenorrhea (8 cycles per year), and 3) polycystic ovaries on ultrasound (12 or more follicles 10 mm in diameter on each ovary or ovarian volume greater than 10 cm). Subjects who were meeting the inclusion criteria and signed informed consent were included in the study. Those with co-morbid endocrinopathies including untreated hypothyroidism, endometriosis, or previously established diabetes mellitus, age > 40 years, and confirmed malignancy were excluded from the study. For the study healthy control group, normoandrogenic women referred to the fertility clinic pertaining to tubal infertility or male infertility without PCOS, age-matched cases with both ovaries present, without morphological abnormalities, having a normal ovarian response, normal menstrual cycle, and with no signs of hyperandrogenism were included. The study received approval from the institutional review committee of the Armed Forces Medical College, Pune (IEC/2018/23).
The demographic data, including age, BMI, gynaecological data such as antral follicle count (AFC), number of menses per year, hormone profile, glucose profile, and biochemical analysis were done before in vitro fertilization (IVF).
Sample collection
Blood samples were obtained on the ovum pick up (OPU) day following an overnight fast. Blood samples were immediately processed, and serum Cp estimation was done by Somani Ambade colorimetric method [31] on a semi-autoanalyzer (Erba Chem 5X, India). Estimation of Mg was performed by the methylthymol blue method on the fully automated analyzer (Dimension EXL 200, Siemens Healthineers) in our National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited laboratory. According to the method used for estimation, the normal reference range for serum Cp was 500–1,000 U/L. A patient was considered hypomagnesemic if the total serum Mg level was 1.7 mg/dL. A fasting glucose level of more than 100 mg/dL was used to define as impaired fasting glucose [32].
Statistical analysis
GraphPad Prism 6.0 statistical software was used to perform all statistical analyses and RegressItPC Excel add-in was used for logistic regression analysis. The distribution of continuous data was assessed using the Shapiro-Wilk normality test. Student’s t test was used to compare means of normally distributed data. Mann-Whitney U test was used if the continuous data were not normally distributed. A simple linear regression was calculated to determine whether the independent variables such as age, BMI, AMH, fasting glucose, hemoglobin (Hb), erythrocyte sedimentation rate (ESR), follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and AFC could predict the continuous dependent variables, namely, Mg and Cp in serum. When three or more independent subgroups were analysed, one-way analysis of variance (ANOVA) was utilized. Spearman correlation was used to study the correlation between Mg and Cp with several biochemical parameters in PCOS. Multivariable linear regression analysis was performed to investigate associations between the Mg, Cp level and biochemical parameters in PCOS. Multivariable logistic regression analysis was applied to calculate the risk of PCOS. Results were expressed as mean ± standard deviation (SD) and 95% confidence interval (CI) if SD is too big. Statistical significance was assumed when P < 0.05.
Results
Biochemical characteristics of the study population
The study population was screened for serum total Mg, Cp level, and other clinical characteristics. The Table 1 shows the summary of their biochemical characteristics. There were 23.46% hypomagnesaemia in the PCOS group and 14.6% in the control group. The mean serum Mg levels in the PCOS and control groups were 1.8 (1.3–2.3) mg/dL and 1.9 (1.8–2.2) mg/dL, respectively (P < 0.03). High level of Cp is present in 74.4% of the total PCOS patients, and 40% of the healthy control, with a significant difference (P > 0.001) between PCOS and control (Figure 1). Compared with the normal Mg group, patients in the low Mg group had a significantly higher mean age (26.9 years) and AMH level (5.8). Differences in other parameters were not statistically significant (P > 0.05).
Descriptive analysis on anthropometric, biochemical, and gynaecological profiles between PCOS cases and controls
| Sl No. | Variable | Median (25–75% percentile) controln = 75 | Median (25–75% percentile) PCOSn = 98 | P value |
|---|---|---|---|---|
| 1 | Menarche (year) | 13 (12–15) | 13 (12–15) | 0.71 |
| 2 | Age (year) | 28 (26–31) | 28 (25–29) | 0.20 |
| 3 | BMI (kg/m2) | 24 (22–25) | 27 (24–30) | < 0.0001$ |
| 4 | PRL (ng/mL) | 11 (6.7–17) | 14 (8.8–23) | < 0.05* |
| 5 | AMH (ng/mL) | 1.8 (1.2–2.1) | 5.1 (3.8–8.6) | < 0.0001$ |
| 6 | FBS (mg/dL) | 90 (85–93) | 98 (81–105) | 0.031* |
| 7 | 2-h PG (mg/dL) | 110 (102–121) | 122 (107–145) | 0.0001$ |
| 8 | Hb (gm/dL) | 12 (12–13) | 12 (10–13) | 0.0003# |
| 9 | ESR (mm) | 20 (14–24) | 14 (10–20) | 0.07 |
| 10 | FSH (IU/L) | 5 (3.7–7) | 4.7 (3.5–6.2) | < 0.0001$ |
| 11 | LH (IU/L) | 4.2 (2.8–6.3) | 6.7 (5.1–8.3) | 0.05* |
| 12 | LH/FSH | 0.9 (0.5–1.5) | 1.5 (1–2.1) | < 0.0001$ |
| 13 | TSH (µIU/mL) | 2 (1.6–3.4) | 2.6 (1.9–3.8) | < 0.0001$ |
| 14 | Progesterone (mol/L) | 0.68 (0.42–1) | 0.84 (0.60–1.3) | 0.038* |
| 15 | Mg (mg/dL) | 1.9 (1.8–2.2) | 1.8 (1.3–2.3) | 0.03* |
| 16 | Cp (IU/L) | 1,140 (845–1,483) | 1,420 (945–1,810) | 0.0003# |
| 17 | AFC (n) | 7 (6.2–8.2) | 13 (11–17) | < 0.0001$ |
| 18 | No mense in a year | 13 (11–13) | 8.2 (6.3–10) | < 0.0001$ |
Statistical analysis was performed using the GraphPad Prism 6.0. The continuous variables are expressed as mean ± SD; the significant differences obtained in PCOS subjects and controls were calculated and compared using ANOVA. * P value < 0.05 was considered to be statistically significant. Values are expressed * P < 0.05, # P <0.01, and $ P < 0.001. 2-h PG: postload 2-hour glucose; FBS: fasting blood glucose; n: number; PRL: prolactin; Sl No.: serial number
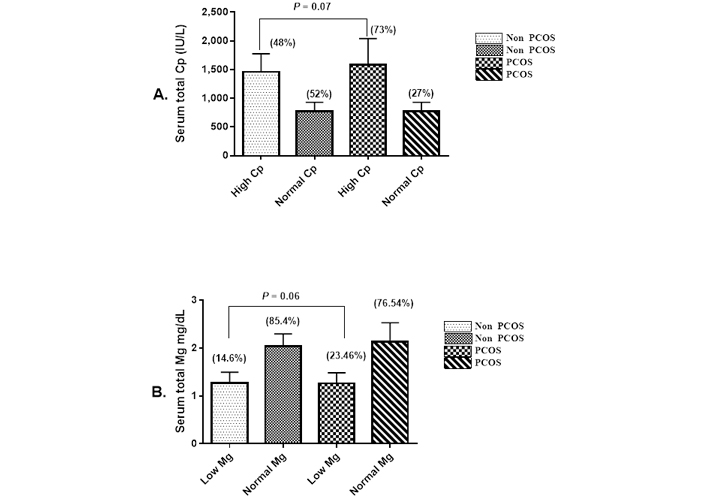
Frequency of hyperceruloplasminemia and hypomagnesaemia in subjects with and without PCOS. A. Expression of serum Cp from patients with PCOS relative to that level in the control; B. expression of serum Mg from patients with PCOS relative to that level in the control. Data are expressed as means ± SD, significance compared to control (one-way ANOVA P ≤ 0.05)
Correlation of serum Mg and Cp level with clinical variables
We performed correlation analysis between serum Mg and Cp levels and selected variables in order to ascertain any association between them. Serum Mg levels were found to be significantly and inversely correlated with FBS (r = –0.2250, P < 0.0259), 2-h PG (r = –0.2693, P < 0.0073), and positively correlated with FSH (r = 0.1992, P < 0.0493) (Figure 2); however, there is no significant correlation with other study variables (Table S1). We next performed correlation analysis between serum Cp level and selected variables. The study found serum Cp level significantly and inversely correlated with TSH (r = –0.2539, P < 0.0116) and PRL (r = –0.2341, P < 0.0204). In the case of non-PCOS healthy control, serum Mg level was negatively correlated only with progesterone (r = –0.3068, P < 0.0074) whereas Cp had no significant correlation with any of the study variables in the control group.
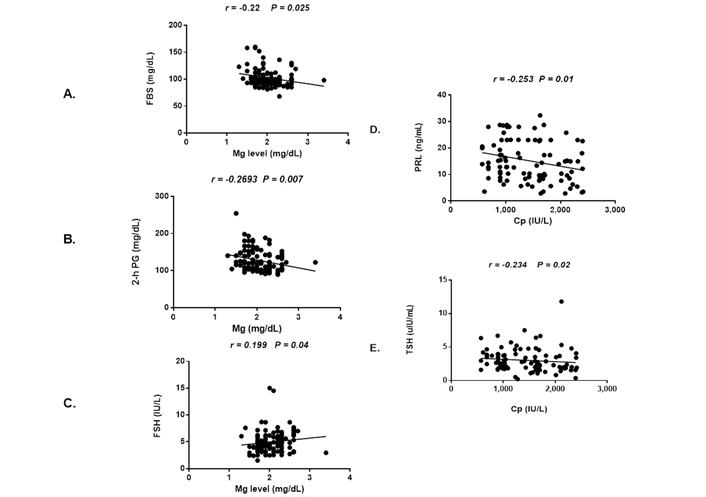
Univariate correlations between serum Mg level and fasting sugar, postprandial sugar, FSH (A, B, C) and univariate correlations between serum Cp level with PRL, TSH (D, E) and in PCOS women
Multiple linear regression studies
To estimate the relationship between independent and the relative expression of Mg and Cp (which is considered as the dependent variable), multiple linear regressions were performed. We observed a direct relationship between Cp and LH and the LH/FSH ratio (Table 2). Cp was positively associated with LH (P = 0.019) and the LH/FSH ratio (P = 0.04). Whereas no significant relationship between Mg with any of the reproductive and metabolic variables was observed (Table S2). Mg level was negatively associated with FBS after adjusting for age and BMI (P < 0.229) with no statistical significance.
Multiple linear regression analysis of serum Cp level with reproductive and biochemical variables in the PCOS subjects
| Variables | B | SE | P value | Lower 95% | Upper 95% |
|---|---|---|---|---|---|
| Menarche | 2.05 | 39.09 | 0.96 | –75.69 | 79.80 |
| Age | 5.68 | 12.28 | 0.65 | –18.75 | 30.10 |
| BMI | 1.55 | 13.47 | 0.91 | –25.24 | 28.34 |
| PRL | –11.30 | 7.54 | 0.14 | –26.30 | 3.71 |
| AMH | –20.59 | 17.07 | 0.23 | –54.54 | 13.36 |
| FBS | 4.26 | 5.20 | 0.41 | –6.08 | 14.61 |
| 2-h PG | –2.67 | 3.05 | 0.38 | –8.75 | 3.40 |
| Hb | –21.06 | 26.93 | 0.44 | –74.61 | 32.50 |
| ESR | –2.73 | 7.14 | 0.70 | –16.94 | 11.47 |
| FSH | 129.99 | 54.58 | 0.02 | 21.42 | 238.56 |
| LH | –64.87 | 42.70 | 0.13 | –149.80 | 20.07 |
| LH/FSH | 425.08 | 208.95 | 0.05 | 9.48 | 840.67 |
| TSH | –11.66 | 36.74 | 0.75 | –84.73 | 61.41 |
| Progesterone | –77.51 | 88.90 | 0.39 | –254.33 | 99.31 |
| Menses per year | 121.29 | 91.63 | 0.19 | –60.65 | 303.23 |
| AFC | –1.87 | 27.59 | 0.95 | –56.66 | 52.91 |
B: regression coefficient; SE: standard error of regression coefficient
Logistic regression analysis and risk predictors of PCOS
Logistic regression showed that PCOS outcomes were associated with high AFC [odds ratio (OR): 2.692] and low frequency of menses (OR: 0.313) and the level of Cp (OR: 1.001) after adjusting for age. Whereas serum Mg did not show any significant association with PCOS status (Table 3). BMI and weight were significantly associated with PCOS status (OR: 0.72, OR: 1.217, respectively) (Table 4).
Multivariate logistic regression analysis on PCOS association with reproductive characteristics, Cp, and Mg levels
| Variable | OR | SE | Lower 95% CI for OR | Upper 95% CI for OR | P value |
|---|---|---|---|---|---|
| AFC (n) | 2.692 | 0.213 | 0.770 | 1.211 | 0.0001 |
| Menses per year | 0.313 | 0.248 | –1.417 | –0.903 | 0.0001 |
| Cp | 1.01 | 0.09 | 0.001 | 0.001 | 0.048 |
| Mg | 1.29 | 0.92 | 0.257 | 0.257 | 0.780 |
Age and FBS levels were used as continuous variables while rest were used as categorical variables. Coding of categorical variables normal Mg level = 0, hypomagnesemia = 1
Multivariate logistic regression analysis on association between PCOS and its anthropometric variables
| Variable | OR | Lower 95% CI for OR | Upper 95% CI for OR | P value |
|---|---|---|---|---|
| Age | 0.934 | –0.112 | –0.025 | 0.105 |
| BMI | 0.724 | 0.389 | 0.255 | 0.0001 |
| PRL | 0.945 | –0.116 | 0.003 | 0.327 |
| Menarche | 0.950 | –0.167 | 0.065 | 0.647 |
| Weight | 1.217 | 0.146 | 0.247 | 0.0001 |
The variables were analysed using multivariate logistic regression with adjusted OR also with a 95% CI, and P value < 0.05 was considered statistically significant. Values are presented as OR coding of categorical variables—non PCOS = 0, PCOS=1
Logistic regression analysis and risk predictors of hypomagnesaemia
Next, the present study explored the possible independent risk factors that were associated with hypomagnesaemia in our studied PCOS subjects. Our result shows age (OR: 1.354), AMH (OR: 0.940), ESR (OR: 1.020), FBS (OR: 1.022), FSH (OR: 0.346), LH (OR: 1.589), PRL (OR: 1.002), PROG (OR: 1.178), and TSH (OR: 1.607). Those variables increase the odds of hypomagnesaemia in our PCOS patients. But the association was not significant except for age and TSH. In addition, we found subjects with hypomagnesemia had a significantly higher OR compared to those with normal Mg for glucose metabolism (OR: 1.022), abnormal menses (OR: 1.093), and AFC count (OR: 1.005). The odd of having high LH in hypomagnesemia is 1.58 and the odd of having low FSH in the same group is 0.34 with P = 0.05 (Table S3).
Logistic regression analysis and risk predictors of high Cp level
The present study also explored the possible independent risk factors that are associated with the high Cp level in PCOS subjects. A logistic regression analysis using dichotomized serum Cp level (normal and low) as the dependent variables identified OR: 1.234 for age, BMI (OR: 1.00), menarche (OR: 1.071), AMH (OR: 0.978), ESR (OR: 1.308), FF (OR: 1.038), FSH (OR: 0.907), progesterone (OR: 1.000), PRL (OR: 0.97), and TSH (OR: 1.308) increases the odds of high Cp in our PCOS patients. However, the association was significant (P < 0.05) only for FSH and progesterone [low OR: 0.907 (3.5–6.2) and OR: 1.00 (0.60–1.3)]. The odd of having high LH in individual with high level of Cp was 0.99 with P < 0.015 and the odd of having low FSH in the same group was 0.907 with P < 0.011. Whereas the odd of having high LH/FSH ratio was 0.911 with statistically significant P value 0.014 (Table S4).
Serum Mg and Cp level in PCOS subgroups
One-way ANOVA analysis revealed that Cp, AMH, and AFC levels were significantly different between groups (P < 0.0001), whereas Mg levels did not differ between the subgroups (P = 0.87) (Figure 3).
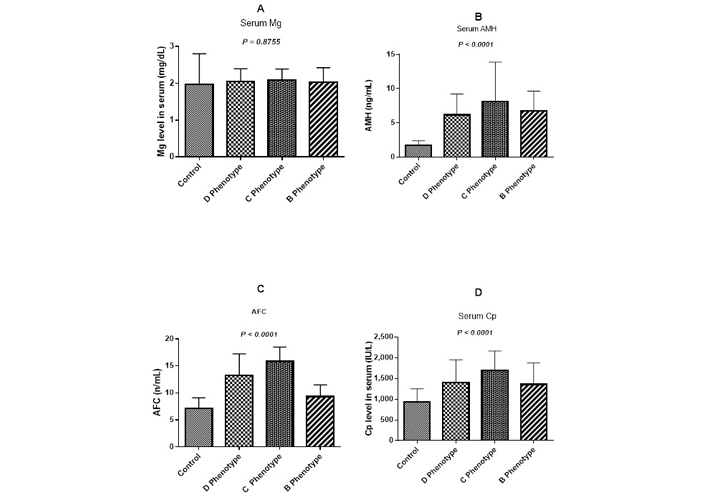
Total serum Mg (A), AMH (B), AFC (C), and Cp (D) levels in normal women and PCOS women with different phenotypes: irregular menses/polycystic ovaries morphology women (D phenotype), hyperandrogenism/polycystic ovaries morphology group (C phenotype), hyperandrogenism/irregular menses (B phenotype). SD bars are shown and P < 0.05 is considered statistically significant
Discussion
The present study reported the frequency of hypomagnesaemia and high serum Cp level and associated risk factors in PCOS women referred to the ART center, CHSC, Pune. We found that 23.46% of our PCOS patients had hypomagnesemia compared to 14.6% in healthy controls. Since hypomagnesemia is linked to IR and hyperinsulinemia [33, 34], type 2 diabetes mellitus [34, 35], and metabolic syndrome [36]. Low serum Mg level in the case of PCOS are thought to cause new or worsening IR by reducing insulin secretion which results in alteration of cellular glucose transport [37]. Our results also showed that lower serum Mg was associated with PCOS. In addition, in correlation test it was seen demonstrated that Mg was negatively associated with FBS and postprandial supporting the previous studies which have implicated Mg deficiency could result in post-receptor IR, decreased cell glucose utilization, and hyperinsulinemia [35, 38].
Our study also indicates serum Mg was directly correlated with FSH (P < 0.04), Mg increases binding of FSH to membrane-bound receptors and increases adenylyl cyclase activity aiding in follicular maturation [39]. It clearly implies that changes in Mg levels have a detrimental impact on FSH levels, resulting in follicular maturation arrest, as seen in PCOS. In the present studied PCOS group, BMI exhibited a relatively higher range when compared to the control group. Overweight individual made up 54% of the population, while lean people made up 3%. We observed an additive effect of BMI and weight which are significantly increased in PCOS group. This effect was mirrored by the additive effect of BMI and weight on serum levels of LH, AFC, and irregular menstrual cycle in PCOS patients. We did observe independent effect of high BMI and weight on the outcome of PCOS with OR of 0.724 and 1.217 consistent with other studies [40–42].
A rise in BMI could influence changes in the biochemical and hormonal systems. Any disturbances in the menstrual cycle in turn disturb the hormonal mechanisms and add to female infertility issues [43]. In the present study, irregular menstrual cycle was observed in 86% of PCOS women and its significance impact was confirmed in the multivariate logistic regression analysis where it stands as risk in PCOS. We also found that the AFC was significantly higher in women with PCOS which led to 2.69 odds times of risk of developing PCOS consistent with other studies [41, 44]. The results clearly justified the feature of PCOS which includes anovulation and rise in AFC resulting in aberrant folliculogenesis [45]. Whereas, though we found decrease in serum Mg level in PCOS women compared to controls, when trying to ascertain an independent risk factor for PCOS, Mg was observed not a risk factor for the disease and thus, it should not be considered as contributing factors for development of PCOS. However, our present study findings indicating age and TSH lead to 1.354 and 1.607-odds times risk of developing hypomagnesemia, whereas decreased FSH and increased LH lead to 0.346 and 1.58-odds times risk of hypomagnesemia.
The augmented Cp level is considered to have high ferroxidase activity and capacity to trap other free radicals as per literature, because of this it acts as an antioxidant and elevated level observed in metabolic syndrome and inflammation [46, 47]. It could be considered in our current study that elevated Cp levels in the PCOS patients indicate either more efficient antioxidant protection or greater oxidative stress, which supports the previous study by Mehde et al. [48]. Furthermore, because our PCOS group’s Cp levels were higher than the controls, we looked into PCOS risk and Cp connection with other clinical factors. The current study result indicates, rise in Cp level was a risk of higher LH/FSH ratio, LH (Table S4), and Cp was positively linked with lower TSH and PRL (Table S1). The combination of the LH/FSH ratio and the LH level has previously been found to have a greater predictive value in diagnosing PCOS [49]. Elevated LH levels stimulate testosterone production by ovaries resulting in disruption in the follicle maturation process, which hampers ovulation [50–52]. Excess androgens increase IR, resulting in elevated insulin levels, which drive androgen production. This worsens PCOS symptoms, making women more prone to diabetes, obesity, and CVD [53, 54]. In the current study, high serum Cp levels were found in 74.4% of women with PCOS, while hyperandrogenism was found in 46.9% of the women. Women with phenotype C which is comprised of hyperandrogenism and polycystic ovaries as per Rotterdam criteria 2003 show significant increase in Cp level compared to B & D phenotypes (Figure 3D), whereas there was no significant different in the level of Mg in all the three studied phenotypes (Figure 3A). The significance was further validated in a multivariate logistic regression analysis, where Cp high level was identified as a risk factor for PCOS.
Next we observed the progesterone of patient with high Cp was associated with an unadjusted OR of 2.112 (IC: 0.022 to 1.472; P = 0.043) and the FSH of patient with high Cp was associated with an unadjusted OR of 0.014 (IC: –7.312 to –1.287, P = 0.005), which clearly implicates progesterone and FSH were risk factors for high Cp, which was supported by a previous study suggesting Cp may be high in states of high oestrogen and progesterone could be considered as major contributing factors for high Cp [48, 55]. Additional studies also specify that an unusual Cp profile could be a sign of abnormal embryonic development [56, 57].
In our study, there was a negative correlation between Cp and TSH, and between Cp and PRL (Figure 2D and 2E). Increased Cp level was reported in hyperthyroid patients in earlier study [58], and a negative correlation between Cp and TSH in our studied cohorts possibly reflects their involvement in the active regulation of metabolic processes and the anti-inflammatory cascade. There is, however, no single established cause that could explain the Cp level correlation with PRL and the need to ascertain it in the future. Although the reason for these discrepancies is unclear, we assumed that, compared with PRL, elevated Cp might reflect acute subclinical inflammation more sensitively or play a more important role in the progression of PCOS. However, the links between these variables and the mechanisms through which they contribute to PCOS pathogenesis are unknown, and more research is needed for further elucidation.
Main strength of the study was that participants were recruited from all over India and were representative of Indian women with PCOS. The limitation of the present study was that the sample size was not calculated for the association between serum Mg and Cp with FBS, metabolic traits, and reproductive traits in PCOS women. Second, we did not use the insulin level to evaluate IR. Moreover, we also could not study the influence of diet and dietary supplements on serum Mg as the recording of daily intake of Mg was not feasible during this study.
Our study clearly indicates serum Mg and Cp levels differ significantly between PCOS and healthy control and are correlated with metabolic and reproductive traits in PCOS. Rise in AFC, BMI, weight, and irregular menses increases the risk of developing PCOS. The clinical implication of our study is that Mg deficiency and high Cp play an important etiological role in PCOS pathogenesis. Elevated serum Cp levels can be considered an additional risk factor for PCOS, whereas Mg, although lower in PCOS women compared with controls, is a risk factor for PCOS. This suggests that women with PCOS are likely to be deficient in high-Mg foods and likely to have low serum Mg levels. Higher FSH has also been shown to increase the risk of hypomagnesemia and elevated Cp in PCOS. Thus, high level of serum Cp in PCOS should be evaluated as an additional risk factor in association with AFC, BMI, weight, and irregular menses, with targeting oxidative stress for therapy. More prospective, large-scale studies are needed to validate whether Mg supplementation can also help reduce the severity of PCOS or even prevent the onset of PCOS. Thus, research evaluating dietary interventions and supplementation is warranted.
Abbreviations
| 2-h PG: |
postload 2-hour glucose |
| AFC: |
antral follicle count |
| AMH: |
anti-Mullerian hormone |
| ANOVA: |
analysis of variance |
| BMI: |
body mass index |
| CI: |
confidence interval |
| Cp: |
ceruloplasmin |
| CVD: |
cardiovascular disease |
| ESR: |
erythrocyte sedimentation rate |
| FBS: |
fasting blood glucose |
| FSH: |
follicle-stimulating hormone |
| Hb: |
hemoglobin |
| IR: |
insulin resistance |
| LH: |
luteinizing hormone |
| Mg: |
magnesium |
| OR: |
odds ratio |
| PCOS: |
polycystic ovarian syndrome |
| PRL: |
prolactin |
| SD: |
standard deviation |
| TSH: |
thyroid-stimulating hormone |
Supplementary materials
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001140_sup_1.pdf.
Declarations
Author contributions
PM: Supervision, Writing—review & editing. RG and SR: Data curation. NN: Investigation. SMK: Writing—review & editing. YV: Conceptualization, Writing—original draft, Validation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. The study was approved by the institutional review committee of the Armed Forces Medical College, Pune reference number IEC/2018/23.
Consent to participate
Informed consent to participate in the study was obtained from all participants.
Consent to publication
Informed consent to publication was obtained from relevant participants.
Availability of data and materials
The data that support the findings of this study will be shared upon reasonable request data policy from the corresponding author.
Funding
Not applicable.
Copyright
© The Author(s) 2023.