Affiliation:
1Santé Cannabis, Montréal, QC H3Z 2Y5, Canada
Email: lrapin@santecannabis.ca
ORCID: https://orcid.org/0000-0003-3603-4168
Affiliation:
1Santé Cannabis, Montréal, QC H3Z 2Y5, Canada
ORCID: https://orcid.org/0000-0002-8043-9915
Affiliation:
1Santé Cannabis, Montréal, QC H3Z 2Y5, Canada
ORCID: https://orcid.org/0000-0001-7227-8456
Affiliation:
1Santé Cannabis, Montréal, QC H3Z 2Y5, Canada
2Faculty of Medicine, University of Sherbrooke, Sherbrooke, QC J1H 5N4, Canada
Affiliation:
1Santé Cannabis, Montréal, QC H3Z 2Y5, Canada
3Faculty of Medicine, McGill University, Montreal, QC H3G 2M1, Canada
Explor Med. 2023;4:363–379 DOl: https://doi.org/10.37349/emed.2023.00148
Received: September 27, 2022 Accepted: February 25, 2023 Published: June 30, 2023
Academic Editor: Staci Gruber, Harvard Medical School, USA
The article belongs to the special issue Beyond Weed: Clinical Applications of Cannabis and Cannabinoids
Aim: Among treatments for chronic non-cancer pain (CNCP), cannabinoid-based medicines (CBMs) have become extremely popular. Evidence remains modest and limited primarily to delta-9-tetrahydrocannabinol (THC) for neuropathic pain; nevertheless, the use of various CBMs, including cannabidiol (CBD) to treat neuropathic, nociceptive, and mixed pain has increased globally. This observational case-series assessed the impact of CBMs as a complementary treatment by pain mechanism and cannabinoid profile over three months.
Methods: An analysis of patients with CNCP and treated with CBMs who consented to an ongoing registry was performed. Outcomes were patient-reported such as the Edmonton Symptom Assessment System-Revised, Brief Pain Inventory-Short Form, and 36-Item Short Form Health Survey. Data from patients with complete outcomes for baseline and 3-month follow-up was extracted. Characteristics of adverse drug reactions (ADRs), including a description of the suspected product were also assessed.
Results: A total of 495 patients were part of this analysis (mean age = 56 years old; 67% women). At 3-month, the proportional use of THC:CBD balanced and THC-dominant products increased. Patients with neuropathic pain had higher pain-severity scores vs. nociceptive pain. In addition to patients with neuropathic pain, patients with nociceptive and mixed pain also reported improvements in pain severity and secondary symptoms such as anxiety, depression, drowsiness, fatigue, sleep disturbances, and overall, health-related quality of life. THC-dominant treatment is more likely to be recommended when pain is severe, whereas CBD-dominant is favored for less severe cases. ADRs were more frequent among cannabis-naive patients and included dizziness, headache, and somnolence among others.
Conclusions: Findings suggest that CBMs can be effective for neuropathic as well as nociceptive and mixed pain. THC is more frequently recommended for neuropathic and severe pain. Future research on CBMs in pain management must include details of CBM composition, and pain mechanism and must consider potential ADRs.
Chronic pain is recognized as an enormous and costly global public health problem. An estimated 1 in 10 adults are diagnosed with chronic pain each year but published population prevalence estimates have been highly variable [1]. In Canada, which the analyzed patient data comes from, the estimated prevalence of chronic pain in individuals over 15 years old is 25% [2].
Pain classification is crucial to determine the most appropriate treatment; this is done by establishing: 1) pain duration (acute or chronic), 2) cause (cancer or non-cancer) and 3) pain mechanism (nociceptive, neuropathic, recently described nociplastic pain and mixed pain) [2]. Chronic non-cancer pain (CNCP) is usually defined as persistent or recurrent pain that lasts longer than three months, causes functional disability and emotional distress, and is not associated with malignant disease [3].
CNCP is difficult to control and frequently requires a multidisciplinary approach and multimodal analgesia. Among pharmacological treatments, opioids are generally considered despite significant notable safety concerns and risk of opioid use disorder [4–6], driving interest in alternative treatment options [3]. Cannabinoid-based medicines (CBMs) may offer a complementary therapeutic option to control refractory pain and associated symptoms, due to their potential analgesic effects [7–11]. Reviews on preclinical studies indicate the antinociceptive effects of delta-9-tetrahydrocannabinol (THC) in various animal pain models and chronic inflammatory models [12, 13]. Although clinical data regarding cannabidiol (CBD) analgesic properties in humans is still very limited, there is preclinical evidence of CBD elicit anti-inflammatory and antinociceptive effects [5, 12].
Chronic pain is the most commonly cited reason for using CBMs in humans, with numerous cohort studies indicating the potential benefits of CBMs for chronic pain management [8, 14–17] although these findings were not always replicated [18]. Furthermore, evidence from randomized clinical trials (RCTs) remains mainly limited to derivatives of cannabis, such as nabiximols, and is inconsistent as shown in numerous systematic reviews and meta-analyses [19–23]. Importantly, most studies and reviews consider only patients with chronic neuropathic pain, and other mechanisms of pain are under-studied, thus limiting the generalization of results [19, 20, 23–26]. During clinical assessment, identification of pain mechanisms may guide the most appropriate management, however, it is rarely considered with respect to CBMs. The effectiveness of CBM to treat different types of CNCP conditions thus remains debatable and requires further investigation.
When indicated and used under clinical monitoring and close supervision by well-trained healthcare practitioners, CBMs are generally considered safe and, in certain cases, have demonstrated a preferable safety profile compared to conventional pharmacological treatments used to treat pain [6, 27, 28].
Medical cannabis products are regulated in Canada by the Cannabis Act [29] and are categorized according to their cannabinoid profile, generalized as the relative concentration of THC and CBD in the product. Treatment recommendations are based on the method of administration and cannabinoid profile, combined with the desire to minimize the risk of adverse effects (AEs). Yet, most studies do not differentiate the cannabinoid profiles and their relative effectiveness to treat CNCP.
With such limited evidence and a lack of clinical guidelines, it is still unclear how clinicians should develop treatment recommendations, often defaulting to initiation with CBD-dominant products and a slow introduction of THC [30]. The extent to which pain etiology and mechanism should be considered in CBMs treatment recommendations remains unknown.
This paper aims to describe the use of CBMs in patients with CNCP on pain severity and pain-related interference with daily activities, associated symptoms intensity, and health-related quality of life (HRQoL), according to treatment cannabinoid profile and pain mechanism.
This study is an uncontrolled case series of patients enrolled in the clinic registry. The clinic registry, set up in July 2020 and still ongoing, captures longitudinal data of consenting adult patients who are prescribed CBM at the clinic.
All participants voluntarily and formally consented to the registry. Participants did not receive any compensation and enrollment in the study did not affect their clinical care.
Inclusion criteria for the registry are being over 18 years old, receiving CBMs, and being able to complete questionnaires in either French or English. Exclusion criteria include current pregnancy or lactation, and current psychotic disorder. Due to the coronavirus disease 2019 (COVID-19) pandemic, all visits were conducted by telemedicine. Eligibility for CBMs and the corresponding treatment plan is determined at the discretion of the clinic physicians based on individualized clinic assessment [31]. Patients coming to the clinic are predominantly referred by their family physician or specialist after not responding to conventional pain management. CBMs are added to the patient’s current regime of prescription medications as a complementary treatment.
Demographics, complete medical history (primary diagnosis according to the International Classification of Diseases-Tenth Revision), primary symptoms, secondary symptoms, concomitant medications, and previous cannabis use (regularly is defined as every day; occasionally as several times during a month) are collected at baseline. All recorded outcomes and adverse drug reactions (ADRs) questionnaires are patient-reported and are administered during routine clinical care visits at baseline, 3 months, 6 months, and 12 months.
For this analysis, registry data collected from July 14th, 2020 to July 17th, 2021 for patients with a primary symptom of CNCP, who were authorized to receive CBMs, and had complete recorded questionnaires at both baseline and follow-up 3 months (FUP3M) were extracted from electronic medical records (EMR). Pain mechanism was clinically assessed (e.g., pain description and characteristics, perceptual qualities of pain, sensory intensity, temporal features, findings in physical exam that includes location and bodily distribution of pain, relationship with the primary diagnosis, etc.) during the initial visit by the clinic physicians and verified during the database review by a pain specialist.
Questionnaires included the Edmonton Symptom Assessment System-Revised (ESAS-r), the Brief Pain Inventory-Short Form (BPI-SF), and the 36-Item Short Form Health Survey (SF-36). The ESAS-r is a self-administered scale, which rates the severity of physical (pain, tiredness, nausea, drowsiness, lack of appetite, and shortness of breath), emotional (depression, anxiety) symptoms and overall well-being from 0 (absence of symptom) to 10 (worst possible severity) at the time of assessment [32]. The BPI-SF is a self-administered scale, rating different aspects of pain severity from 0 (no pain) to 10 (pain as bad as you can imagine), as well as how pain interferes with seven different activities (0 = no interference, 10 = completely interferes) [33]. The SF-36 is a 36-item survey assessing physical and mental HRQoL (0 = very poor, 100 = excellent) [34].
ADRs were either self-reported or reported during routine clinic visits and included information on ADR terms, severity, causality, outcome, and seriousness.
CBM information included type of CBM (medical cannabis and/or pharmaceutical cannabinoids), method of administration (oral extracts vs. inhaled dried flower vs. other such as topical and edibles), and cannabinoid profiles (CBD-dominant, THC-dominant or THC:CBD balanced).
The product selection based on the cannabinoid profile and the methods of administration is displayed in Figure 1.
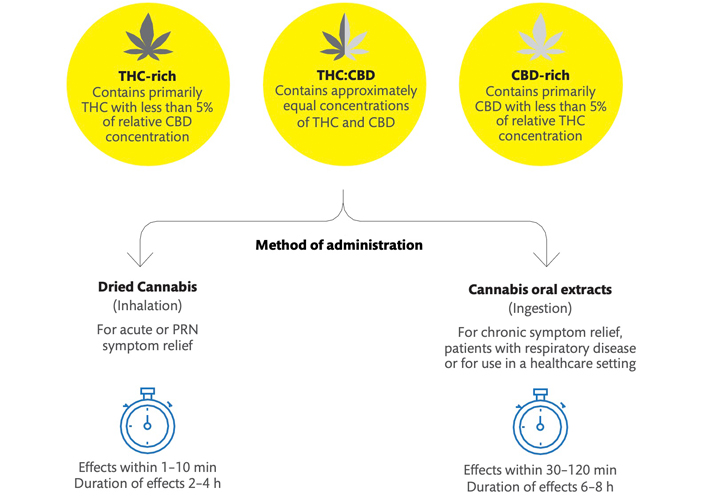
Product selection, methods of administration with respective time to effect, and duration of effect characteristics. PRN: pro re nata (as needed)
Pharmaceutical cannabinoids are approved medications containing synthetic or plant-derived cannabinoids; in Canada, nabilone and nabiximols are available. Medical cannabis products are plant-derived cannabinoids that have been legalized and regulated for medical use but are yet considered unapproved treatments [29]. Medical cannabis products include dried flowers and concentrates for inhalation, oral formats such as oils, sprays, and edibles as well as topical and suppository products. Multiple formulations of each product format are available in Canada, with distinct concentrations of THC and CBD. Products may also contain very limited concentrations of other cannabinoids, terpenes, and flavonoids.
CBD-dominant products contain a high concentration of CBD and are limited in THC concentration. In the Canadian medical cannabis program, CBD-dominant ingested cannabis oils contain approximately 0.5–1 mg/mL of THC and 20–25 mg/mL of CBD. CBD may be extracted from both hemp plants and other varieties of cannabis plants and products must be manufactured in accordance with the Cannabis Act [29]. THC-dominant products contain a high concentration of THC and limited CBD. THC-dominant oral cannabis oils generally contain 20–25 mg/mL of THC and 0.5–1 mg/mL of CBD. The category THC:CBD balanced indicates that the treatment plan includes both THC and CBD in significant concentration, which may be a product with a similar concentration of each cannabinoid (for example 10 mg/mL of THC and 10 mg/mL of CBD) or a personalized combination of CBD-dominant, THC-dominant, and THC:CBD products. For participants taking multiple cannabis products, the cannabinoid profile was determined by the overall THC:CBD concentration of all products. The delineation of the composition of each product and dosing schedule requires more investigation, which is beyond the focus of this study.
Quantitative data was summarized using mean and range, and qualitative data using frequency and proportion. Separate linear mixed effect models were used to assess the clinical impact of CBMs between baseline and 3 months on core outcomes (scores of each symptom for ESAS-r, pain severity and pain-related interference respective scores for the BPI-SF and physical health and mental health respective scores on the SF-36). For each model (total = 14), the participant was a random intercept and time, pain mechanism (nociceptive, neuropathic, mixed, undefined), and initial treatment cannabinoid profile (CBD-dominant, THC-dominant, THC:CBD balanced) were modeled as fixed factors. Questionnaires completed at the 3-month visit assess the self-reported effectiveness of initial visit treatment recommendations. Based on these responses, treatment adjustments or additional recommendations were proposed during the 3-month visit. Therefore, the initial treatment cannabinoid profile was assessed in the analyses. Statistical significance was set at P = 0.01 to account for potential type-I errors associated with testing multiple models and interactions terms were dropped if non-significant. The association between the presence of ADRs and socio-demographic variables was assessed with Chi-squares tests (χ2). All statistical analyses were performed using IBM Statistical Package for Social Sciences (SPSS) software [35].
Data extraction of registry patients with CNCP and complete outcome baseline and FUP3M data resulted in 495 patients [mean age = 56.26 years old, standard deviation (SD) = 15.72, range = 20–92; 67.1% women]. Chronic pain syndromes were the most prevalent pain-related medical conditions (33.1%) followed by chronic spine disorders (23.2%) and rheumatic disorders (20.4%). Neuropathic pain was reported in 43.6% of patients and nociceptive pain in 41%. Outlines demographics, primary diagnoses, and pain mechanism distribution are reported in Table 1.
Demographics, diagnoses, and pain mechanism information
| Variable | Data [N (%)] |
|---|---|
| Sex | |
| Female | 332 (67.1) |
| Male | 163 (32.9) |
| Occupational status | |
| Retired | 179 (36.2) |
| Employed full-time | 111 (22.4) |
| Long-term disability | 103 (20.8) |
| Employed part-time | 32 (6.5) |
| Unemployed | 27 (5.5) |
| Short-term disability | 22 (4.4) |
| Student | 11 (2.2) |
| Not reported | 10 (2.0) |
| Marital status | |
| Married or living with a partner | 280 (56.6) |
| Single | 113 (22.8) |
| Divorced or separated | 62 (12.5) |
| Widowed | 32 (6.5) |
| Not reported | 8 (1.6) |
| Annual income | |
| Under $20,000 CAD | 115 (23.3) |
| $20,000–$49,999 CAD | 210 (42.4) |
| $50,000–$99,999 CAD | 119 (24.1) |
| 100,000 CAD$ and more | 15 (3) |
| Not reported | 36 (7.3) |
| Education level | |
| Less than high school | 48 (9.7) |
| Highschool diploma | 102 (20.6) |
| Post-high school education | 332 (67.1) |
| Not reported | 13 (2.6) |
| Previous cannabis use | |
| Never tried before | 119 (24) |
| Self-attempted medical treatments | 196 (39.6) |
| Medical and non-medical (“recreational”) | 74 (14.9) |
| Non-medical | 106 (21.4) |
| Primary diagnosis | |
| Chronic pain syndromes | 164 (33.1) |
| Fibromyalgia | 102 (20.6) |
| Unspecified chronic pain syndromes | 62 (12.5) |
| Chronic spine disorders | 115 (23.2) |
| Low back pain | 49 (9.9) |
| Degenerative disc disorders | 21 (4.2) |
| Spinal stenosis | 13 (2.6) |
| Cervicalgia | 10 (2) |
| Other spine disorders (lumbar radiculopathy, spondylolisthesis, dorsopathies, etc.) | 22 (4.4) |
| Rheumatic disorders | 101 (20.4) |
| Osteoarthritis | 52 (10.5) |
| Spondylosis and spondylopathies | 14 (2.8) |
| Rheumatoid arthritis | 12 (2.4) |
| Other arthritis (including polyarthritis) | 10 (2) |
| Other rheumatic disorders | 13 (2.6) |
| Trauma-related chronic pain | 48 (9.7) |
| Work related accident | 16 (3.2) |
| Post motor vehicle accident | 12 (2.4) |
| Complex regional pain syndrome | 6 (1.2) |
| Unspecified post traumatic chronic pain | 12 (2.4) |
| Neurological disorders | 28 (5.6) |
| Multiple sclerosis | 11 (2.2) |
| Other neurological disorders | 17 (3.4) |
| Chronic musculoskeletal pain disorders | 21 (4.2) |
| Soft tissues disorders | 9 (1.8) |
| Systemic connective tissue disorders | 5 (1) |
| Osteomyelitis and other osteopathies | 4 (0.8) |
| Tendinitis and synovitis | 4 (0.8) |
| Other musculoskeletal pain disorders | 3 (0.6) |
| Gastrointestinal disorders | 8 (1.6) |
| Crohn’s disease | 3 (0.6) |
| Diverticular disease of the intestine | 2 (0.4) |
| Other gastrointestinal disorders | 3 (0.6) |
| Other disorders | 10 (2) |
| Other uncategorized disorders | 5 (1) |
| Mental disorders | 3 (0.6) |
| Infectious and parasitic diseases | 2 (0.4) |
| Pain mechanism | |
| Neuropathic | 216 (43.6) |
| Nociceptive | 203 (41) |
| Mixed | 58 (11.7) |
| Undefined | 18 (3.6) |
The age range of the statistics objects is 20–92, and the mean is 56.26. N (%): the number of people under current conditions (percentage of people under current conditions)
Patients can report multiple secondary symptoms; 471 patients (95.2%) reported at least one secondary symptom. Most patients reported sleep disturbances (87.1%), 64% fatigue, 55.4% anxiety, and 32.9% depression.
At baseline, patients were taking 8.4 medications on average (SD = 4.86, range = 0–33). Only 6 patients were not taking any conventional medication. Antidepressants were used by 72% of patients. Over half of patients were currently using non-steroidal anti-inflammatory drug (54%), opioids (52%), or other analgesics (acetaminophen, gabapentinoids, other anticonvulsants, baclofen; 51%).
About three-quarters (76%) of patients had tried cannabis (dried flower and/or oral oil) in the past, and almost 40% for self-attempted medical treatments. About a quarter of participants (119, 24%) participants had no previous exposition to CBM before getting recommended at least one product during their initial visit. Current regular use of oral oil was reported by 77 patients (15.6%), 7.5% occasionally. Current regular use of dried cannabis was reported by 85 patients (17.2%), and 5.3% occasionally.
The frequency of the type of CBMs, the cannabinoid profile and the formulation characteristics per visit are displayed in Table 2. At baseline, a large majority (73.9%) of patients were authorized exclusively medical cannabis products. Overall, oral oil extracts were the preferred formulation (81.6%) followed by a combination of oral oil extracts and dried flower (14.5%).
Type of CBM and cannabinoid profile for each time-point
| CBM information | Baseline [N (%)] | 3-Month [N (%)] |
|---|---|---|
| Treatment was discontinued at 3 month | 0 | 11 (2.2) |
| Type of CBM | ||
| Medical cannabis (plant-derived) | 366 (73.9) | 363 (75) |
| Pharmaceutical cannabinoids (all nabilone) | 2 (0.4) | 6 (1.2) |
| Medical + pharmaceutical | 127 (25.7) | 115 (23.8) |
| Cannabinoid profile | ||
| CBD-dominant | 246 (49.7) | 174 (35.2) |
| THC:CBD balanced | 247 (49.9) | 293 (59.2) |
| THC-dominant | 2 (0.4) | 17 (3.4) |
| Formulation characteristics of medical cannabis | ||
| Ingested oil products | 404 (81.6) | 382 (77.2) |
| Dried flower products | 4 (0.8) | 7 (1.4) |
| Ingested oil and dried flower products | 72 (14.5) | 71 (14.3) |
| Other combinations of formulations (topical, edible, oil, dried) | 15 (3) | 35 (7.1) |
The total sample size for baseline is 495 and 484 for 3-month (11 participants discontinued treatment at 3-month). N (%): the number of people under current conditions (percentage of people under current conditions)
The proportion of each cannabinoid profile differed according to the visit [χ2(3) = 39.10, P < 0.001], CBD-dominant products proportion decreased at the FUP3M whereas THC:CBD and THC-dominant proportion products increased. There is no association between pain mechanism and baseline treatment recommendations [χ2(6) = 1.32, P < 0.97] which indicates that pain mechanism is not a primary consideration in treatment recommendations relative to other factors, such as pain severity and previous cannabis use.
Mean differences and 95% confidence interval (CI) Bonferroni-adjusted for multiple post hoc pairwise comparisons for all outcomes measures (total = 14) are reported in Table 3.
Mean difference and 95% CI Bonferroni-adjusted for multiple comparisons from pairwise comparisons for all outcomes measures
| Pairwise comparisons (mean differences, CI)a | Pain mechanism | Baseline cannabinoid profile | Visit | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neuropathic vs. nociceptive | Neuropathic vs. mixedb | Neuropathic vs. undefinedb | Nociceptive vs. mixedb | Nociceptive vs. undefinedb | Mixed vs. undefinedb | CBD-dominant vs. THC:CBD | CBD-dominant vs. THC-dominantc | THC:CBD vs. THC-dominantc | Baseline vs. FUP3M | |
| BPI-SF pain severity | 0.49 (0.15; 0.82)* | –0.06 (–0.57; 0.44) | 0.24 (–0.6; 1.08) | –0.55 (–1.06; –0.04) | –0.25 (–1.09; 0.59) | 0.30 (–0.62; 1.23) | –0.33 (–0.61; –0.05) | –0.24 (–2.44; 1.97) | 0.09 (–2.11; 2.30) | 0.79 (0.56; 1.02)* |
| BPI-SF pain-related interference | 0.44 (0.04; 0.84) | 0.05 (–0.55; 0.65) | 0.07 (–0.93; 1.07) | –0.39 (–1.00; 0.21) | –0.37 (–1.37; 0.63) | 0.03 (–1.07; 1.12) | 0.42 (–0.75; –0.09)* | –0.55 (–3.17; 2.07) | –0.13 (–2.75; 2.49) | 1.46 (1.19; 1.73)* |
| ESAS-r pain | 0.43 (0.06; 0.81)* | –0.23 (–0.80; 0.33) | 0.15 (–0.79; 1.10) | –0.67 (–1.24; –0.10)* | –0.28 (–1.22; 0.67) | 0.39 (–0.65; 1.43) | –0.29 (–0.61; 0.02) | –0.27 (–2.75; 2.20) | 0.02 (–2.45; 2.49) | 1.02 (0.76; 1.27)* |
| ESAS-r anxiety | 0.30 (–0.25; 0.86) | 0.31 (–0.54; 1.15) | –0.30 (–1.70; 1.09) | 0.00 (–0.85; 0.85) | –0.61 (–2.01; 0.79) | –0.61 (–2.15; 0.93) | –0.49 (–0.96; –0.03) | 0.99 (–2.67; 4.66) | 1.48 (–2.18; 5.15) | 0.70 (0.32; 1.08)* |
| ESAS-r depression | 0.27 (–0.25; 0.79) | 0.05 (–0.55; 0.65) | 0.36 (–0.96; 1.69) | –0.21 (–1.01; 0.58) | 0.1 (–1.23; 1.43) | 0.31 (–1.14; 1.76) | –0.56 (–1.00; –0.12)* | 0.02 (–3.41; 3.45) | 0.58 (–2.85; 4.01) | 0.62 (0.26; 0.98)* |
| ESAS-r fatigue | 0.79 (0.33; 1.25)* | 0.01 (–0.68; 0.70) | 0.07 (–1.08; 1.22) | –0.78 (–1.48; –0.08)* | –0.72 (–1.87; 0.43) | 0.06 (–1.2; 1.33) | –0.17 (–0.56; 0.21) | 2.38 (–0.64; 5.40) | 2.55 (–0.46; 5.57) | 0.93 (0.61; 1.24)* |
| ESAS-r drowsiness | 0.77 (0.22; 1.31)* | 0.66 (–0.16; 1.49) | 0.10 (–1.19; 1.57) | –0.10 (–0.93; 0.72) | –0.57 (–1.96; 0.81) | –0.47 (–1.98; 1.04) | –0.21 (–0.67; 0.24) | 2.29 (–1.28; 5.86) | 2.50 (–1.07; 6.073) | 0.69 (0.32; 1.06)* |
| ESAS-r sleep | 1.71 (–1.01; 4.43) | 0.40 (–1.67; 2.46) | –0.74 (–3.1; 1.61) | –1.32 (–3.38; 0.75) | –2.46 (–4.81; –0.10) | –1.14 (–2.69; 0.41) | –0.45 (–1.20; 0.30) | 1.71 (–1.99; 5.42) | 2.16 (–1.55; 5.87) | 1.60 (1.22; 1.98)* |
| ESAS-r nausea | 0.31 (–0.09; 0.71) | 0.10 (–0.5; 0.71) | 0.20 (–0.80; 1.20) | –0.20 (–0.81; 0.40) | –0.11 (–1.11; 0.90) | 0.10 (–1.01; 1.20) | –0.26 (–0.59; 0.08) | 0.51 (–2.12; 3.15) | 0.77 (–1.86; 3.40) | 0.11 (–0.16; 0.39) |
| ESAS-r shortness of breath | 0.16 (–0.38; 0.70) | –0.21 (–1.02; 0.60) | –0.40 (–1.74; 0.95) | –0.36 (–1.18; 0.45) | –0.55 (–1.90; 0.79) | –0.19 (–1.67; 1.29) | 0.10 (–0.35; 0.55) | 0.07 (–3.46; 3.60) | –0.03 (–3.56; 3.49) | 0.38 (0.02; 0.75) |
| ESAS-r lack of appetite | 0.28 (–0.22; 0.79) | 0.47 (–0.30; 1.24) | –0.41 (–1.69; 0.87) | 0.18 (–0.59; 0.96) | –0.69 (–1.98; 0.59) | –0.88 (–2.28; 0.53) | –0.65 (–1.07; –0.22)* | 1.21 (–2.14; 4.57) | 1.86 (–1.49; 5.21) | 0.16 (–0.18; 0.51) |
| ESAS-r wellbeing | 0.45 (–0.01; 0.91) | –0.17 (–0.87; 0.52) | 0.01 (–1.15; 1.16) | –0.62 (–1.32; 0.08) | –0.44 (–1.60; 0.71) | 0.18 (–1.09; 1.44) | –0.04 (–0.42; 0.35) | 0.4 (–2.62; 3.43) | 0.44 (–2.58; 3.46) | 0.84 (0.53; 1.16)* |
| SF-36 physical HRQoL | –4.77 (–8.09; –1.44)* | 2.90 (–2.13; 7.94) | 3.39 (–4.97; 11.74) | 7.67 (2.6; 12.74)* | 8.16 (–0.22; 16.53) | 0.48 (–8.7; 9.67) | 1.53 (–1.25; 4.31) | –3.05 (–24.98; 18.88) | –4.58 (–26.51; 17.35) | –6.75 (–9.02; –4.48)* |
| SF-36 mental HRQoL | –3.79 (–7.70; 0.12) | –2.34 (–8.26; 3.57) | 1.33 (–8.49; 11.15) | 1.45 (–4.51; 7.41) | 5.26 (–4.72; 14.97) | 3.68 (–7.12; 14.48) | 3.08 (–0.18; 6.36) | –19.05 (–44.83; 3.64) | –22.13 (–47.91; 3.64) | –7.92 (–10.59;–5.25)* |
a A narrow CI indicates a more precise estimate of the mean difference whereas a wider interval points to a less precise estimation. CI that crosses through zero, with a negative lower bound value and a positive upper bound value suggests there is not enough evidence to conclude on a difference between the means; b an estimate of the modified population marginal mean for mixed and undefined categories; c An estimate of the modified population marginal mean for THC-dominant category; * significance set at P < 0.01
For both models related to BPI-SF outcomes variables, all interaction terms were removed due to non-significance. The models included intercept, initial treatment, pain mechanism and visit timepoint as fixed factors for a total of 8 parameters. Scores on pain severity and pain-related interference improved between baseline and FUP3M (Figure 2) independent from initial treatment and pain mechanism [F (1,990) = 45.75; P < 0.001 and F (1,990) = 111.49; P < 0.001 respectively)]. Pain mechanism was a significant predictor of pain severity [F (1,990) = 5.90; P < 0.001] but did not reach the required significance for pain-related interference (P = 0.02). Across visits pain severity scores from patients with neuropathic pain were significantly higher than scores of those with nociceptive pain (P < 0.001). Initial treatment did not reach the required statistical significance for pain severity (P = 0.017), however, it was a significant predictor of pain-related interference [F (1,990) = 4.63; P < 0.01]. Patients with an initial THC:CBD treatment had significantly higher pain-related interference scores than those with a CBD-dominant treatment (P < 0.01). Of note, patients with THC:CBD treatment also had higher pain-severity scores than those with a CBD-dominant treatment (P = 0.013).
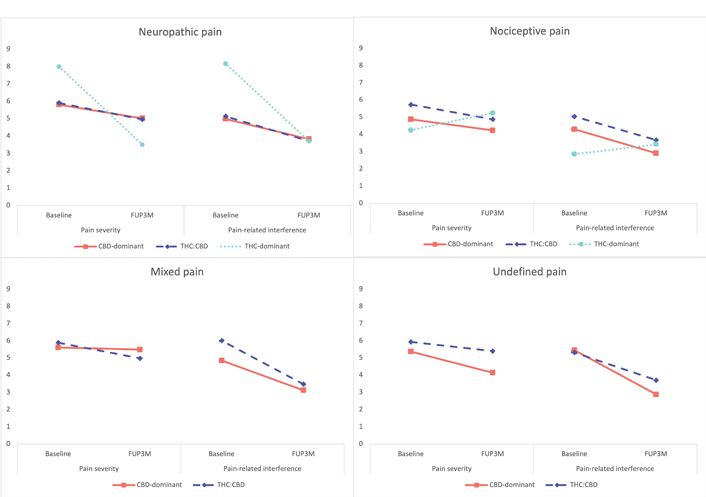
BPI-SF pain severity average scores for baseline and FUP3M, according to pain mechanism and initial cannabinoid profile. The group of THC-dominant is comprised of only 2 participants (one with neuropathic pain and one with nociceptive pain). A decrease in average scores means a report of improvement, the patient with neuropathic pain and THC-dominant products reported a large improvement in both pain severity and pain-related interference
For all but one (sleep) model related to ESAS-r scores variables, all interaction terms were removed due to non-significance for a total of 8 parameters (including residual). The model for the sleep variable included the interaction between the pain mechanism and initial treatment (P < 0.001) for a total of 12 parameters.
ESAS-r average scores on anxiety [F (1,987) = 13.13; P < 0.001], depression [F (1,986) = 11.66; P < 0.001], drowsiness [F (1,982) = 13.38; P < 0.001], fatigue [F (1,988) = 33.83; P < 0.001], pain [F (1,988) = 60.76; P < 0.001], sleep problems [F (1,988) = 67.65; P < 0.001] and wellbeing [F (1,986) = 27.75; P < 0.001] improved over time (Figure 3). Nausea, lack of appetite, and shortness of breath average scores did not statistically change between visits (P > 0.05).
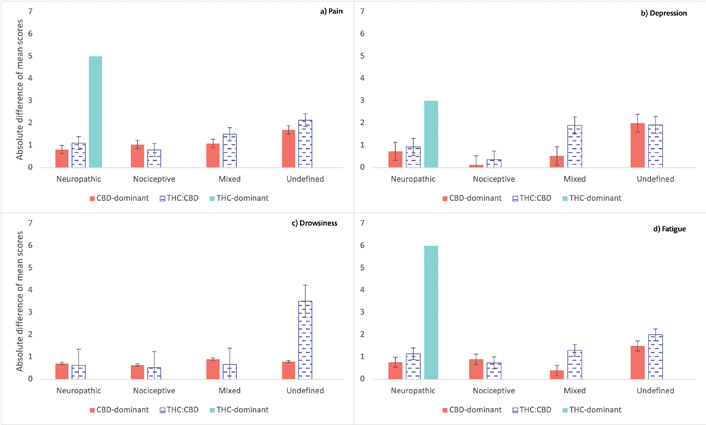
ESAS-r absolute difference of mean scores. a) Pain; b) depression; c) drowsiness; d) fatigue symptoms. The group of THC-dominant is comprised of only 2 participants (one with neuropathic pain and one with nociceptive pain, for which there was no difference in scores between visits, so it is not appearing in the Figure 3). There was a strong improvement in pain, depression, and fatigue from baseline to FUP3M for the patient with initial THC-dominant product and neuropathic pain. Patients with neuropathic and mixed pain taking THC:CBD products had bigger improvements than CBD-dominant treatments on pain, depression, and fatigue. Errors bars represent standard errors
Pain mechanism was a significant predictor of pain [F (1,988) = 4.69; P < 0.01], drowsiness [F (1,982) = 5.01; P < 0.002], and fatigue [F (1,988) = 7.81; P < 0.001] ESAS-r scores. Patients with nociceptive pain had significantly lower drowsiness (P < 0.001), pain (P = 0.014), and fatigue (P < 0.001) compared to patients with neuropathic pain, and lower pain compared to patients with mixed pain (P = 0.012).
Initial treatment was a significant predictor of depression [F (1,986) = 4.75; P < 0.01), and lack of appetite [F (1,987) = 7.23; P < 0.001]. Patients with an initial THC:CBD treatment had higher depression (P < 0.01) and lack of appetite (P < 0.001) scores compared to patients with an initial CBD-dominant treatment.
For both models related to SF-36 outcomes variables, all interaction terms were removed due to non-significance for a total of 8 parameters. Scores on both physical and mental HRQoL scales improved over time [F (1,990) = 34.03; P < 0.001 and F (1,990) = 33.92; P < 0.001 respectively]. Pain mechanism was a significant predictor of physical health [F (1,990) = 8.45; P < 0.01], Patients with nociceptive pain had significantly higher physical HRQoL scores compared to patients with neuropathic pain but had significantly smaller HRQoL scores compared to patients with mixed pain (both P < 0.001).
During the study period, 158 (31.9%) patients reported a total of 262 AEs which were assessed to have some level of relationship to CBMs and therefore were classified as ADRs. Over half (54%) of ADRs were reported about a month after treatment initiation, 3% within the first 2 weeks after baseline, and 43% at FUP3M. The ADRs severity, causality, outcome, and action taken according to the initial cannabinoid profile are reported in Table 4. Over three-quarters (77.5%) of ADRs were recovered or recovering and 14.9% were not recovered at the time of data extraction.
ADRs severity, causality, outcome, and action taken criteria
| ADR description criteria | CBD-dominant [N (%)] | THC-CBD balanced [N (%)] | THC-dominant [N (%)] | Total [N (%)] |
|---|---|---|---|---|
| Total ADRs | 112 (42.7%) | 148 (56.5%) | 2 (0.8%) | 262 |
| Severity | ||||
| Mild | 86 (32.8%) | 99 (37.8%) | 2 (0.8%) | 187 (71.4%) |
| Moderate | 24 (9.2%) | 44 (16.8%) | 0 (0%) | 68 (26.0%) |
| Severe | 2 (0.8%) | 5 (1.9%) | 0 (0%) | 7 (2.7%) |
| Causality | ||||
| Definitely related | 6 (2.3%) | 10 (3.8%) | 0 (0%) | 16 (6.1%) |
| Possibly related | 65 (24.8%) | 81 (30.9%) | 1 (0.4%) | 147 (56.1%) |
| Probably related | 37 (14.1%) | 54 (20.6%) | 1 (0.4%) | 92 (35.1%) |
| Unlikely related | 4 (1.5%) | 3 (1.1%) | 0 (0%) | 7 (2.7%) |
| Outcome | ||||
| Not recovered | 17 (6.5%) | 22 (8.4%) | 0 (0%) | 39 (14.9%) |
| Recovered | 45 (17.2%) | 72 (27.5%) | 2 (0.8%) | 119 (45.4%) |
| Recovering | 41 (15.6%) | 43 (16.4%) | 0 (0%) | 82 (32.1%) |
| Stabilized | 8 (3.1%) | 11 (4.2%) | 0 (0%) | 19 (7.3%) |
| Unknown | 1 (0.4%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Action taken | ||||
| Dose decreased | 26 (9.9%) | 32 (12.2%) | 1 (0.4%) | 59 (22.5%) |
| Dose delayed | 6 (2.3%) | 3 (1.1%) | 0 (0%) | 9 (3.4%) |
| Dose increased | 19 (7.3%) | 9 (3.4%) | 0 (0%) | 28 (10.7%) |
| Dose interrupted or discontinued temporarily | 19 (7.3%) | 25 (9.5%) | 0 (0%) | 44 (16.8%) |
| Dose not changed | 21 (8.0%) | 39 (14.9%) | 0 (0%) | 60 (22.9%) |
| Dose withdrawn permanently | 21 (8.0%) | 37 (14.1%) | 1 (0.4%) | 59 (22.5%) |
| Unknown | 0 (0%) | 3 (1.1%) | 0 (0%) | 3 (1.1%) |
N (%): the number of people under current conditions (percentage of people under current conditions)
Overall, dizziness and giddiness, headache, and somnolence were the most reported ADRs (13.4%, 12.2%, and 11.1% respectively). Fatigue accounted for 7.6% and buzz sensation for 6.1%. Dyspepsia and nausea accounted for 5.7% each of total ADRs, diarrhea for 4.2%, and dry mouth for 3.4%. Other ADRs (for a total of 30.6%) accounted for less than 3% of total ADRs reported and included constipation, palpitations, polyphagia, and shortness of breath among others.
The majority of ADRs (71.4%) were evaluated as mild, 26% as moderate, and 2.7% as severe (Table 4). Three serious ADRs were reported to health regulations as they led to hospitalization, one was due to pulmonary embolism (assessed as unlikely related to the CBD-dominant ingested oil treatment), one due to tachycardia (possibly related to the THC-dominant ingested oil and CBD-dominant ingested oil treatment) and one due to vomiting (probably related to CBD-dominant ingested oil and THC:CBD balanced inhaled dried flower). Treatment was interrupted temporarily for the pulmonary embolism, ceased completely for the tachycardia, and ceased during the day for the vomiting ADR.
The patients who reported at least one ADR did not differ from those who did not in terms of age, sex, and CBM cannabinoid profile category. However, over half of the patients who reported at least one ADR had neuropathic pain. Previous cannabis use was also associated with the presence of ADRs [χ2(3) = 8.57, P < 0.036]; patients who had never tried cannabis accounted for 31% of patients who reported at least one ADR. Among patients who had previously tried both dried flower and/or ingested cannabis oil extracts, there was a higher proportion of no ADRs (75%) vs. at least one ADR (25%) reported.
This analysis indicates that after a medium-term assessment of three months following CBM treatment initiation, independently from their pain mechanism or initial treatment, patients report modest improvement in pain and associated symptoms such as anxiety, depression, drowsiness, fatigue, sleep disturbances, and overall HRQoL. Our findings also show that the mechanism of pain has an impact on patients’ reports of pain management and symptom burden. Patients with neuropathic pain report higher pain intensity, drowsiness, and fatigue than those with nociceptive pain, concordant with another recent study [14]. Furthermore, patients reporting either higher pain intensity, pain-related interference, depression, or lack of appetite scores were mainly prescribed at baseline with THC:CBD treatments, suggesting that THC is more likely to be recommended when pain is severe, whereas CBD-dominant treatment is favored by clinicians for less severe cases, in line with other reports [36].
While defining pain mechanism is essential during clinical assessment, it remains a major limitation of medical cannabis studies that usually generalize their results to any type of CNCP [25]. This analysis provides a novel contribution to support that CBMs could be used as a complementary treatment not only for neuropathic pain but also for nociceptive and mixed pain. This observation adds information to current literature citing insufficient evidence to recommend medical cannabis for other types of chronic pain [37, 38].
Initial treatments were predominantly oral cannabis oils with either CBD-dominant or THC:CBD balanced concentrations, corroborating clinicians’ preference for non-smoked products [15, 39, 40] and for limiting THC intake at baseline [39]. A product profile change at FUP3M is observed with a significant addition of THC and a decrease in the use of CBD-dominant products. The strategy to ‘start low and go up slowly’ remains a standard of care in clinical practice to limit AEs [41, 42].
Pharmaceutical cannabinoid products are generally manufactured from purified extracts or synthetic cannabinoid preparations and contain specific concentrations of CBD, THC, and, in certain formulations, limited concentrations of other cannabinoids, terpenes, and flavonoids. Many of these components are known to be pharmacologically active and may have beneficial effects on symptoms or on the function of the endocannabinoid system, however, pharmacokinetic and pharmacodynamic impacts of these components, alone or in combination, remain largely unidentified [43, 44].
About a third of patients reported at least one ADR during the study period, the majority were mild and produced by both THC and CBD, in line with previous reports [27, 39, 40] and systematic reviews [19, 45]. As is standard clinical practice, CBMs were added as an adjunct to the patient’s complex makeup of concomitant medications making causality assessment more challenging. The most frequently reported ADRs were dizziness, headache, somnolence, and fatigue. Cannabis naive patients were more likely to experience AEs despite the conservative approach to treatment recommendations, indicating the importance of a thorough assessment of previous cannabis use. While safety considerations generally drive treatment recommendations, further study is required to refine clinical decision criteria [42].
Almost all patients were taking conventional pharmacological treatment when CBM was introduced which presents potential for drug-drug interactions primarily via the hepatic cytochrome P450 (CYP450) system. Significant interactions have been observed between cannabinoids and warfarin, rifampin, ketoconazole, clobazam, and valproate; thus the use of these medications in combination with cannabinoids requires careful monitoring and assessment of risk-benefit [43, 46]. Further research is needed to better understand how the addition of CBMs may impact the pharmacological effects of conventional pain medications.
Standard limitations of registry studies apply to this study, such as population heterogeneity and lack of placebo-control. The registry takes place at a network of clinics in a specific province within the Canadian public healthcare system. The current context of medical cannabis access, including social stigma, high out-of-pocket cost, and limited insurance coverage can increase the patient selection bias. Patients who continue CBM treatment despite these barriers are likely to be those who find CBM helpful. The high cost as well as the privileged environment with supported, efficient access to supportive healthcare professionals may also increase the placebo effect of CBM. The use of complete case analysis, in which only data from patients with both complete baseline and FUP3M questionnaires were extracted, may reduce potential bias and adjust for attrition, however, it further limited the sample size. The small sample size of the THC-dominant product category limits the ability to model it as a separate group, however, the exclusion of this group did not change the results of the analysis, therefore, the three-category model was presented as it is more representative of clinical practice. Furthermore, the pain mechanism groups of both mixed and undefined pain are smaller relative to the other respective categories, which contributes to a somewhat unequal weighting of the statistical models.
Treatment discontinuation is a recurring issue in clinical practice [14, 47, 48]. Patient discontinuation is influenced by various factors, including social and financial challenges, lack of effectiveness, and AEs. The COVID-19 pandemic has increased stress, financial and technological repercussions for many patients and may also have an impact on our population during the study period.
The categorization of initial treatments by the overall THC:CBD ratio instead of by estimated exposure to each cannabinoid limits the generalizability of the results. However, this categorization represents common clinical practices and the tendency to maintain simple initiation regimens during a patient’s first 3 months of medical cannabis treatment.
The cannabis plant has a complex composition of more than 100 unique cannabinoids including CBD, THC, and others such as cannabinol (CBN), cannabichromene (CBC), and cannabigerol (CBG) [49]. Future research is needed to better understand their mechanisms of action and unique effects which will support clinicians as these components become more predominant in medical cannabis products.
This analysis investigated the use of CBM treatment during the initial three-month period according to the mechanism of pain and the initial CBM treatment. It serves as an example of the use of real-world evidence to bridge gaps of limited RCTs and provides a preliminary indication of the effectiveness of specific CBM treatments for nociceptive and mixed pain as well as neuropathic pain. Future research on the role of medical cannabis in pain management must detail the product characteristics and must consider the pain mechanism. Study results indicate that countries where regulatory frameworks limit medical cannabis products to CBD or otherwise continue the prohibition of THC likely fall short of the needs of patients and healthcare professionals in today’s social and cultural climate and escalating rates of CNCP. While CBM treatments are generally considered safe; this study shows that a significant number of ADRs, including some serious ADRs, can occur even within a controlled, conservative supervision of treatment initiation and titration. This emphasizes that CBMs require medical supervision by trained healthcare professionals to support patient safety and ensure appropriate follow-up.
ADRs: adverse drug reactions
AEs: adverse effects
BPI-SF: Brief Pain Inventory-Short Form
CBD: cannabidiol
CBMs: cannabinoid-based medicines
CI: confidence interval
CNCP: chronic non-cancer pain
ESAS-r: Edmonton Symptom Assessment System-Revised
FUP3M: follow-up 3 months
HRQoL: health-related quality of life
SF-36: 36-Item Short Form Health Survey
THC: delta-9-tetrahydrocannabinol
χ2: Chi-squares tests
The authors thank the participants in this study and the medical professionals and clinic staff for collection of data. The authors also thank Youri Drozd and Charlotte Bastin for technical support.
LR, MFA, and EP: Conceptualization. LR: Data curation, Formal analysis, Writing—original draft. MFA: Resources. EP: Supervision. MFA, EP, CS, AW, and DD: Writing—review and editing.
All authors have a relationship with Santé Cannabis, a medical cannabis clinic and research centre based in Montreal, Québec, Canada. Santé Cannabis operates within the public healthcare system, patients do not pay fees to receive care at the clinic. Santé Cannabis does not sell, or receive financial benefit from the sale of medical cannabis.
The registry, from which the submitted data is extracted, was approved by the McGill University Faculty of Medicine and Health Sciences Ethics Committee (ID A07-E47-20B/20-07-004). The study was conducted in accordance with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets for this manuscript are not publicly available because they are not part of a public repository. Requests for accessing the datasets should be directed to Lucile Rapin, lrapin@santecannabis.ca.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gregory Giordano ... Angela D. Bryan
Elizabeth N. R. Schjelderup ... Alasdair M. Barr
Hannah Thurgur ... David J. Nutt
Amanda Stueber, Carrie Cuttler
Cassandra L. Taylor, Schuyler A. Pruyn
Gerhard Nahler
Trevor R. Norman
