Affiliation:
1Drug Science, EC2V 6DL London, UK
Email: hannah.thurgur@drugscience.org.uk
ORCID: https://orcid.org/0000-0001-7714-3446
Affiliation:
2Stroke and Neurorehabilitation, Nuffield Health, YO31 8TA York, UK
ORCID: https://orcid.org/0000-0002-6637-5441
Affiliation:
3Bod Australia Limited, Sydney 2028, Australia
ORCID: https://orcid.org/0000-0003-2560-3628
Affiliation:
1Drug Science, EC2V 6DL London, UK
4Faculty of Medicine, Department of Brain Sciences, Division of Psychiatry, Centre for Neuropsychopharmacology, Imperial College London, W12 0NN London, UK
ORCID: https://orcid.org/0000-0002-1286-1401
Explor Med. 2023;4:487–503 DOI: https://doi.org/10.37349/emed.2023.00158
Received: December 05, 2022 Accepted: April 13, 2023 Published: August 31, 2023
Academic Editor: Kelly Sagar, Harvard Medical School, USA
The article belongs to the special issue Beyond Weed: Clinical Applications of Cannabis and Cannabinoids
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection can result in a range of persistent symptoms impacting everyday functioning for a considerable proportion of patients, a condition termed Long coronavirus disease (COVID) or post COVID-19 syndrome. The severity and set of symptoms vary between patients, and include fatigue, cognitive dysfunction, sleep disturbances, palpitations, tachycardia, pain, depression, and anxiety. The high prevalence of Long COVID combined with the lack of treatment approaches has resulted in considerable unmet clinical needs. There is a growing body of evidence that cannabis-based medicinal products (CBMPs) can be used to treat symptoms including pain, anxiety, depression, fatigue, sleep, headaches, and cognitive dysfunction, which are commonly reported in Long COVID. This article provides an overview of the pathophysiology of Long COVID and discusses preliminary pre-clinical, clinical trials, and real-world evidence (RWE) for CBMPs in the context of Long COVID. This review summarises current clinical trials and studies exploring CBMPs in Long COVID. The current evidence provides a rationale to further explore CBMPs as a treatment for Long COVID symptoms. In addition to further randomised controlled trials (RCTs), the increasing availability of CBMPs globally, coupled with the continued prevalence of Long COVID in the population, also highlights the value of real-world data in the research of CBMPs in Long COVID. Critically, there is an evident need for multidisciplinary approaches of CBMPs and Long COVID in real-world clinical practice settings.
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is responsible for the coronavirus disease-19 (COVID-19) pandemic which has a catastrophic impact globally and a death toll in the millions [1]. Since the initial detection of SARS-CoV-2 in 2019, understanding of the disease pathogenesis has rapidly evolved, acute medical treatment has improved, and vaccines have been developed [2]. However, challenges remain in treating the long-term symptoms and health problems that persist after the acute infection, a condition termed Long COVID, post COVID-19 syndrome or post COVID-19 condition [2]. The high prevalence of Long COVID (estimates vary between 10–50% of those infected) combined with the lack of treatment approaches has resulted in considerable unmet clinical needs [3–6]. Pre-clinical and clinical findings suggest a potential role for cannabis-based medicinal products (CBMPs) in the treatment of Long COVID symptoms and recovery for the millions of people living with Long COVID. This review provides an overview of the current evidence base for the use of CBMPs in Long COVID based on a systematic ascertainment of the literature. Multiple search engines were interrogated using the search terms ‘Long COVID’ or ‘post COVID-19 syndrome’ or ‘post COVID-19 condition’ and ‘medical cannabis’ or ‘cannabis’ or ‘cannabidiol’ or ‘tetrahydrocannabinol’ or ‘cannabis-based medicinal products’ for literature published between January 2020 and March 2023. This search initially identified 38 potential papers and the relevance of these for inclusion in the current review was assessed.
The World Health Organization (WHO) defines post COVID-19 as symptoms that occur 3 months from the onset of an acute COVID-19 infection and that last for at least 2 months and estimates that 10–20% of people develop Long COVID post-acute infection [7]. Similar to acute COVID-19 infection, multiple organ systems have been implicated including respiratory, cardiovascular, and neurological, and there is increasing recognition of the potential long-term disability associated with recovery from COVID-19 infection [8]. Symptoms can be new, returning, or ongoing, and there is a spectrum of symptoms and severity. A meta-analysis deemed the five most common Long COVID symptoms to be fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnoea (shortness of breath; 24%) [9]. A further meta-analysis assessed the five most common symptoms in hospitalised and non-hospitalised patients reporting as fatigue (28.4%), pain/discomfort (27.9%), impaired sleep (23.5%), breathlessness (22.6%), and impaired usual activity (22.3%) in hospitalised patients, whilst fatigue (34.8%), breathlessness (20.4%), muscle pain/myalgia (17.0%), affected sleep (15.3%), and loss of sense of smell (12.7%) in non-hospitalised patients [10]. The most reported symptoms across hospitalised and non-hospitalised patients were fatigue (25.2%), breathlessness (18.2%), impaired usual activity (14.9%), loss of sense of taste (14.9%), and loss of sense of smell (14.1%) [10]. Other commonly reported symptoms include cognitive dysfunction, sleep disturbances, palpitations, tachycardia, chest pain, joint pain, and mood disturbances including depression and anxiety [9, 11, 12]. One survey identified symptoms that are not commonly mentioned in public discussion of Long COVID, including anaphylaxis and new allergies, seizures, suicidality, changes in sensitivity to medication, vision loss, hearing loss, and facial paralysis [11]. The study showed 85.9% of patients with Long COVID experienced relapses, primarily triggered by exercise, physical or mental activity, and stress [11]. Long COVID also significantly impacts daily lives including difficulties with usual activities, reduced levels of exercise, and impaired ability to work [11, 13].
There is considerable uncertainty about the prevalence of Long COVID. Differences in Long COVID recognition and diagnosis by general practitioners (GPs), symptom presentation/severity, and variable reporting by GPs contribute to the disparity in prevalence figures reported [14]. Estimates of the prevalence of Long COVID vary between 10–50% of people infected with the virus, with a higher rate in patients hospitalised [3–6]. A large retrospective study demonstrated that the severity of acute COVID infection is associated with the severity of Long COVID symptoms [15]. However, individuals who were asymptomatic or only mildly affected with COVID may also develop Long COVID symptoms [16, 17]. A systematic review reported low-level evidence that vaccination may reduce the risk of Long COVID, but the impact of vaccinations for those with pre-existing Long COVID symptoms remains uncertain [18].
Long COVID is recognised as a distinct clinical condition from acute SARS-CoV-2 infection [19]. Acute infection is associated with the initial infiltration of SARS-CoV-2 into cells via the angiotensin-converting enzyme 2 (ACE2) receptor [20, 21]. This ACE2 receptor is present in multiple cell types across the body including brain, oral and nasal mucosa, lungs, heart, gastrointestinal tract, liver, kidneys, spleen, and arterial and venous endothelial cells [20, 21]. Hence, infection can lead to multi-organ disease [20, 21]. Once SARS-CoV-2 enters the host cell, a local inflammatory response is triggered and there is a cascade of cell damage and cell death, followed by a subsequent immune response [21, 22].
The pathogenesis of Long COVID is thought to be multifactorial, with several suggested mechanisms contributing to the multiple clinical manifestations and array of symptoms [19]. Potential mechanisms include prolonged inflammation, immune-mediated vascular dysfunction, thromboembolism, and nervous system dysfunction [19]. SARS-CoV-2 infection causes endothelial dysfunction in the vasculature, which can result in the development of multi-organ tissue injury [23–25]. Furthermore, endothelial dysfunction has been demonstrated in Long COVID patients [26–28]. There is research interest in identifying risk factors for Long COVID and four Long COVID-anticipating risk factors at the time of the initial COVID diagnosis have been identified: type 2 diabetes, SARS-CoV-2 RNAemia, Epstein-Barr virus (EBV) viremia, and specific auto-antibodies [29]. Other research indicates that Long COVID symptoms may be related to a reactivation of the EBV after COVID-19 infection [16].
Considerable uncertainty about the pathophysiology and prognosis of this disease remains.
Critically, there is a lack of knowledge of what treatment approaches are likely to be most effective in Long COVID, and an urgent need to identify and trial potential treatments for this emerging clinical condition. To date, the main treatment for Long COVID has been the management of symptoms, active fatigue management, and rehabilitation via an experienced multidisciplinary team of healthcare professionals [30].
Cannabis can be described as a family of medicines rather than one medicine [31]. CBMPs include pharmaceutical preparations that can contain a broad spectrum of the bioactive chemical constituents of the cannabis plant (‘whole-plant’ or ‘full-spectrum’ products), only individual chemical constituents of the plant (isolates), or in a varied combination of constituents. The cannabis plant synthesises many cannabinoids including cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC), terpenes, and flavonoids [32]. Cannabinoids act on the endocannabinoid system, a complex widespread neuromodulator system involved in multiple functions throughout the body [33], highlighting the array of potential targets for CBMPs.
The constituents of the cannabis plants are thought to have distinct therapeutic benefits, and there is a further layer of complexity whereby there may be an entourage effect of some constituents [32]. The entourage effect is a pharmacological synergy whereby the entirety of the effect is greater than the sum effects of each individual component of the cannabis extract [34]. This term was first coined in 1998 after in vitro and in vivo observations that various seemingly inactive metabolites could alter the activity of the endocannabinoid system [35]. Currently, most medicinal cannabis research has focused on CBD and THC, and therefore further research is needed into the specific roles of minor cannabinoids and other constituents, as well as the entourage effect [36, 37]. The potential of cannabinoids to have distinct therapeutic benefits has been demonstrated in the context of acute COVID-19 infection, whereby cannabigerolic acid and cannabidiolic acid prevented the entry of SARS-CoV-2 into epithelial cells in vitro [38]. Although there is a limited investigation of the entourage effect in treating Long COVID symptoms, it is necessary to highlight this synergy whereby whole plant CBMPs may offer therapeutic superiority over isolates [36, 39].
Given that the preparation of the CBMP and its constituents will influence the potential effects, the details on the constituent of the CBMPs used in studies throughout this review are provided (when this information is available).
Understanding the medical contexts in which CBMPs are currently used and the existing evidence highlights why CBMPs may offer a potential treatment of Long COVID symptoms. It outlines some of the key overlapping symptoms and conditions CBMPs are used to treat and those commonly reported in Long COVID (Figure 1). It is not exhaustive as Long COVID presents a vast array of symptoms [10], some of which are not included in Figure 1. Similarly, CBMPs are used in the real-world setting for a variety of conditions and symptoms which may not be presented in Figure 1 [40, 41].
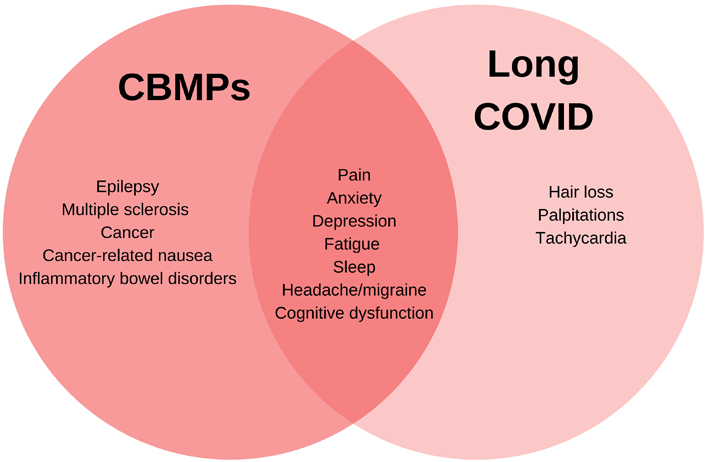
Venn diagram showing the overlapping symptoms and conditions CBMPs are used to treat and commonly reported Long COVID symptoms
CBMPs are typically used to try and manage symptoms to provide symptomatic relief for patients [42]. They are used in chronic complex conditions where patients are often on multiple medications [41], including multiple sclerosis (MS) and fibromyalgia, and patients with these diagnoses commonly have more than one symptom [41, 43]. Due to the nature of the cannabis plant and differing types of CBMPs, the selection of CBMPs is usually individualised and tailored to the patient’s symptoms. Globally, the most common symptoms for which medical cannabis is used are pain, muscle stiffness (spasticity), nausea, fatigue, anxiety, insomnia, mood disorders, and seizures [41, 44, 45]. Some of these symptoms, particularly anxiety, mood disorders, fatigue, and pain are commonly reported in patients with Long COVID [9–12].
Multiple lines of evidence support the potential use of CBMPs in Long COVID (see Figure 2 for an overview of the areas of evidence). Firstly, some of the most commonly reported symptoms of Long COVID include sleep disturbance, fatigue, pain, and anxiety: these symptoms are common to many of the disorders currently being treated with CBMPs. There is burgeoning literature suggesting the efficacy and effectiveness of cannabinoids in treating pain, anxiety/depression, and other symptoms [40, 46–50] while also improving quality of life [40, 46–51]. In the sections below, key evidence for CBMPs use in commonly reported symptoms is discussed.
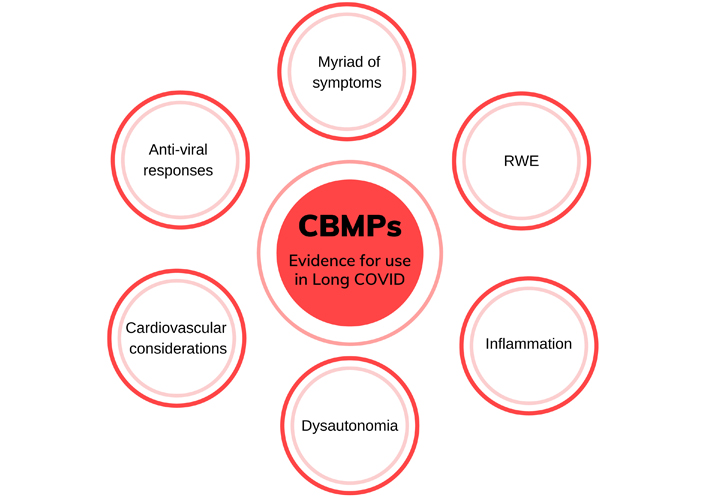
Overview showing the areas of evidence which provide the rationale for the use of CBMPs in treating Long COVID symptoms. This includes the myriad of symptoms reported in Long COVID some of which are shared with conditions that CBMPs are currently used to treat, RWE of CBMPs for various symptoms, anti-inflammatory properties of some cannabinoids, commonality of dysautonomia in Long COVID and conditions treated with CBMPs, cardiovascular considerations of CBMPs, as well as potential anti-viral properties of cannabinoids. RWE: real-world evidence
Increasing RWE from global large-scale databases highlights that patients are successfully using medicinal cannabis to treat pain, anxiety, depression, migraines/headaches, insomnia, post-traumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), nausea, and more see [41] and [40] for summaries from two United Kingdom (UK)-based registries. RWE provides an alternative to controlled trials to study the effects of CBMPs and has a broad range of advantages [43]. RWE offers better ecological validity than randomised controlled trials (RCTs) as it can be acquired from a much larger demographic range of patients and is inclusive of those with significant comorbidities [43]. Furthermore, RWE enables the investigation of a broad range of CBMPs and is therefore more reflective of the variety of CBMPs used by patients [43].
Long COVID is associated with a myriad of symptoms, and therefore these patients present similarities to current medical cannabis patients who typically have co-morbid symptoms and conditions secondary to their primary condition. The multiple suggested effects of CBMPs are thought to contribute to overall improvement in overall quality of life, a key issue in chronic conditions [41]. In the United Kingdom Medical Cannabis Registry, treatment with CBMPs was associated with an improvement in quality of life, sleep- and anxiety-specific symptoms after 12 months in patients with chronic illness [40]. This highlights how CBMPs are already used to manage patients with complex conditions and symptoms in chronic illness, presenting similarity with the complexity of Long COVID.
Meta-analyses demonstrate low to moderate-quality evidence for a role of CBMPs in the reduction of chronic pain [52–55]. The most frequent primary condition in Project Twenty21, a UK based real-world data registry of prescribed cannabis patients, is chronic pain [41]. These chronic pain patients reported high levels of comorbidity; people reported an average of 4.6 comorbid conditions with the most common comorbid conditions being anxiety, depression, insomnia, and stress [41]. These high levels of comorbidity are also reported in the United Kingdom Medical Cannabis Registry [40]. These registries include patients being treated with a broad set of CBMPs: various formulations (oil, capsules, lozenges, and dried flower), preparations (isolated cannabinoids or broad/full spectrum extract), and constituents of cannabinoids/terpenes [40, 41]. Project Twenty21 has demonstrated consistent reductions in pain symptomatology and improvements in quality of life, general health, mood, and sleep among chronic pain patients [56]. Additionally, there was a substantial reduction in the use of prescribed opioids in these patients [56]. Further observational studies of patients using CBMPs have reported improvements in pain [57–62].
Headaches and migraines are other common symptoms of Long COVID [10]. Systematic reviews report reduced frequency [63, 64] and length [63] of migraine headaches after treatment with CBMPs.
Emerging evidence suggests that the immune-inflammatory response triggered by acute COVID-19 infection is associated with the neuropsychiatric symptoms of Long COVID including anxiety, depression, and fatigue [65]. Furthermore, preclinical data show that prolonged, sustained inflammation in COVID-19 animal models correlates with anxiety-like behaviour [66]. Focusing on one component of CBMPs, CBD has been proposed as a potential treatment for anxiety disorders, despite a lack of studies investigating chronic CBD dosing [67]. A systematic review of the concentration effects of CBD reported that the majority (70.3%) of studies found no effect of CBD on anxiety outcomes, however, the authors stated that it would be premature to conclude that CBD does not have anxiety-reducing properties [68]. There are several limitations of the existing literature, including the assessment of anxiety symptomatology, cross-species analysis, and missing pharmacokinetics (PK) data [68]. Studies of particular interest include the use of a 300 mg daily dose of CBD for a period of 4 weeks for the treatment of social anxiety disorder in teenagers which demonstrated a reduction in anxiety measures [69]. Furthermore, a small tolerability clinical trial investigating a full spectrum high-CBD CBMP in 14 patients with anxiety demonstrated improvements in anxiety, mood, sleep, quality of life, and cognition [70]. It is suggested that low doses of THC are anxiolytic and can offer therapeutic benefits, however, THC may be anxiogenic at higher doses [71].
The potential role of CBD in COVID-19-related anxiety and post-traumatic stress symptoms (PTSS) or PTSD, as well as sleep disturbances often concomitantly reported, has been hypothesised and discussed in the context of current evidence [72]. The authors discuss a growing body of small randomized controlled clinical trials using CBD for anxiolytic effects; however, many were in healthy volunteers rather than relevant patient cohorts [72]. RWE highlights the potential value of CBMPs to reduce anxiety symptomatology and improve patients’ well-being and sleep [40, 56] and observational studies of CBMPs have also demonstrated improvements in anxiety [59, 61, 62, 73]. There is a need for further investigation into which CBMPs may offer therapeutic benefits in anxiety, as well as the dosing schedule and treatment duration.
The evidence for CBMPs in the treatment of depression is more limited than for anxiety. A meta-analysis reported that CBMPs did not improve symptoms of depression, however, depression was often recorded as an outcome measure in RCTs with a different primary indication [74]. Observational studies demonstrate the potential role of CBMPs in alleviating depression symptoms in patients, including those seeking treatment for other primary conditions [40, 56, 75, 76].
Fatigue is commonly reported in Long COVID [10] and while there’s a lack of research specifically investigating CBMPs and fatigue, conditions with fatigue as a symptom, such as fibromyalgia can provide potential insights. Fibromyalgia is a pain disorder characterised by chronic widespread pain, fatigue, and sleep disturbance, in the absence of any well-defined underlying organic disease. Although there is limited data from RCTs for CBMPs in fibromyalgia [77], fibromyalgia is a common condition for which patients seek CBMPs in the real-world setting [40]. An observational study investigating CBMP in pain conditions, including 132 patients with fibromyalgia out of 751, reported reductions in fatigue as well as decreases in pain, headaches, fatigue, anxiety, and nausea [62].
Sleep disturbances are commonly reported by Long COVID patients [10]. Meta-analyses report a potential improvement in sleep from CBMPs treatment in chronic pain patient groups [74, 78], particularly noting the effect of nabiximols [74, 78]. RWE from registries and observational studies elucidate a potential role of CBMPs in improving sleep often presented alongside other symptoms [40, 56, 59, 61, 73, 75, 76].
Cognitive dysfunction is commonly reported by Long COVID patients [10]. CBD has the potential to impact brain function [79] and although CBD has been shown to improve cognition in preclinical models of cognitive impairment [80], some uncertainty remains for the potential effects of CBMPs on cognitive function due to the heterogeneity of constituents and limitations across the studies [81]. An improvement in cognition after 4 weeks of treatment with a high-CBD CBMP has been demonstrated [70] and a small clinical trial of the CBMP nabiximols in ADHD demonstrated a trend towards significance in favour of the CBMP for improving cognitive performance and activity level after 6 weeks of treatment [82]. Despite the limited evidence from RCTs for ADHD, CBMPs are currently used in clinical practice for ADHD [40]. An observational study demonstrated cognitive improvements after 3 months of treatment with CBMPs and reported improved task performance accompanied by changes in brain activation patterns within the cingulate cortex and frontal regions that were more reflective of healthy controls from previous studies than at pre-treatment [75]. A further observational longitudinal study demonstrated that CBMPs may improve cognitive function over the course of 12 months [83].
CBD, a key component of many CBMPs, has anti-inflammatory properties [84–87] and the potential role of CBD or high CBD CBMPs as an anti-inflammatory treatment for acute COVID-19 infection has been highlighted [88–90]. Inflammation is one of the hypotheses regarding the aetiology of Long COVID [91] and therefore CBD dominant CBMPs may offer direct benefits for Long COVID patients if this is proven to be the case. In an animal study on asthma, CBD was able to reduce the production of proinflammatory cytokine production, actually reducing airway inflammation [92]. In the same study, CBD also reduced pulmonary fibrosis, a condition where lung tissue becomes damaged and scarred, thickening lung tissue and making breathing more difficult [92], which mirrors COVID-19 symptoms such as serious pulmonary fibrosis [93]. Through in silico investigation, the cannabis derivatives CBD and cannabivarin have been suggested to have the potential to bind to and downregulate central nervous system (CNS) proteins related to Long COVID symptoms [94].
Despite limited research into the potential anti-viral properties of cannabinoids [95], the potential antiviral effects of cannabinoids and terpenes in the context of SARS-CoV-2 virus have been highlighted [96]. The potential anti-viral properties of cannabinoids could be of relevance due to the potential role of EBV in Long COVID. Studies have demonstrated an increased incidence of reactivation of the EBV in patients following SARS-CoV-2 infection [16]. This is hypothesised to be one of the contributing aetiologies of Long COVID [16], and THC has been shown to suppress activation of the EBV in vitro [97].
One current hypothesis is that some of the Long COVID symptoms could be attributed to dysautonomia [98] and the potential connection has become increasingly recognised amongst health care professionals [99, 100]. Dysautonomia refers to a disorder of autonomic nervous system (ANS) function that generally involves failure or excessive activation of the sympathetic or parasympathetic components of the ANS. Several studies indicate a high prevalence of dysautonomia in Long COVID patients [101, 102], including a global survey which reported moderate to severe autonomic dysfunction in 66% of patients [101]. Dani and colleagues [103] have discussed the scientific rationale for an underlying impaired autonomic physiology in Long COVID proposing that virus- or immune-mediated disruption of the autonomic nervous system results in orthostatic intolerance syndromes. Common reported symptoms of dysautonomia in Long COVID include palpitations, fatigue, sleep difficulties, cognitive impairment, breathlessness, and dizziness. Dysautonomia is also observed frequently in other post-viral states and other conditions such as diabetes [104], MS [105], fibromyalgia [106], and Parkinson’s disease [107]. Medical cannabis is used to treat the symptoms of some of these conditions including fibromyalgia and MS [41, 108]. A recent study suggested that Long COVID patients with fatigue may exhibit dysautonomia characterised by dysregulation of heart rate variability, compared to the control group of participants with previous COVID infection but no fatigue [109].
Several cohort studies indicate that acute COVID-19 survivors exhibit an increased 12-month risk of cardiovascular diseases including cerebrovascular disorders, dysrhythmias, heart disease, heart failure, thromboembolic disease, and other cardiac disorders [24, 110]. Therefore, it is important to consider any potential adverse cardiovascular effects of CBMPs in Long COVID patients. The composition of cannabis can influence its therapeutic and cardiovascular adverse effects [111]. While cardiovascular complications, such as tachycardia and acute coronary events, can be associated with cannabis smoking, it is thought to be linked to the high THC content of recreational cannabis [111]. CBD has been suggested to have therapeutic potential in the treatment of the cardiovascular diseases such as stroke, myocardial infarction, myocarditis, cardiomyopathies, and cardiovascular complications of diabetes, which is connected with vasodilatory, cardioprotective, antioxidant, anti-inflammatory, and neuroprotective properties of CBD [112, 113]. Furthermore, the literature suggests that CBD has no or little effect on blood pressure and heart rate under physiological conditions [113].
A search of the ClinicalTrials.gov database to identify studies investigating CBMPs as effective treatments for Long COVID was performed (see Table 1). The search was conducted on March 9th, 2023 and included the following search terms: Long COVID, post COVID-19 syndrome, post COVID-19 condition, medical cannabis, cannabis, cannabidiol, and tetrahydrocannabinol. The search identified a total of 3 studies (NCT04997395, NCT05467904, and NCT04828668) specifically investigating CBMPs in the context of Long COVID.
Long COVID clinical trials/studies involving CBMPs (also including CBD/hemp-based supplements)
| NCT number | Title | Country | Status | Study design | Participants | Condition | Intervention | Publications |
|---|---|---|---|---|---|---|---|---|
| NCT04997395 | Feasibility of cannabidiol for the treatment of Long COVID | UK | Active, not recruiting | Phase 2, open label | Adults over 18. N = 30 | Long COVID | MediCabilis 5% CBD Oil (UK trade name: MediCabilis™ Cannabis sativa 50). Daily dose up to 150 mg CBD, 6 mg THC for 5 months | Not published |
| NCT05467904 | Double-blind randomized placebo-controlled trial of a proprietary full hemp flower formulation for Long COVID | USA | Not yet recruiting | Placebo controlled. Phase not stated | Adults 18–65. N = 111 | Post-acute COVID-19 syndrome (Long COVID) | Full hemp flower formulation (Xltran Plus™ and Xltran™) for 28 days | Not published |
| NCT04828668 | Study to evaluate benefits & safety of Endourage Formula C™ oral drops in people with post-acute COVID-19 syndrome | USA | Completed | Placebo controlled. Phase not stated | Adults 18–75. N = 60 | Post-acute COVID-19 syndrome (Long COVID) | Formula C™ sublingual drops for 28 days (Extracted from hemp flower) | Published [114] |
USA: United States of America; N: refers to the number of study participants
The three Long COVID studies utilise different interventions and study designs. Two of the studies (NCT05467904 and NCT04828668) investigate wellness products isolated from whole hemp flower (Xltran Plus™ and Xltran™; Formula C™). The phase 2 feasibility clinical trial investigates MediCabilis 5% CBD Oil (UK trade name: MediCabilis™ Cannabis sativa 50), a full spectrum CBD dominant cannabis oil, with the study reaching a total daily dose up to 150 mg CBD and 6 mg THC. An interesting common feature between all three studies is the choice of CBD dominant products [Xltran Plus™, Formula C™, and MediCabilis 5% CBD Oil (UK trade name: MediCabilis™ Cannabis sativa 50)] and the use of whole flower extracts. However, the product Xltran™ is different in that it contains only terpenes and no cannabinoids or THC. A noteworthy observation is the difference in duration of treatment; two of the studies (NCT05467904 and NCT04828668) have a shorter treatment duration of 28 days compared to the longer duration of 5 months in the feasibility clinical trial (NCT04997395). The longer duration of treatment is more reflective of how CBMPs are typically used for long periods for the management of other chronic symptoms. Although the results from the Formula C™ study (NCT04828668) demonstrated improvement in both the placebo and treatment group, the investigators state that their placebo may have had active components that contributed to the benefits observed [114]. These early studies provide insights into different approaches to study design including the choice of CBMP, type of placebo, and duration of treatment.
Long COVID is a complex multisystem condition associated with an array of neurological, respiratory, cardiovascular, and gastrointestinal symptoms. Preliminary evidence reviewed here demonstrates a rationale for exploring CBMPs for the treatment of Long COVID symptoms. This approach is supported by evolving evidence, both from clinical trials and from RWE that CBMPs may be effective in reducing symptoms often associated with Long COVID including pain, anxiety, depression, fatigue, sleep, headaches, and cognitive dysfunction. Although clinical trials of CBMPs for treating Long COVID are in progress, the large population diagnosed with Long COVID coupled with a lack of proven treatments, highlight the importance of further researching the potential of CBMPs for treating Long COVID. Given the unique situation in which CBMPs are increasingly becoming accessible via legal routes across multiple jurisdictions, observational and real-world data offer considerable potential for elucidating the role of CBMPs in treating Long COVID. The benefits of RWE for studying the effectiveness of CBMPs for treating a range of conditions have been previously discussed [43]. These advantages include cost effectiveness, more representative patient samples, and providing an understanding of the potential effectiveness of interventions delivered in typical patient settings rather than the highly idealized setting of a clinical trial. The augmentation of RCTs with observational and patient-reported outcomes offers further advantages.
Long COVID is a common and debilitating long-term condition for which there are few available treatments. Given current evidence that the use of CBMPs may ameliorate symptoms in Long COVID, there is rationale to further explore how cannabinoids might interact with the SARS-Cov-2 virus and how CBMPs may work in Long COVID patients. There is value in the role of RWE in the context of CBMPs and Long COVID to progress much-needed research in this large population of patients, contributing to not only the evidence base for Long COVID but also current knowledge of CBMPs.
ADHD: attention deficit hyperactivity disorder
CBD: cannabidiol
CBMPs: cannabis-based medicinal products
COVID: coronavirus disease
EBV: Epstein-Barr virus
MS: multiple sclerosis
RCTs: randomised controlled trials
RWE: real-world evidence
SARS-CoV-2: severe acute respiratory syndrome coronavirus-2
THC: delta-9-tetrahydrocannabinol
UK: United Kingdom
HT, AKS, and EI: Conceptualization, Writing—original draft, Writing—review & editing. AH and ML: Conceptualization, Writing—review & editing. DJN: Conceptualization, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Author DJN is the Chair of the charity Drug Science. Author ML is the Chief Research Officer of Drug Science. Author AKS is the Head of Research of Drug Science and scientific advisor to the Primary Care Cannabis Network, and an executive member of the Cannabis Industry Council, both unpaid roles. Author HT is a Senior Research Officer and Study Coordinator of the medical cannabis and Long COVID clinical trial at Drug Science. Author EI is an expert member of the Drug Science Medical Cannabis Working and Principial Investigator of medical cannabis and Long COVID clinical trial at Drug Science. Drug Science receives an unrestricted educational grant from a consortium of medical cannabis companies to further its mission, which is the pursuit of an unbiased and scientific assessment of drugs regardless of their regulatory class. All Drug Science committee members, including the Chair, are unpaid by Drug Science for their effort and commitment to this organization. None of the authors would benefit from the wider prescription of medical cannabis in any form. AH was Chief Scientific Officer at Bod Australia Limited which is the sponsor of a medical cannabis and Long COVID clinical trial at Drug Science.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gregory Giordano ... Angela D. Bryan
Lucile Rapin ... Michael Dworkind
Elizabeth N. R. Schjelderup ... Alasdair M. Barr
Amanda Stueber, Carrie Cuttler
Cassandra L. Taylor, Schuyler A. Pruyn
Gerhard Nahler
Trevor R. Norman
