Abstract
Oral cancer is the most common carcinoma of head and neck cancers. The majority of oral cancers are oral squamous cell carcinoma (OSCC). Among the various etiological factors, oral microbes—bacteria are also associated with pathogenesis of OSCC. But only few studies have been done associating the presence of oral bacteriome with OSCC. The main aim of this review is to focus on association of microbes with OSCC, the pathogenesis, variation in bacteriome profiling in different geographic conditions, their role in pathogenesis of OSCC, and different samples and methods that are used to study their association with habits and tumour node metastasis (TNM) staging. To conclude, the imbalance in the oral bacteriome could be considered an etiological factor for OSCC. Since the bacteriome profiling varies greatly with geographic location and even in an individual in different locations of the oral cavity, it advocates more research. The study on identifying bacteria associated with OSCC will also enable their use as diagnostic markers and preventive management of OSCC.
Keywords
Oral bacteriome, oral squamous cell carcinoma, variationIntroduction
Oral cancers constitute 75% of head and neck cancers, and oral squamous cell carcinoma (OSCC) forms 90% of oral cancers [1]. The various etiological factors associated with OSCC include smoking, alcohol usage, betel nut use, tobacco chewing, diet, viruses, and genetic factors [2]. The oral cavity harbours more than 700 species of microbes, which include commensal and opportunistic bacteria, viruses, and fungi. They establish symbiotic relationships with one another and with the host inside the oral cavity [3]. Bacteria form the main population of the oral cavity. Proteobacteria, Firmicutes, Actinomycetes, and Bacillus are the primary bacteria that are seen inside the oral cavity [4]. This review focuses on (i) the association of microbes with OSCC; (ii) their role in the pathogenesis of OSCC; (iii) geographic variation in bacteriome profiling; (iv) bacterial association with different habits and “tumour node metastasis” (TNM) staging; (v) different samples and methods used to study these bacteria.
The primary factors that influence the existence of microorganisms inside the oral cavity are saliva and intraoral conditions that are present during tooth development. The additional factors that influence the existence of microbes inside the oral cavity are temperature (35°–36°C), redox potential (Eh, between –200 mV and +200 mV), oxygen (0–21%), pH (6.75–7.25), and the nutrients which may be exogenous or endogenous. The oxygen concentration is higher on mucosal surfaces, whereas it has gradients in plaque, favouring the existence of obligate anaerobes. Low Eh also paves the way for the existence of obligate anaerobes, with the lowest value at the gingival crevices. Endogenous nutrients that influence microbes include proteins, glycoproteins, and peptides from saliva and gingival crevicular fluid. Exogenous nutrients include foods like dietary sugars that favour acidogenic bacteria in plaque [5].
Microbiome as commensal
Microbes with antioxidant and anti-inflammatory properties are present in the human body. Microorganisms are known to contribute significantly to the maintenance of healthy digestive tract, regulation of the cardiovascular system, enhancement of the host’s defence mechanism, prevention of colonisation by harmful microorganisms, regulation of fat storage and metabolism, and provision of additional metabolic potential, as documented in previous studies [6].
The term “oral microbiome” pertains to microorganisms that inhabit the oral cavity [7]. The microbiome is composed of two distinct categories, namely the core microbiome and the variable microbiome. The term “core microbiome” pertains to the predominant microbial species observed in diverse anatomical locations of the human body in all individuals who exhibit sound health. On the other hand, the “variable microbiome” refers to microorganisms that are unique to each other and are influenced by factors such as the individual’s genetic composition, lifestyle, immune system, physiology, pathobiology, and surroundings [8].
There exists inter-individual variation in microbial composition across diverse racial and ethnic groups [9]. The oral microbiome is known to exert significant influence over cariogenic bacteria through the conversion of dietary nitrates into nitrites and nitric oxide. Nitric oxide demonstrates an anticariogenic property. When proteins, carbohydrates, and lipids from oral bacteria combine with those from saliva and gingival crevicular fluid, they form a pellicle that protects teeth from acid-induced demineralization [6].
The microflora maintains a largely stable composition at a site [5]. Alterations in the microbial ecosystem, such as changes in the microbe-host relationship, increased microbial abundance, or the acquisition of virulence factors, can trigger disease processes. The etiological factors that may precipitate these alterations encompass inadequate oral hygiene, immune system dysfunctions, and genetic predisposition [8].
Pathogenesis
Oral bacteria causing caries and periodontitis
In the oral cavity, the bacteria cause demineralization of tooth structure and infect the gums, causing caries and periodontitis respectively. Common caries-producing bacteria include Lactobacillus spp., Prevotella spp., Dialister spp., and Filifactor, and those that cause periodontitis are Fusobacterium nucleatum, Carbachia, Helicobacter, Clostridium, Porphyromonas, Actinomycetes, Tannella, Hurdella, Micromonas, Eugenia, and Streptococcus pneumoniae [4]. The identification of metabolites in the saliva produced due to oxidative stress has been observed to be a potential biomarker for the early detection of periodontitis [10].
Oral bacteria and associated systemic diseases
Oral bacteria are associated with various systemic diseases. Oral bacteria associated with the systemic diseases like diabetes, obesity, and rheumatoid arthritis are mentioned in Table 1 associated with them. Studies have identified that bacterial concentrations may vary significantly in specific systemic diseases. Some bacteria are present in high concentrations while others are present in low concentrations in specific systemic diseases studied [4]. These studies indicate that systemic diseases could be treated by altering the oral microbiota and pharmacologically targeting specific microbes.
Oral bacteria and associated systemic diseases
| Systemic diseases | Bacteria | |
|---|---|---|
| Increased abundance | Decreased abundance | |
| Diabetes | Selenomonas, Actinomyces, Capnocytophaga, Veillonella, Fusobacterium, Streptococcus | |
| Obesity | Plasmodium, Streptococcus mutans | Corynebacterium, Haemophilus, carbonophilic phage, Staphylococcus |
| Rheumatoid arthritis | Lactobacillus salivarius, Leptotrichia, Atopobium, Cryptobacterium curtum, Prevotella | Streptococcus, Corynebacterium |
Bacteria and carcinogenesis
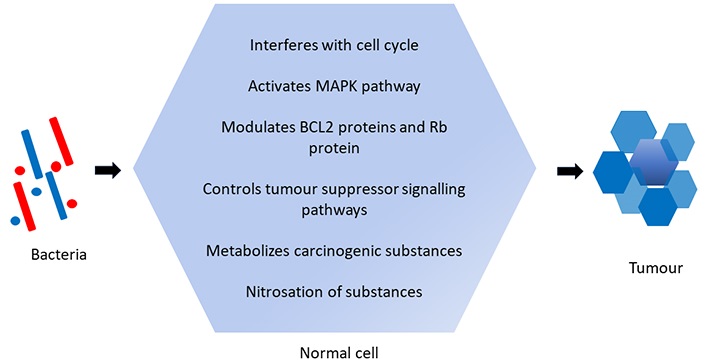
Bacteria are also known to cause cancer. More research is being carried out on bacteria and their role in carcinogenesis [2]. The various mechanisms by which bacteria can induce carcinogenesis include (Figure 1):
(i) Promoting modified cellular growth: The bacterial toxins interfere with the cell cycle and induce modified cellular growth.
(ii) Increased cellular proliferation and transformation: The presence of long-standing infections can induce cellular proliferation and replication by activating the mitogen-activated protein kinases (MAPK) pathway and cyclin D1. Genetic mutation results in cellular change.
(iii) Apoptosis inhibition: Bacteria modulate the B-cell lymphoma 2 (BCL2) protein and the retinoblastoma (Rb) protein, thereby interfering with apoptosis.
(iv) Suppression of signalling pathways: The signalling pathways that retard tumour growth are suppressed.
(v) Carcinogen metabolism: Microbes metabolise carcinogenic substances like ethanol that exist in the oral cavity into acetaldehyde which causes damage to DNA.
(vi) Nitrosation: Bacteria carry out nitrosation of substances inside the oral cavity, like the conversion of nitrites and amides to N-nitroso compounds that induce cell transformation [2].
The list of oral bacteria that are associated with cancer in the liver and pancreas is given in Table 2 [4].
Oral bacteria and associated cancers
| Cancer | Bacteria | |
|---|---|---|
| High percentage | Low percentage | |
| Liver cancer | Oribacterium, Clostridium, Ciliate, Actinomycetes, Campylobacter | Streptococcus, Pseudomonas, Haemophilus |
| Pancreatic cancer | Porphyromonas gingivalis, Actinobacillus actinomycete | |
Oral bacteriome and OSCC
Bacteria are also known to cause oral cancer. Various studies (Table 3) have been done to confirm the association of bacteria with OSCC. Bacteria like Streptococcus spp., Peptostreptococcus spp., Prevotella spp., Porphyromonas gingivalis spp., and Capnocytophaga gingivalis are strongly associated with OSCC. Fusobacterium, Clostridium, Enterobacteriaceae, Veillonella, Actinomyces, and Haemophilus are also associated with oral cancer and other epithelial precursor lesions [11]. Mager et al. [12] demonstrated high counts of bacteria in the saliva of OSCC patients when compared to normal subjects. Increased counts of Capnocytophaga gingivalis, Prevotella melaninogenica, and Streptococcus mitis were observed in the oral cancer patients when compared to non-cancer patients. The study also indicated that when the concentration of each species were ≥ 0.4 × 105 /mL, it could be used as a diagnostic indicator for OSCC with 80% diagnostic sensitivity and ≥ 82% diagnostic specificity.
Studies associating oral bacterial flora with oral cancer and factors that influence the type of flora
| Study subject | Author | Reference | Study purpose | Microbes present/role of microbes | Observation |
|---|---|---|---|---|---|
| Study associating oral bacterial flora with oral cancer | Karpiński | [11] | Review article | Streptococcus spp., Peptostreptococcus spp., Prevotella spp., Porphyromonas gingivalis, Capnocytophaga gingivalis | Strongly associated with cancer |
| Fusobacterium, Veillonella, Actinomyces, Clostridium, Haemophilus, Enterobacteriaceae | Associated with oral cancer and epithelial precursor lesions | ||||
| Mager, et al. | [12] | To determine salivary microbiota as a diagnostic indicator of oral cancer | Capnocytophaga gingivalis, Prevotella melaninogenica, Streptococcus mitis | Each of the 3 species when concentration ≥ 0.4 × 105 /mL, could be used as a diagnostic indicator for OSCC with diagnostic sensitivity and specificity ≥ 80% | |
| Study on factors that influence bacterial flora in oral cavity | Mason, et al. | [13] | To identify ethnicity (African Americans, Caucasians, Latinos, and Chinese) affects oral microbiome composition | Filifactor, Mycoplasma, Staphylococcus, Treponema | Ethnicity specific oral microbiome was identified. Filifactor, Mycoplasma, Staphylococcus, and Treponema were increased in abundance in Chinese and Latinos |
| Li, et al. | [14] | To illustrate distinctiveness of saliva microbiome under different climatic conditions (Alaskans, Germans, and Africans) | Bacterial genera were uniquely present in only one human group: 48 in the Alaskan group, 37 in the German group, and 22 in the African group. Gemella-Granulicatella, Capnocytophaga-TM7_genera_incertae_sedis, Actinomyces-Veillonella, and Haemophilus-Veillonella were seen in all the three groups | Saliva of humans under different climate presents different composition | |
| Kato, et al. | [15] | To determine the role of diet in oral microbiome | Saturated fatty acids showed positive association with Betaproteobacteria and Fusobacteria. Vitamins exhibited positive association with Leptotrichiaceae and Lachnospiraceae. Lactobacillaceae was observed in abundance in people with glycaemic load | Oral bacteria are associated with diet intake and diet effects are habitat specific | |
| Simón-Soro, et al. | [16] | To identify bacteria in various sites in oral cavity | Genera Streptococcus were seen in 40% to 70% on the vestibular surface of incisors and canines but were almost absent on the lingual side | Bacterial composition varies between different teeth surfaces | |
| Demmitt, et al. | [17] | To find association between human genes and oral microbiome | Most heritable was OTU4483015 of Granulicatella species | The loci that could have an effect on microbial phenotypes were identified to be located on chromosomes 7 and 12 |
Though the association between bacteria and cancer and its role in carcinogenesis is understood and established, the bacterial profile is known to vary among individuals of different races and ethnicities. Various factors that could contribute to changes in the microbiome include age, lifestyle, environment, medication, and nutrition [9]. In a study by Mason et al. [13] to identify the role of ethnicity as a determinant factor of oral microbial flora composition, 192 healthy individuals of 4 different ethnicities were studied in the U.S. In the study, the subgingival microbial flora was identified as being specific to each ethnic group and individuals were identified of their ethnicity based on the subgingival microbial composition. Machine learning classifier detected Americans with sensitivity and specificity of 100% and 74%, respectively. Caucasians in the study were identified with sensitivity and specificity of 50% and 90%, respectively [13]. Furthermore, a study by Li et al. [14] also demonstrated the difference in salivary microbial profiles in people who lived in different climatic conditions and locations. The study involved Alaskans, Germans, and Africans. It was observed that Germans and Africans shared 3 genera in common—Actinobacillus, Capnocytophaga, and Aggregatibacter. Alaskans and Africans shared 6 genera in common—Campylobacter, Granulicatella, Neisseria, Actinomyces, Selenomonas, and Megasphaera. While three genera (Streptococcus, Fusobacterium, and Leptotrichia) were of the same abundance in all groups, Gemella, Rothia, Escherichia, Veillonella, Citrobacter, and Enterobacter were of various abundances in all groups [14].
Variation in bacterial profile was also noticed with diet in the studies. In a study to determine the correlation between diet and oral microbiome, Kato et al. [15] demonstrated saturated fatty acids showing a positive association with Betaproteobacteria and Fusobacteria. Vitamins exhibited positive associations with Leptotrichiaceae and Lachnospiraceae. Likewise, Lactobacillaceae was observed in abundance in people with high glycaemic load. Even within an individual, differences in the microbial niche are noticed at different locations inside the oral cavity. Within the oral cavity, various factors like oxygen, pH, Eh, and temperature are known to influence the microbial compartment. The study by Simón-Soro et al. [16] showed great differences in the microbial niche between teeth and their surfaces. The presence of the genera Streptococcus was seen in 40% to 70% of vestibular surface of incisors and canines while it was nearly absent on the lingual side [16]. This difference in the microbiome inside the oral cavity is attributed to the “lock and key” mechanisms. The bacteria contain adhesins and they can bind only to specific receptors located on the oral mucosal surfaces [12].
Apart from external factors that could influence the components of the oral bacterial flora, genetic factors are also known to influence the components. Genetic influence on the oral microbiome was noted in the largest twin oral microbiome study by Demmitt et al. [17]. In this study, 752 twin pairs were studied and it was observed that some microbiome phenotypes were heritable. The loci that could influence microbial phenotypes were identified to be located on chromosomes 7 and 12, which are near the gene inner mitochondrial membrane peptidase subunit 2 (IMMPL2) and the non-coding RNA gene inhibin subunit beta A antisense RNA 1 (INHBA-AS1), respectively.
Diagnostic methods
New methodologies to study bacteria
The literature reveals various methods that are used to detect microbes in the sample. These include culture and microscopy, gel-based techniques, polymerase chain reaction-based methods, and DNA microarrays. The other new techniques that are used to study the DNA sequence of the organism include 16S ribosomal RNA (rRNA) sequencing, whole metagenome shotgun (WMS) sequencing, metabolomics, metaproteomics, and metatranscriptomics.
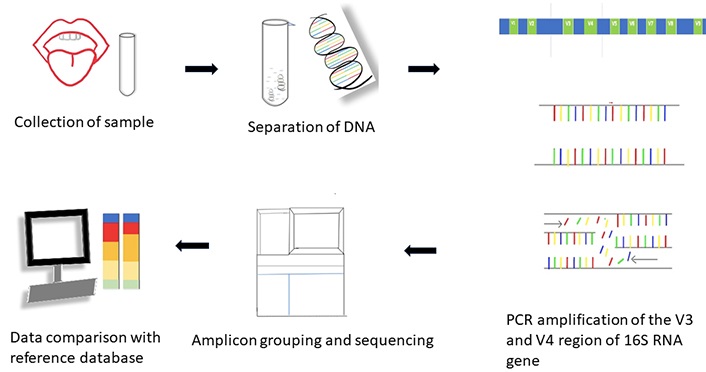
16S rRNA amplicon sequencing (Figure 2): The 16S rRNA gene is present in all bacteria and archaea with highly conserved regions. It comprises hypervariable regions from V1 to V9. V3 and V4 are the target regions in bacterial studies because the sequences in these regions are species-specific. This enables the use of 16S rRNA as a valuable marker gene and allows the use of universal primer sequences to separate it for sequencing. In this method, the DNA sequences are read from the samples and compared with a database of sequences to identify the organisms present. This method is also known as 16S barcoding [18].
In WMS sequencing, DNA sequences are read and compared with database sequences. DNA is fragmented, sequenced, and then reassembled by connecting the overlapping ends into full or partial genomic sequences. Information on the total DNA content of the organisms is obtained by this method.
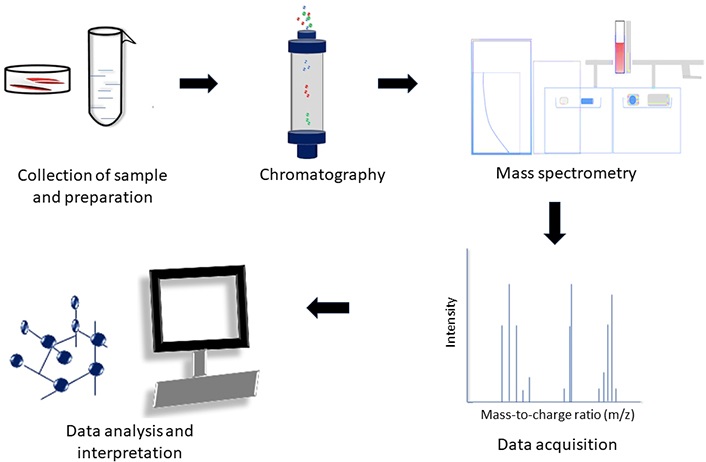
Metabolomics (Figure 3) is the study of metabolites in the sample. This technique involves chromatography and mass spectrometry. The metabolites in the sample are identified and quantified. Metabolites identified can act as an indicator of the status of the sample. In research involving microbial metabolites in the samples, extensive study is required to identify the source of the metabolites (whether the metabolites identified were produced by the host or the microorganisms) and to associate the metabolites with the relevant pathways.
Metaproteomics is used to list the proteins in the sample and employs protein extraction and tandem mass spectrometry analysis. The challenges associated with this method are data processing due to incomplete protein databases and the presence of similar peptides in homologous proteins in various organisms.
Metatranscriptomics involves obtaining the entire microbial gene expression of the sample by targeting the mRNA content. But in this method, the instability of mRNA and excess rRNA can pose challenges. The availability of reference databases is also limited. Both metaproteomics and metatranscriptomics determine the functional activity of the microbiome [18].
Samples used to study the oral microbiome
Various sample sources are used to study the microbial flora. This includes tissues, swabs, and saliva. Tissue and saliva samples are used in many studies. The study by Zhao et al. [19] involved the use of a swab to study the microbial profile. The study revealed greater microbial diversity in OSCC samples than on the normal mucosal surface [19]. Oral rinse is also used to determine the bacterial profile. The sensitivity and specificity for the identification of oral cavity and oropharyngeal cancer through a microbial profile using oral rinse have been proven to be 100% and 80%, respectively [20].
These studies indicate that in conditions when a biopsy cannot be performed, saliva, swabs, and oral rinse could be used to study oral microbial flora.
Bacteria and carcinogenesis associated with staging, metabolites, and habits
Various studies have been conducted on OSCC and bacteria in relation to metabolites present, viruses, TNM staging, habits, and molecular analysis (Table 4).
Studies associating bacteria and tumour with metabolites, HPV virus, and treatment
| Author | Reference | Study purpose | Microbes present/role of microbes | Observation |
|---|---|---|---|---|
| Lohavanichbutr, et al. | [21] | To identify salivary metabolites profiling in oral cavity squamous cell carcinoma/oropharyngeal squamous cell carcinoma and normal cases, and between oral cavity squamous cell carcinoma with and without nodal metastasis. | Oral microbes could also influence the type and level of metabolites. | Glycine, proline, ornithine, and citrulline are associated with early stage of oral cavity squamous cell carcinoma. |
| Guerrero-Preston, et al. | [22] | To identify microbiota associated with oropharyngeal and oral cavity squamous cell carcinoma patients, HPV, and before and after surgical treatment. | Tumour samples exhibited increased bacterial flora, including Dialister, Streptococcus, and Veillonella. Only HPV positive patients have an abundance of genus Gemellaceae and Leukonostoc. Abundance of Proteobacteria, Firmicutis, and Bacteroidetes present before surgery. | Difference in Bacterial flora abundance in tumour samples and normal samples. Bacterial flora’s abundance varies in HPV positive and negative patients. Bacterial flora’s abundance varied before and after surgery. |
| Kadam, et al. | [23] | To identify role of oral microbiome as a tool of management in tobacco associated oral cancers. | Increased bacteria in oral cancer due to smokeless form of tobacco—Porphyromonas gingivalis, Streptococcus anginosus, Veillonella, Prevotella intermedia, Clostridium, Fusobacterium nucleatum, Actinomyces, Escherichia coli, Haemophilus parainfluenza, Streptococcus anginosus, Abiotrophia species, Enterococcus faecalis. | Microbial profiling could be used as screening tool and in treatment strategies. |
| Hooper, et al. | [24] | To characterize bacterial flora inside oral cancer tissue. | Fusobacterium naviforme, Clavibacter michiganensis subsp. tessellarius, and Ralstonia insidiosa were in > 30% more of tumourous samples than non-tumourous samples. Saccharolytic and aciduric species: Proteobacteria and members of genera Streptococcus, Prevotella, Fusobacterium, and Veillonella. | Most of the bacteria identified were saccharolytic and aciduric species. |
In a study to identify the use of salivary metabolites as biomarkers in oral cavity squamous cell carcinoma, it has been demonstrated that the levels of metabolites like glycine and proline are different in oral cavity squamous cell carcinoma cases than in controls. Also, glycine, proline, ornithine, and citrulline were found to be associated with the early stage of oral cavity squamous cell carcinoma. The metabolites are observed to vary in similar studies done before due to variations in the population studied, site of tumour, sample collection method, and also the type of microbes in the oral cavity [21]. This study signifies the importance of further research required for the identification of metabolites produced by bacteria and their differentiation from the metabolites that are produced by humans in conditions of OSCC. Research on the identification of specific metabolites will enable the use of metabolites as diagnostic markers in OSCC. The treatment method can also be made more efficient and customised for patients following their identification.
Guerrero-Preston’s study identified the association between bacteria Lactobacillus spp. and head and neck squamous cell carcinoma (HNSCC) samples [22]. The presence of Lactobacillus was highly detectable in cancer samples and the abundance of the bacteria increased along with increasing TNM staging. The study implied that in saliva, an abundance of Lactobacillus and a depletion of Gemellaceae, Hemophilus, Neisseria, or Aggregatibacter may serve as HNSCC biomarkers. The study also demonstrated that Megasphaera, Veillonella, and Gemelaceae could be possible biomarkers for HPV positive HNSCC tumours. The study found a reduction in alpha diversity measure in the samples taken before and after surgery, suggesting their use as potential biomarkers [22].
Tobacco has been shown to modify the microbiome inside the oral cavity, resulting in chronic inflammation and cellular invasion. Additionally, the altered microbiota induces the proliferation of cells and prevents apoptosis. All these factors, along with the production of carcinogens, lead to carcinogenesis. High levels of bacteria like Fusobacterium nucleatum are found in smokers. They produce sulphur compounds that stimulate cellular proliferation, migration, invasion, and angiogenesis. Therefore, microbial profiling can be used as a screening tool, that will help in identifying the precursor lesions and carcinogenic process. Similarly, intervention with these microbial growths and the discovery of drugs against them can prevent carcinogenesis [23].
In a study on molecular analysis of bacteria present in OSCC, tumourous and non-tumourous tissue samples were collected from OSCC patients. The specimens were subjected to PCR cloning and 16S rRNA gene sequencing. Fifty-two phylotypes were identified from tumourous tissue and 37 taxa from non-tumourous tissue. Organisms like Ralstonia insidiosa, Clavibacter michiganensis subsp. tesselarius, and Fusobacterium naviforme were present ≥ 30% of the tumourous sample. The majority of the species that were identified in the tumour samples in the study were aciduric and saccharolytic, indicating the presence of an aciduric and hypoxic environment in the tumour area [24]. The higher richness and diversity of bacteria in tumour areas when compared to non-tumour areas (opposite normal tissues of the same OSCC patients) were also demonstrated in a work by Zhang et al. [25]. The tumour tissues were enhanced with Peptostreptococcaceae, Prevotellaceae, Flavobacteriaceae, Campylobacteriaceae, Lachnospiraceae, Fusobacteriaceae, and 13 other genera. Similarly, genes associated with pathological processes like bacterial chemotaxis were also increased in OSCC [25]. The preceding studies demonstrate that understanding bacterial profiles enables their use as diagnostic markers and treatment targets.
Although numerous investigations on the microbial—bacterial communities in the OSCC were conducted, the findings were different in terms of the species that are associated with OSCC. This could be attributed to the fact that studies relied on the compositional analysis and not on the functional analysis of the bacteriome. A study on functional analysis by Perera et al. [26] identified an increase in lipopolysaccharide biosynthesis and peptidases in OSCC which are proinflammatory bacterial inflammatory attributes. The study indicated that dysbiotic, inflammatory bacteriome is linked to OSCC, and microbial communities with varying species can exhibit similar functionality [26].
Prevention of bacteria associated carcinogenesis
Various substances are being studied to control carcinogenesis that is induced by microorganisms. Candida and bacteria convert alcohol to a carcinogenic substance like acetaldehyde. In cases of acetaldehyde associated carcinogenesis, a non-essential acid, cysteine has been found to prevent carcinogenesis by forming a stable—thiazolidine-carboxylic acid compound. Additionally, cysteine has been demonstrated to lessen metastasis in animal models. Similarly, compounds like carotenoids, retinoids, and vitamins A and E are known to exhibit antimicrobial activity [27]. Studies were conducted on the use of antibodies to control tumour growth. TLR is a toll-like receptor family that signals the body about the pathogens. Farnebo et al. [28] in their study have proven their pro-tumourigenic role. The pathogen associated molecular patterns (PAMPs) motifs, like components of the cell wall and DNA, attach to these receptors and result in activation of the MAPK and nuclear factor kappa B (NF-κB) pathways [28].
The NF-κB pathway has been observed to play a function in the process of tumourigenesis through various mechanisms such as preventing apoptosis, regulating tumour angiogenesis, promoting metastasis, and modulating tumour metabolism. NF-κB inhibits apoptosis by inducing the expression of antiapoptotic genes such as FLICE like inhibitory protein (FLIP), an inhibitor of caspase-8. The genes targeted by NF-κB have been found to play a role in the process of angiogenesis. NF-κB is responsible for the regulation of cell adhesion molecules, including selectins and integrins, which in turn govern the process of metastasis. The upregulation of glucose transporter 3 (Glut3) expression has been observed to increase glucose uptake in p53–/– mouse embryonic fibroblasts through the activation of NF-κB [29].
MAPK through early gene encoded c-Jun and c-Fos proteins, has been observed to promote cell cycle progression from G1 to S phase. Furthermore, it promotes the proliferation of tumours through the inhibition of the expression of the cell cycle inhibitory protein p27 [30].
In the study conducted by Fornebo et al. [28], it was identified that activation of the receptor TLR2 in HNSCC cell lines results in tumour growth. Therefore, the use of antibody against this receptor could greatly constrain tumour growth [28]. These antibodies could be very useful in treating cases of infection-derived inflammation that induces carcinogenesis.
Research is also being conducted with attenuated bacterial vaccines, bacteria containing nanoparticles carrying genes, and nano sensors to detect and treat cancer. It is essential to detect cancer early, as it is known to improve the 5-year survival rate to 80–90% [31].
Prevention of oral cancer
The incidence of oral cancer continues to rise despite advancements in diagnostic and therapeutic approaches aimed at its management and prevention. The early detection of tumours is becoming increasingly important due to its significant impact on the survival rate of patients. The emphasis is also placed on the identification of a cost-effective diagnostic approach that can facilitate timely detection.
Various studies have been conducted with saliva as a research tool in the study of HNSCC. A study by Sayal et al. [32], demonstrated that cell free mitochondrial DNA in saliva can serve as potential predictor of the overall survival of patients with HNSCC. Also, the study has established that saliva can be considered a non-invasive and dependable source [32].
Zhou et al. [33] conducted a study which revealed that the detection of microorganisms present in saliva can result in a diagnosis of OSCC.
The “salivaomic” profile, which is the result of integrating data from the microbiome, transcriptome, proteome, metabolome, and exosome analysis, is a very promising area of research to explore for translational biomarkers [34].
Though the primary strategy for preventing the occurrence of oral cancer is to increase public awareness of habits, the studies mentioned above highlight the significance of acknowledging the role of an imbalanced oral microbiome in the pathogenesis of OSCC and the feasible methods to identify it. Extensive research on the oral microbiome’s diversity between racial groups and geographical regions using samples like saliva may facilitate early, non-invasive, and cost-effective detection of OSCC. Additionally, it could contribute to the development of customised medical interventions with the potential to substantially decrease the prevalence of oral cancer. The various levels at which preventative measures may be implemented is illustrated in Figure 4.
Conclusions
Bacteria exist as commensals inside the oral cavity and the type of flora varies with ethnicity and geographic area. With a change in the environment inside the oral cavity, bacteria could result in carcinogenesis. With proper sample collection methods and advanced analytical techniques, the type of bacteria, its abundance, and the corresponding metabolites could be used as diagnostic markers.
Abbreviations
| Eh: |
redox potential |
| HNSCC: |
head and neck squamous cell carcinoma |
| MAPK: |
mitogen-activated protein kinases |
| NF-κB: |
nuclear factor kappa B |
| OSCC: |
oral squamous cell carcinoma |
| rRNA: |
ribosomal RNA |
| TNM: |
tumour node metastasis |
Declarations
Author contributions
KP: Conceptualization, Writing—original draft, Visualisation. SKM, RK, and MM: Supervision, Writing—review & editing. RR: Visualization, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2023.