Affiliation:
1Department of Veterans Affairs, Veterans Health Administration, Phoenix, AZ 85012, USA
2Department of Medical Sciences, Ross University School of Medicine, Bridgetown BB11093, Barbados
3Department of Neurology, University of Arizona College of Medicine, Phoenix, AZ 85724, USA
Email: ZackMerhavy@gmail.com
ORCID: https://orcid.org/0000-0002-8860-1980
Affiliation:
1Department of Veterans Affairs, Veterans Health Administration, Phoenix, AZ 85012, USA
2Department of Medical Sciences, Ross University School of Medicine, Bridgetown BB11093, Barbados
ORCID: https://orcid.org/0009-0002-9444-6670
Affiliation:
1Department of Veterans Affairs, Veterans Health Administration, Phoenix, AZ 85012, USA
2Department of Medical Sciences, Ross University School of Medicine, Bridgetown BB11093, Barbados
Affiliation:
1Department of Veterans Affairs, Veterans Health Administration, Phoenix, AZ 85012, USA
2Department of Medical Sciences, Ross University School of Medicine, Bridgetown BB11093, Barbados
Affiliation:
1Department of Veterans Affairs, Veterans Health Administration, Phoenix, AZ 85012, USA
2Department of Medical Sciences, Ross University School of Medicine, Bridgetown BB11093, Barbados
Affiliation:
1Department of Veterans Affairs, Veterans Health Administration, Phoenix, AZ 85012, USA
3Department of Neurology, University of Arizona College of Medicine, Phoenix, AZ 85724, USA
4Colangelo College of Business, Grand Canyon University, Phoenix, AZ 85017, USA
ORCID: https://orcid.org/0000-0002-3586-2909
Explor Med. 2023;4:739–746 DOI: https://doi.org/10.37349/emed.2023.00174
Received: April 17, 2023 Accepted: July 26, 2023 Published: October 27, 2023
Academic Editor: Shannon L. Risacher, Indiana University School of Medicine, USA
The patient is a 58-year-old male who presented with chief complaints of right-sided numbness, tingling, and loss of temperature sensation in the upper and lower extremities. The patient’s symptoms began around the face and right corner of the mouth [maxillary/mandibular (V2/V3) distribution] before descending to the arm, trunk, and followed by the lower leg and foot. His home medication regimen included lisinopril, atorvastatin, long and short-acting insulin, and amlodipine. During the interview, the patient admitted to abstinence from his medications. Upon examination, the patient was found to have a loss of hot and cold touch on the right side and expressed 2+ reflexes (brisk response; normal) on both upper and lower extremities. In the initial work-up of the patient, he received a computed tomography (CT) scan which demonstrated an area of potential ischemic infarct of one of the left sided pontine perforator arteries. Immediately at that time he was given a loading dose of 325 mg aspirin and started on 81 mg daily. Because of the patient’s symptoms and risk factors, he was hospitalized for further additional work-up and eventually discharged on dual antiplatelet therapy. This case is intriguing as both neuroradiological reading and neurological examination helped with localization of the lesion and changing the treatment strategy of the patient. With a pontine perforator ischemic event, the harms of treatment with thrombolytics would have outweighed the benefits. This interprofessional work between neuroradiology, internal medicine, and neurology ensured that the patient received the best care for his specific ailments.
Etiologies of cheiro-oral-pedal syndrome (COPS) are ischemic stroke, hemorrhagic stroke, and less commonly: neoplasm, intracranial bypass complications, cervical cord disorder, vascular malformation, aneurysm, infection/abscess, seizure, middle cerebral artery (MCA) stenosis, dermoid cyst, stereotactic surgery, drug, and other idiopathic causes [1]. Reported areas of involvement in COPS include the capsule-striatum, thalamus, medulla oblongata, corona radiata, pons, fronto-parietal cortex, multiple sites, or other unknown sites [2, 3]. Six rare cases have pointed to spinal cord involvement after findings of aggravated paresthesia when flexion and extension manipulations to the neck were performed [2]. Risk factors contributing to COPS include hypertension, hyperlipidemia, diabetes mellitus, trauma, and vessel malformations [3].
A hemispheric or brainstem infarct may be labeled as large or small depending on the extent of the area of occlusion, where a brainstem infarct is classified as large when it occupies over one-fourth of the specific brainstem section, and small when it is any less [1]. A hemispheric infarct is considered large when it occupies over one-third of the area inhabited by the MCA and small if it is less than one-third [1]. Classic presenting symptoms include paresthesia, numbness, and loss of temperature sensation in regions of the face—particularly the corner of the mouth, the trunk, and extremities [4]. The medial lemniscus, thalamocortical projections, or thalamic areas could all play a role in the sensory manifestations of COPS. The medial lemniscus fibers in the pons work as afferent pain and temperature sensation receptors and are in close proximity to sensory fibers from the head, trunk, and extremities [4].
Symptoms are further classified as type I, type II, type III, or type IV depending on the localization and size of the infarct as discussed earlier. Type I involves sensory impairment to the perioral area and ipsilateral hand/foot, type II involves perioral and hand/foot sensory deficits bilaterally (typical bilateral), type III is a sensory impairment in either the perioral area or the hand/foot bilaterally with the other area having unilateral impairment (atypical bilateral), and type IV being a sensory impairment confined to the perioral area and the contralateral hand/foot in a crossed pattern [3, 5].
Ischemic strokes follow a specific resolution timeline, where the first step is development of a red nucleus, occurring 12–24 h after infarction [1]. Following the development of the red nucleus is liquefactive necrosis due to neutrophil infiltrate 24–72 h after infarction [1]. Increased microglial activity occurs 3–5 days post-infarction, and up to two weeks later, reactive gliosis and vascular proliferation are achieved [1]. Glial scarring is the last step to occur in this timeline of ischemic recovery [1]. Signs of recovery may be seen on follow-up imaging within one to two weeks of infarction, and in instances where infarction reoccurs, it is likely due to insufficient vessel healing time [5].
The therapy of choice for a large-vessel ischemic stroke treatment is tissue plasminogen activator (tPA), given within three hours of symptom onset [6]. COPS involvement of small vessels makes this treatment option less favorable due to the outweighed harm of increased chance of serious bleeding [6]. An antiplatelet drug such as aspirin then becomes an effective alternative treatment with less chance of complication compared to tPA [6].
The patient is a 58-year-old male who presented with chief complaints of right-sided numbness, tingling, and loss of temperature sensation in the right upper and lower extremities. The patient’s symptoms began three hours prior to admission in regions of the face including the right corner of the mouth [maxillary/mandibular (V2/V3) distribution] before descending to the arm, trunk, and finally the lower leg and foot. Past medical history is significant for type 2 diabetes, hyperlipidemia, hypertension, a 47-pack-year smoking history, ethanol (EtOH) abuse disorder, and cocaine use. The patient denied any headache, dizziness, nausea, or vomiting on presentation.
Upon initial exam, the patient was found to have a loss of hot and cold touch on the right side and expressed 2+ reflexes (brisk response; normal) on both upper and lower extremities. His home medication regimen included 25 mg lisinopril, 80 mg atorvastatin, 30 units glargine, 10 units aspart, and 10 mg amlodipine. Upon interview, the patient admitted to abstinence from his medications.
In the initial work-up of the patient, he received a computed tomography (CT) scan which demonstrated an area of potential ischemic infarct of one of the left sided pontine perforator arteries. Immediately at that time he was given a loading dose of 325 mg aspirin and started on 81 mg daily.
This patient’s pure sensory presentation with complete hemisensory syndrome (with a facio-brachio-crural distribution of sensory deficit) is typically due to a lacunar infarct in the thalamic ventral posterolateral nucleus topography as can be seen in many cases [7].
Early in the process of performing a thorough history, differential diagnosis of hypothyroidism, syphilis, vitamin B deficiency, stroke, and other conditions that frequently present with sensory deficits in the unilateral distribution described by patients may remain highly probable. Upon completion of a detailed neurological exam exploring the existence of any motor deficits along with the characteristic unilateral or bilateral sensory deficits located at the corner of the mouth, hand, and/or foot, with or without thoracic flank/trunk involvement is highly suggestive of cheiro-oral syndrome (COS) or cheiro-oral-pedal syndrome caused by an underlying central nervous system pathology. The differential diagnosis begins to narrow towards cerebral or brainstem infarction, intracranial hemorrhage, brain tumor, migraine, and unique presentation of encephalopathy/encephalitis (if corresponding history applies), and referral for high quality imaging and interpretation from a neuroradiologist is the preferred next step in evaluation [2]. Since the location and deterioration of COS/COPS cannot be predicted by clinical symptoms alone, COS/COPS should be considered an emergent condition for aggressive investigation until the fatal cause is substantially excluded [3].
The quality of the imaging and interpretation is crucial for the exclusion of more significant underlying conditions such as cavernous malformation [4]. The diagnostic modality of choice in evaluating for a suspected COPS diagnosis is magnetic resonance imaging (MRI), with the use of susceptibility weighted imaging (SWI) sequence that shows prior hemorrhage and multifocal lesions when done at 3-Tesla (3T) [4]. This particular high-resolution imaging modality is useful in monitoring and appropriately managing the progression of the disease as resolution and reinfarction are common. If repeated hemorrhages lead to progressive neurological morbidity, surgery may be considered [4]. The patient in this case received a CT scan likely due to insurance authorization limitations, which demonstrated a poorly defined area of mild hypodensity in the area supplied by the left pontine perforator branches.
The areas that are most probable for the evaluating radiologist to discover an offending lesion are the thalamus, pons, and cortex (> 80% of cases) [5]. Subtypes of COS/COPS exist to further distinguish both location of lesion as well as likelihood of occurrence (types I, II, III, or IV). For type I COS/COPS, the most commonly identifiable lesions were found first in the thalamus, then pons, and cortex [5]. For types II and III, the pons is the most likely affected area, and type IV is most likely to present with a medulla lesion [5]. However, it should be noted that a significant portion of type I and II COS/COPS presentations (> 18%) may be classified as “unknown”, which includes etiologies such as isolated MCA stenosis, post intracranial bypass surgery complications, or rare drug side-effects, but still the largest proportion of cases included in the “unknown” category presented without identifiable etiology or an identifiable lesion upon imaging (approximately 10%) [5]. Irrespective of the specific subtype of COS/COPS, the most common location of lesions is found at the thalamus, followed by the pons, cortex, internal capsule, cervical cord, corona radiata, medulla oblongata, midbrain, or at multiple sites [2, 5, 8].
Blood analysis tests consisting of: complete blood count (CBC), comprehensive metabolic panel (CMP), thyroid-stimulating hormone, serum lead and copper, vitamin B1, B9, and B12, rapid plasma re-antigen for syphilis, liver function test, erythrocyte sedimentation rate, and QuantiFERON tuberculosis (TB) Gold test were assessed. Results indicated an elevated hemoglobin A1c measurement of 14.4%. The patient’s laboratory tests also indicated cholesterol levels elevated at 298 mg/dL, elevated triglycerides at 497 mg/dL, and low-density lipoprotein (LDL) at 203 mg/dL. Liver function test indicated a slightly elevated alanine transaminase (ALT) to aspartate transaminase (AST) ratio. The patient had decreased high-density lipoprotein (HDL) levels at 34 mg/dL. Vitamin D was low, indicating levels less than 13 ng/mL. The other test results were within normal limits (WNL), as indicated in Table 1.
Blood analysis test results
| Test | Findings | Reference values | Interpretation |
|---|---|---|---|
| Hemoglobin A1c | 14.4% | < 5.7% | Elevated |
| Thyroid-stimulating hormone | 1.198 mIU/L | 0.4–4.0 mIU/L | Normal |
| Serum lead and copper | Lead: 0 μg/dL Copper: 95 μg/dL | Lead: < 10 μg/dL Copper: 62–140 μg/dL | Normal |
| Vitamin B1, B9, B12 | B1: 5 μg/dL B9: 8 ng/mL DFE B12: 307 pg/mL | B1: 2.5–7.5 μg/dL B9: 2.7–17.0 ng/mL DFE B12: 160–950 pg/mL | Normal |
| Rapid plasma re-antigen for syphilis | Negative | Positive/negative | Non-reactive |
| Liver function test ALT/AST | ALT: 49 U/L AST: 52 U/L | ALT: 4–36 U/L AST: 8–33 U/L | Elevated |
| QuantiFERON TB Gold | Negative | Positive/negative | No TB |
| Vitamin D | < 13 ng/mL | 20–40 ng/mL | Low |
| Erythrocyte sedimentation rate | 16 mm/h | 0–22 mm/h | Normal |
| Cholesterol | 298 mg/dL | < 200 mg/dL | Elevated |
| LDL | 203 mg/dL | < 130 mg/dL | Elevated |
| HDL | 34 mg/dL | > 40 mg/dL | Low |
| Triglycerides | 497 mg/dL | < 150 mg/dL | Elevated |
DFE: dietary folate equivalent
The following physical examinations were conducted on the patient: head ears eyes neck throat (HEENT), cardiac, respiratory, abdominal, and neurologic motor and sensory. Significant findings included a cardiac exam which indicated tachycardia with an irregular rhythm. Neurologic exam indicated cranial nerves I–XII were intact bilaterally. Motor exam showed full 5/5 strength (normal) bilaterally to the biceps, triceps, grips, quadriceps, hamstrings, dorsiflexors, and plantar flexors. Tendon reflexes were 2+ graded strength at the biceps, triceps, Achilles, and quadriceps. The patient initially reported a loss of hot and cold touch on the right side, however, the sensory exam found that the patient’s temperature insensitivity had resolved, and the results of both the right and left sensations were WNL. Cerebellar exam showed normal finger-to-nose testing, normal rapid motion testing, and normal stereognosis. The patient also had a negative Romberg, Babinski, and Hoffman test.
Imaging was required in order to properly assess the patient by locating the exact location of the infarct. A cranial CT scan was obtained. As seen in Figure 1, the results from this scan indicated a potential ischemic infarct of the left pontine perforator arteries, and no other observations were noted on the CT. Localization of the potential infarct allowed for the construction of the patient’s treatment plan. Due to rapid symptom resolution and associated financial burden on the patient, more detailed imaging was not pursued. As stated, MRI is considered the gold standard in cases such as this, however, due to identification of the patient’s lesion on CT, the care team deemed MRI unnecessary at the time.
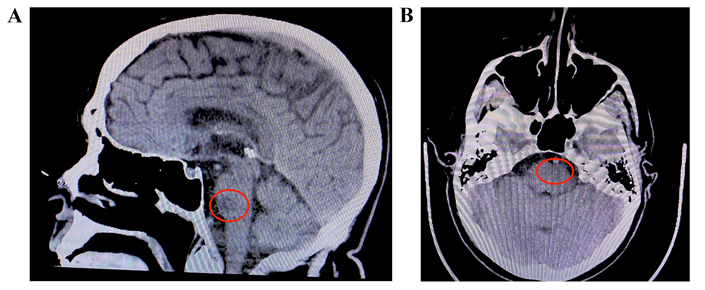
A) Sagittal view of infarct. Red circle indicates the potential ischemic infarct in the left pontine perforator region; B) transverse view of infarct. Red circle indicates the potential ischemic infarct in the left pontine perforator arteries with no additional lesion
An echocardiogram was ordered at the time of the neurological symptoms to rule out a patent foramen ovale. The echocardiogram did not demonstrate any evidence of an intracardiac shunt, and the left ventricular ejection fraction was deemed to be 60% to 65%. A carotid artery ultrasound with Doppler was also ordered which did not demonstrate any significant level of carotid artery stenosis.
Management options for similar infarcts include observation or surgery (surgical resection, radiosurgery, or stereotactic laser ablation) [4]. In this case, observation was selected as this patient had only presented with sensory symptoms, and the lesion was located in the deep pons, where the morbidity associated with such intervention is high [9]. In the case that the patient presented with progressing symptoms, surgical options would have been more highly considered.
The patient was prescribed dual antiplatelet therapy of 81 mg of aspirin and 75 mg of clopidogrel to take at home for 21 days and restarted his home medication regimen. Nevertheless, the patient returned to the hospital one week later with continued abstinence from medication. Surprisingly, the symptoms of the initial infarct had begun to self-resolve before a new infarct and symptoms arose another week later. This infarct-resolving pattern continued a total of three times before the patient was discharged on a statin regimen and scheduled neurology follow-up. Throughout the entire clinical course, the patient only complained of his initial symptoms of numbness on the right side and continued to deny headache, nausea, vomiting, or dizziness.
COPS is a rare neurologic syndrome characterized by paresthesia of the corner of the mouth, hand, and foot. The literature has described cases where COPS presents secondary to midbrain and pontine hemorrhage [10], secondary to thalamic infarctions [11], and secondary to brainstem hematomas [12]. This variety of presentations makes the diagnosis of COPS difficult and potentially delayed. In this case, COPS is presented uniquely and has seldom been documented as this patient’s syndrome occurred secondary to a left pontine perforator artery infarction, which caused contralateral symptoms [13]. As stated previously, COPS is typically due to damage in the brainstem ipsilateral to the symptoms. In the pons, the sensory fibers from the mouth, arm, and leg are located from the medial to lateral side in the area of the medial lemniscus [14]. The ventral trigeminothalamic tract, which transmits epicritic sensation from the face, is medially situated and is dorsal to medial lemniscus [15]. Damage to the medial lemniscus and ventral trigeminothalamic tract in the mid-pons could explain our patient’s sensory deficit. A critical point to note is the rarity of incomplete pure hemisensory symptoms resulting from medial lemniscus pontine lesions. The significance of this case report lies in its unique presentation that is not commonly observed in clinical practice. This is further supported by recent research, such as the study published in Acta Neurologica Belgica in 2020, which found that none of the 19 consecutive incomplete pure sensory stroke patients from a total of 99 patients with pure sensory stroke included in a stroke registry over a 19-year period showed the pons as the brain lesion lacunar site [16]. Therefore, this case report highlights the importance of recognizing the atypical presentation of neurological symptoms and the need for further research to better understand the underlying mechanisms of such rare cases.
With this unique presentation and the involvement of such small arteries, the radiologist’s evaluation of the imaging was crucial to arrive at the correct diagnosis. Though the use of 3T MRI with SWI sequence is currently considered the best option when diagnosing COPS [4], in this case, the diagnosis was made using a CT scan which may still be used when suspecting COPS, especially when there is limited access to higher-quality imaging modalities.
The patient abstinence from medication and hesitancy to lifestyle modifications played an important role in the progression and worsening of his condition. Treatment of COPS includes management of risk factors, especially smoking cessation; changing diet; and the initiation of appropriate preventative statin and antiplatelet therapies [17]. Poor compliance with these treatment methods likely led to the second infarction just weeks later of the initial infarct, further highlighting the importance for patients to properly follow treatment plans when being diagnosed with COPS.
This case can be emphasized by three main clinical pearls, which may further help healthcare providers better understand future cases of COPS. These clinical pearls are as follows:
Clinical pearl 1: Patients may not be the best historians when discussing medications, they may or may not be taking as prescribed as their response can dramatically impact the care received. In many cases, patients are fearful of judgment from providers and require time to build a proper rapport. Taking the time to speak with patients and providing non-judgmental statements such as, “Many patients struggle to remember taking all their medications, how many times a week does this happen to you?” can often help provide that history. In other cases, patients can struggle to remember how often they actually take their medications, in helping to advise compliance the provider can help by providing support with a pharmacy consultation and pill organizers which can help improve compliance.
Clinical pearl 2: Historical information can help one dictate location of the lesion when paired with proper ordering and reading of imaging. Thus, it is important for providers to remember to collect all necessary lab values, perform a thorough interview and physical exam, and run any diagnostic imaging before diagnosing and treating their patients. Just as important, having the properly trained staff, in this case, radiologists, are a crucial part of the care team as they are the individuals best suited to know where/how to look for lesions. In most cases of COPS, the thalamus is the typical site of the lesion, however, as this case presented with a highly specific pontine location of the infarct, it could have easily been missed [5].
Clinical pearl 3: Transient ischemic attacks (TIAs) are warning signs of potential worsening of the diseased state. The patient presented a week later in a worse condition because he was not taking his antiplatelet therapy at home. It is well known that within 21 days after a stroking event, patients should be continuing 2 antiplatelet medications: clopidogrel/aspirin, ticagrelor/aspirin, ticagrelor/clopidogrel, as noted in the Clopidogrel in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events (CHANCE) and Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trials [18].
Clinical pearl 4: The last key clinical pearl from this case is considering the rare diagnosis as part of the differential [19]. With COPS, because of the rarer constellation of symptoms, this disease state is often misdiagnosed as something that is more common and there is a failure of the team to ensure a proper work-up and provide appropriate subsequent outpatient follow-up. As a result of this disease state being considered rarer and the constellation often missed or not taken seriously by either the patient or the provider that sees the patient, the suffering individual often does not get the proper work-up, making COPS a potentially underrepresented illness [3–5, 7, 8, 12–17]. Therefore, because of the desire to ensure proper care of patients, it is the authorial team’s highest recommendation, that in cases where “weird” symptoms present, providers ensure that a proper work-up is conducted to ensure the safety and treatment of these patients who do suffer from debilitating consequences where these are not taken seriously [19].
In this unique case presentation of COPS, the 58-year-old patient presented to the clinic with classic signs of type I COPS; however, it was discovered that rather than the typical thalamic infarct, or even the lesser major pontine infarct, this patient was suffering from a left pontine perforator infarct. Additionally, this patient uniquely presented with contralateral symptoms rather than the normal ipsilateral presentation. Lastly, most cases of COPS present with hemorrhagic infarcts, and yet, this patient presented with repeated ischemic infarcts, which lead to waxing and waning neurologic symptoms. From this case, the major clinical pearls discussed included cautiously taking a patient history regarding their medication compliance; the importance of collecting all necessary lab values, thorough interviewing, physical exams, and ordering all necessary diagnostic imaging; and understanding the proper antiplatelet therapy regimen based on the CHANCE and POINT trials.
ALT: alanine transaminase
AST: aspartate transaminase
COPS: cheiro-oral-pedal syndrome
COS: cheiro-oral syndrome
CT: computed tomography
MCA: middle cerebral artery
MRI: magnetic resonance imaging
TB: tuberculosis
This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration.
ZIM: Conceptualization, Writing—original draft, Writing—review & editing. GDB, LD, ZE, and EF: Writing—original draft, Writing—review & editing. TCV: Conceptualization, Investigation, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The ethical approval of this case study is exempted by the Institutional Review Board (IRB) of the Phoenix Veterans Health Administration (VA) Health Care System.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from the participant.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3655
Download: 32
Times Cited: 0
