Abstract
Aim:
The aim of this study is to investigate the prevalence of human papillomavirus (HPV) genotypes in Moroccan women diagnosed with invasive cervical cancer and to assess the association between HPV infection and some socio-demographic characteristics and clinicopathological features.
Methods:
In this study, 80 fresh biopsies from patients with confirmed diagnoses of cervical cancer during the study period (2020–2021) were collected. All cases were subject to HPV detection by nested PCR using MY09/11 and GP5+/6+ primers. HPV genotyping was performed by type-specific PCR targeting HPV 6, 11, 16, 18, 31, and 33.
Results:
The average age of patients was 54 years. Most patients were diagnosed with squamous cell carcinoma (SCC; 82.5%) at stage II (71.3%). Overall, 91.3% of cervical cancer cases were HPV-positive. HPV 16 is the most prevalent genotype, reported in 60.3% of HPV-positive cases, followed by HPV 18, 33, and 31 genotypes, identified in 20.5%, 12.3%, and 6.8%, respectively. No double infection with these genotypes was observed. Statistical analysis showed a significant correlation between HPV infection and age at menarche (P = 0.028), parity (P = 0.004), childbirth delivery (P = 0.040), and marital status (P = 0.042).
Conclusions:
HPV-DNA was prevalent in most examined cervical cancer tissues and HPV 16, HPV 18, HPV 33, and HPV 31 were present, at single infection, in all HPV-positive cases. These results emphasize already reported data on HPV distribution in Morocco and may contribute significantly to promoting the use of HPV DNA-based screening tests and available vaccines to limit HPV infection, viral dissemination, and cancer cervical development.
Keywords
Morocco, cervical cancer, oncogenic human papillomavirus, PCR, genotypingIntroduction
Cervical cancer (CC) is a global public health burden. It is the fourth most diagnosed female cancer and the second leading cause of cancer death [1]. In 2020, 604,127 (6.5%) new cases of CC were diagnosed and about 342,000 (7.7%) deaths were reported to be associated with CC, 90% of them occurring in low and middle-income countries, and the highest incidence and mortality are reported in sub-Saharan Africa [2, 3].
In Morocco, CC is the second most common female cancer after breast cancer and the fifth deadliest human cancer. According to the International Agency for Research on Cancer, age-standardized incidence and mortality rates of CC in Moroccan women are estimated at 14.3 and 7 per 100,000, respectively [4, 5]. This high mortality rate is a result of late and poor diagnosis often due to the lack of effective methods of detection (diagnosis and screening) at an early stage of the disease. Indeed, 65.7% of CC cases are diagnosed at high grades and advanced stages [5, 6].
Currently, it’s widely accepted that CC is a virus-induced cancer. Extensive clinical and epidemiological evidence has reported that approximately 99.7% of CC is due to persistent infection of high-risk human papillomavirus (HR-HPV), which induces the progression of benign warts to precancerous lesions and then to invasive malignant tumors [7]. However, if this persistent infection is the etiologic factor for CC development, it’s still not sufficient, and multiple events are involved during carcinogenesis, including immunological, host genetic, and epigenetic alterations, as well as other exogenous cofactors [active smoking, oral contraceptives, human immunodeficiency virus (HIV), Chlamydia trachomatis, etc.], which potentially increase the risk of developing CC [8, 9].
Formerly, there were more than 200 human papillomavirus (HPV) genotypes, but the interest is still given to about 40 genotypes, encompassing HR-HPV and low-risk HPV (LR-HPV), associated with CC and precancerous lesions. Among them, 13 HR-HPV: HPV 16 and 18, are involved in 70% of CC cases, and HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 are associated with 22% of cases [10].
Scientific evidence has shown that HPV vaccination is an important step in reducing the burden of CC globally. Overall, 4 prophylactic vaccines, mainly directed against HPV 16 and HPV 18, were developed and are used to prevent CC development. In Morocco, two vaccines are already available; the bivalent vaccine CervarixTM against HPV 16 and 18 and the quadrivalent vaccine Gardasil® against HPV 16, 18, 6, and 11 [11]. Moreover, Morocco has developed a global strategy to extend HPV vaccination to 11-year-old female students. Nevertheless, epidemiological studies have shown that the HPV prevalence and the genotype distribution differ according to geographical regions [7], which disagree with the relative effectiveness of these vaccines due to the absence of the targeted genotype in a given region. It is necessary to emphasize the importance of implementing the currently available quadrivalent vaccine alongside the nonvalent vaccine, which is already on the market. If gaps in coverage or efficacy persist, consideration can be given to the development of new, more inclusive vaccines.
Thus, the present study was conducted to identify HR-HPV genotypes in Moroccan women diagnosed with CC and further, assess the clinicopathological features associated with cancer development, to reinforce our knowledge on circulating HPV genotypes and evaluate the efficacy of such vaccination to prevent CC in Morocco.
Materials and methods
CC specimens
Our study was carried out on 80 fresh biopsies obtained from patients aged 27 to 85, with histopathologically confirmed CC who had undergone surgery in the Onco-gynecology Department of the Mohammed IV Oncology Center-Casablanca, Morocco, between January 2020 and December 2021. Epidemiological, clinical, and histopathological parameters of recruited cases were also collected. Data collection was conducted with respect to Strengthening The Reporting Of Cohort Studies in Surgery (STROCSS) guidelines to improve the quality and usefulness of obtained information [12]. The study protocol was approved by the Ethics Committee for Biomedical Research of the Faculty of Medicine and Pharmacy of Casablanca, Morocco (Reference 3/2018 on 30.04.2018). Free and informed consent was obtained from all patients involved in this research and the confidentiality of their personal information was well-respected according to ethical rules. The study included patients who were diagnosed with CC based on histopathological examination. Patients younger than 25 years or older than 65 years, as well as those who had received chemotherapy and/or radiotherapy, were excluded from the study. These exclusion criteria aimed to ensure that the study focused on a specific age range and excluded potential concerns such as prior treatment that could affect the results of the study.
Tissue sampling and DNA extraction
Biopsies were collected according to standard protocols by physicians and then immediately stored at –80°C until processing and analysis. DNA extraction was performed on a subsample of ≤ 25 mg of frozen tissue using the PurelinkTM Genomic DNA Mini Kit (K182001, Thermo Fisher Scientific, USA), according to the manufacturer’s instructions. The DNA extract is used immediately or stored at –20°C until use.
Qualitative and quantitative DNA analysis
DNA concentration and purity were assayed using the NanoDropTM 2000 Spectrophotometer (Thermo Fisher Scientific, USA).
To assess the quality of the extraction, the integrity of the DNA extract, and the absence of PCR inhibitors, PCR amplification of the β-globin gene, used as a reference housekeeping gene, was performed using the primers PCO4 and GH20, as previously described [1].
HPV-DNA detection and genotyping
The L1 gene of HPV was targeted by nested PCR using the MY09/MY11 and GP5+/GP6+ consensus primers sets (Invitrogen, USA), as previously described [2]. Briefly, the PCR reaction mixture with a total volume of 25 µL consisted of 2 µL of concentrated DNA (8 ng/µL) and 6.5 µL of ultra-pure water, 2 µL each of forward and reverse primers, and 12.5 µL of Taq PCR Master mix (Vazyme Biotech, China). Primer sequences of HPV L1 gene amplification as previously described [2]. The PCR amplification was performed in a Perkin Elmer Thermal Cycler 2400 (Perkin Elmer, USA) according to the following cycling conditions: initial denaturation at 94°C for 10 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at the specific temperature 55°C (MY09/MY11)/40°C (GP5+/GP6+) for 1 min, elongation at 72°C for 1 min, and final incubation at 72°C for 10 min. For each PCR amplification, a PCR reaction mixture containing no DNA was used as a negative control, and a commercially available MaximeTM PCR positive control was used (HPV-C001, Maxime Biotech, USA).
To evaluate the outcome of each PCR, all PCR products were analyzed by electrophoresis BlueMarineTM 100 (SERVA Electrophoresis GmbH, Germany) on 2% agarose gel at 70 V for 1.5 h, stained with ethidium bromide, and visualized under UV light on DIGIDOC-IT® Imaging System (Thomas Scientific, USA). Typing was performed by type-specific PCR for HPV 6, 11, 16, 18, 31, and 33 as previously described [3]. All primers of these HPV types are provided elsewhere [4–6].
In our study, we employed a specialized genotyping protocol with a primary emphasis on detecting HR-HPV genotypes 16, 18, 31, and 33, as well as LR-HPV genotypes 6 and 11. However, to target other HPV genotypes not explicitly investigated in our research, a distinct protocol is essential, requiring the use of specific primers tailored to each of these different genotypes.
Statistical analysis
Statistical analysis of the data was generated using jamovi software (version 2.3.21). The correlation between HPV-positive CC cases and the clinicopathological parameters was carried out by the chi-square test or Fisher’s exact test (when one of the theoretical numbers is less than 5). The difference was considered statistically significant when the P-value was ≤ 0.05.
Results
Clinicopathological data of CC cases
The clinicopathological data are summarized in Table 1. Overall, the patient’s average age at diagnosis was 54 years, with extreme ages at 27 years and 85 years. The most common age group ranges from 42 years old to 61 years old, accounting for 56.3% of cases. At diagnosis, 82.5% of patients had squamous cell carcinoma (SCC) and 17.5% had adenocarcinoma [1].
Distribution of CC Moroccan patients according to clinicopathological characteristics (n = 80)
| Characteristics | Type | Sample number (n) | Percentage (%) |
|---|---|---|---|
| Age at diagnosis (year, mean age = 54) | 27–41 | 15 | 18.8 |
| 42–61 | 45 | 56.3 | |
| > 61 | 20 | 25.0 | |
| FIGO clinical stage | I | 16 | 20.0 |
| II | 57 | 71.3 | |
| III | 7 | 8.8 | |
| IV | 0 | – | |
| Histological type | SCC | 66 | 82.5 |
| Adenocarcinoma | 14 | 17.5 | |
| Age at menarche (in years) | ≤ 12 | 55 | 68.8 |
| > 12 | 25 | 31.3 | |
| Menopause | Yes | 54 | 67.5 |
| No | 26 | 32.5 | |
| Age at menopause (in years) | < 45 | 14 | 25.9 |
| ≥ 45 | 40 | 74.1 | |
| Oral contraceptives use | Yes | 56 | 70 |
| No | 24 | 30 | |
| Duration of oral contraceptives use (in years) | ≥ 5 | 33 | 58.9 |
| < 5 | 23 | 41.1 | |
| ABO blood group | A+ | 17 | 21.25 |
| A– | 5 | 6.25 | |
| B+ | 11 | 13.75 | |
| B– | 7 | 8.75 | |
| AB+ | 2 | 2.50 | |
| AB– | 0 | – | |
| O+ | 32 | 40.00 | |
| O– | 6 | 7.50 | |
| Parity | Yes | 70 | 87.5 |
| No | 10 | 12.5 | |
| Number of children | 1–2 | 30 | 42.9 |
| ≥ 3 | 40 | 57.1 | |
| Childbirth delivery | Vaginal birth | 59 | 84.3 |
| Caesarean section | 11 | 15.7 | |
| Residence area | Urban and sub-urban | 58 | 72.5 |
| Rural | 22 | 27.5 | |
| Marital status | Single (separated or widowed) | 18 | 22.5 |
| Married | 62 | 77.5 | |
| Health insurance | Covered (RAMED) | 75 | 93.8 |
| Not covered | 5 | 6.3 |
FIGO: International Federation of Gynecology and Obstetrics; –: not applicable; RAMED: Regime d’Assistance Medicale
Clinical staging was performed according to the FIGO classification and revealed the predominance of stage II with a significant percentage of 71.3%. Clinical stages I and III represent 20.0% and 8.8% of recruited cases, respectively.
In the present cohort, the most common blood group was O, representing 47.5% of CC patients, followed by group A, reported in 27.5% of cases. Blood groups B and AB were reported in 22.5% and 2.5% of cases, respectively.
According to reproductive factors, 68.8% of patients had ≤ 12 years of menarche and 67.5% were in menopause, of which 74.1% reached menopause at ≥ 45 years. In addition, a high percentage of pregnancies, 87.5%, were noticed. Interestingly, 84.3% of patients gave birth vaginally and only 15.7% gave birth by caesarean section, and 57.1% of them had three or more children.
Regarding contraceptive methods, the use of oral contraceptives prevails and was reported in 70% of cases, and 58.9% of people have reported long-term contraceptive use (≥ 5 years).
The socio-demographic evaluation showed that most recruited cases were married (77.5%), residents in urban or peri-urban areas (72.5%), and medically covered by the Medical Assistance Plan (93.8%).
HPV DNA prevalence
In the present study, an amplifiable β-globin gene was obtained in all 80 specimens and therefore all DNA samples were adequate for further analysis. Nested PCR amplification of a conserved region of the HPV L1 gene DNA, using the consensus MY09/11 and GP5+/6+ primers, revealed the presence of HPV DNA in 91.3% of cases (73/80).
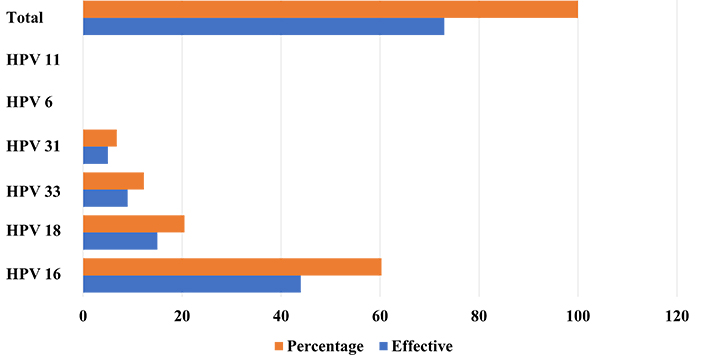
HPV genotyping showed that among the 6 HPV investigated (16, 18, 31, 33, 6, and 11) only the 4 HR-HPV (16, 18, 31, and 33) were detected in CC cases. HPV 16 was the most prevalent genotype, identified in 60.3% of HPV-positive samples (44/73). HPV 18, 33, and 31 were identified in 20.5% (15/73), 12.3% (9/73), and 6.8% (5/73) of cases, respectively. HPV 6 and 11 were not detected in any CC cases (Figure 1). However, our genotyping protocol exhibits limitations in its ability to identify cases of coinfections involving multiple HPV genotypes, which could lead to an underestimation of the prevalence of HPV genotypes occurring in our population.
Association between HPV infection and clinicopathological features
In this study, the association between HPV infection and socio-demographic data and clinic-pathologic characteristics was also assessed and clearly showed a significant association between the presence of viral DNA and age of menarche (P = 0.028), parity (P = 0.004), childbirth delivery (P = 0.040), and marital status (P = 0.042). Moreover, a borderline association was found between viral infection and age at diagnosis, as all women diagnosed after 61 years are HPV-positive and HPV DNA was detected in only 80% of younger women with CC (Table 2).
Correlation between HPV infection and clinicopathologic features of patients
| Characteristics | Effective | HPV-positive | P | |
|---|---|---|---|---|
| Samples number (n) | Percentage (%) | |||
| Age at diagnosis (year) | ||||
| 27–41 | 15 | 12 | 80.00 | 0.081 |
| 42–61 | 45 | 41 | 91.11 | |
| > 61 | 20 | 20 | 100.00 | |
| Histological type | ||||
| SCC | 66 | 61 | 92.42 | 0.600 |
| Adenocarcinoma | 14 | 12 | 85.71 | |
| FIGO clinical stage | ||||
| I | 16 | 14 | 87.50 | 0.511 |
| II | 57 | 53 | 92.98 | |
| III | 7 | 6 | 85.71 | |
| IV | 0 | 0 | – | |
| ABO blood group | ||||
| A+ | 17 | 15 | 88.24 | 0.277 |
| A– | 5 | 5 | 100.00 | |
| B+ | 11 | 10 | 90.91 | |
| B– | 7 | 5 | 71.43 | |
| AB+ | 2 | 2 | 100.00 | |
| AB– | 0 | 0 | – | |
| O+ | 32 | 31 | 96.88 | |
| O– | 6 | 5 | 83.33 | |
| Age at menarche (in years) | ||||
| ≤ 12 | 55 | 53 | 96.36 | 0.028 |
| > 12 | 25 | 20 | 80.00 | |
| Menopause | ||||
| Yes | 54 | 51 | 94.44 | 0.206 |
| No | 26 | 22 | 84.62 | |
| Age at menopause (in years) | ||||
| < 45 | 14 | 14 | 100.00 | 0.560 |
| ≥ 45 | 40 | 37 | 92.50 | |
| Parity | ||||
| Yes | 70 | 67 | 95.71 | 0.004 |
| No | 10 | 6 | 60.00 | |
| Number of children | ||||
| 1–2 | 30 | 28 | 93.33 | 0.573 |
| ≥ 3 | 40 | 39 | 97.50 | |
| Childbirth delivery | ||||
| Vaginal birth | 59 | 56 | 94.92 | 0.040 |
| Caesarean section | 11 | 8 | 72.73 | |
| Oral contraceptives use | ||||
| Yes | 56 | 53 | 94.64 | 0.189 |
| No | 24 | 20 | 83.33 | |
| Duration of oral contraceptives use (in years) | ||||
| ≥ 5 | 33 | 32 | 96.97 | 0.562 |
| < 5 | 23 | 21 | 91.30 | |
| Residence area | ||||
| Urban and sub-urban | 58 | 52 | 89.66 | 0.667 |
| Rural | 22 | 21 | 95.45 | |
| Marital status | ||||
| Single (separated or widowed) | 18 | 14 | 77.78 | 0.042 |
| Married | 62 | 59 | 95.16 | |
| Health insurance | ||||
| Covered (RAMED) | 75 | 69 | 92.00 | 0.375 |
| Not covered | 5 | 4 | 80.00 | |
RAMED: Regime d’Assistance Medicale; –: not applicable
Potential risk factors related to HPV-positive CC patients
Regarding associational statistical analysis depicted in Table 3, an increased risk for CC and HPV infection was found among patients with an SCC histological type [odds ratio (OR) = 2.03; 95% confidence interval (CI) = 0.352; 11.700], who had their first period at ≤ 12 years (OR = 6.63; 95% CI = 1.19; 36.90) and who are at menopause (OR = 3.09; 95% CI = 0.638; 15.000).
Potential risk factors and protective factors of HPV-positive CC patients
| Factors | OR value | 95% CI |
|---|---|---|
| Histological type | 2.030 | [0.352; 11.700] |
| Age at menarche | 6.630 | [1.19; 36.90] |
| Menopause (yes/no) | 3.090 | [0.638; 15.000] |
| Age at menopause | 0.369* | [0.0179; 7.610] |
| Parity | 14.900 | [2.68; 82.70] |
| Number of children | 2.790 | [0.241; 32.300] |
| Childbirth delivery | 7.380 | [1.27; 43.00] |
| Oral contraceptives use | 3.530 | [0.726; 17.200] |
| Duration of oral contraceptives use | 3.050 | [0.260; 35.800] |
| Residence area | 0.413 | [0.0468; 3.640] |
| Marital status | 0.178 | [0.0357; 0.887] |
*: Haldane-Ascombe correction applied
Increasing parity is associated with a higher risk of developing CC linked to HPV infection (OR = 14.9; 95% CI = 2.68; 82.70). Patients with 3 or more children had a higher risk of developing HPV-positive CC than those who had < 3 children (OR = 2.79; 95% CI = 0.241; 32.300), as well as those who gave birth vaginally (OR = 7.38; 95% CI = 1.27; 43.00).
The use of oral contraceptives (OR = 3.53; 95% CI = 0.726; 17.200), especially when these are used for a long period ≥ 5 years (OR = 3.05; 95% CI = 0.260; 35.800) also increases the risk of HPV-related CC.
Discussion
Worldwide, great efforts are made to control CC development and decrease the number of positive cases and deaths. In this field, many countries have introduced the prophylactic vaccination against HPV in their national programs and have implemented molecular detection of HPV in the overall management of CC lesions. In Morocco, as it’s the case in many developing countries, the main problem faced by clinicians is the delay in the diagnosis.
In the present study, most patients were diagnosed with SCC at stage II. These results are in agreement with those already reported in patients with CC cases [7–9]. Of note, stage IV was not found because in this case, the carcinoma has already spread to adjacent pelvic organs (stage IVA) or even presented distant metastatic dissemination (stage IVB), which is often not operable and therefore not included in our study since we cannot have a biopsy in this case.
Worldwide, many studies have addressed the potential association between blood groups and the risk of CC development. Our findings showed a predominance of blood group O (47.5%) followed respectively by A (27.5%), B (22.5%), and a small percentage of AB (2.5%). These results are consistent with Saxsena and Gupta’s [10] observational study on the association between gynecologic cancers and ABO blood groups, which was found to be highly significant in blood type A, followed by B, O, and a weaker association in AB. Similarly, Yuzhalin and Kutikhin [11] found a predominance of blood groups A and O (36.01%) in southeast Siberia with no significant correlation between cancer development and blood groups. Of note, the predominance of blood groups O+ phenotype in our study is more likely to be related to the fact that this group is the more widespread phenotype in the Moroccan population [12].
At diagnosis, the most common age of recruited patients ranges between 42 and 61. Data reported around the world converge on the fact that women in this age group are more likely to develop CC [13, 14]. Age serves as a pivotal determinant in the progression of CC, acting as an insidious catalyst and propelling increased risks with each passing year [15]. In fact, it’s widely accepted that younger women may engage in unprotected sexual activities, exposing them to infectious agents, including HPV, requiring more than 10 years to initiate, develop, and promote cancer [15].
Correlation between HPV infection and socio-demographic information and clinicopathological features showed a close association between HR-HPV positivity and histological type, parity, menopause status, birth delivery mode, and the use of oral contraceptives. These associations have already been reported and discussed and are considered as the main risk factor for CC development [16–18]. Also, it is important to note that the prevalence of HPV infection and HPV-type distribution in women within the general population varies significantly by country, regions within the same country in our case Morocco, and its population [19]. In addition, other risk factors such as genetic variation, sexual behavior, lifestyle, and population immunity may influence the prevalence of HPV [20]. Accordingly, many awareness campaigns are widely carried out to reduce the impact of the risk factors and hence limit CC development.
Many reports showcased that multiple clinicopathological features and risk factors may affect significantly the progression and clearance rate of HPV infection, the primary causative agent of CC [21].
Our results elucidated 84.3% of women that gave birth vaginally and 57.1% of them had three or more children. These results are consistent with those of El Maazouzi et al. [16] and Kennedy et al. [21] who found that the risk of HPV-related CC was higher in women with more than 3 children (54%) and more than 5 children (63%). Reports that go in hand with our results that women who are at menopause (OR = 3.09; 95% CI = 0.638; 15.000) have a higher risk of HPV exposure, discussed the aspect of menopausal status and its impact on CC risk, suggesting that postmenopausal women may experience changes in the cervical epithelium, making it more susceptible to HPV infection [16].
Long-term use of oral contraceptives may be connected to a slight increase in the risk of CC [16]. The synthetic hormones in these contraceptives, such as estrogen and progestin, could impact the development of this type of cancer. Estrogen specifically has been linked to the growth of HPV-infected cervical cells, a major cause of CC. However, it’s important to note that while there is an overall small increase in risk associated with using oral contraceptives, their benefits in preventing unintended pregnancies and managing other health conditions generally outweigh this potential risk [16].
Other important long-term risk factors didn’t take part in our studies like smoking and sexual activity because knowing that in the Moroccan culture, smoking among women is still considered taboo, so only very few Moroccan women do smoke, therefore, including this factor in our study wouldn’t have much significance, the same thing goes for the sexual factors, the prevailing cultural and religious norms lead to a majority of Moroccan women refraining from multiple sexual partnerships and delaying the initiation of their sexual lives, but these long-term factors should be mentioned due to their close link with the clinicopathological parameters studied, such as smoking playing a major role in augmenting exposure risk and contributing to the recurrence and/or persistence of HPV related infections and lesions. According to many reports, smoking, which is a known significant behavior contributing to the development of CC [16], brings carcinogenic substances into contact with the cells lining the cervix, causing harm at a genetic level and creating an environment conducive to inflammation [16], which elevates the likelihood of malignant cell transformation. Furthermore, research has shown that women who smoke and are infected with high-risk types of HPV have a higher likelihood of developing precancerous lesions and eventually CC [16]. Altogether, these effects undermine immune system functionality in managing HPV infections while obstructing cellular repair mechanisms within the body [16]. This weakened immune system constitutes another vibrant ally that allows HPV infections to persist and progress to cancerous lesions, increasing the risk of CC development.
Another important risk factor is sexual behavior, as numerous reports suggest, affects the acquisition of HPV infections [16]. Early sexual debuts and having multiple sexual partners increase the risk of exposure to HPV. This heightened risk for initial HPV infections can lead to a greater likelihood of persistent infection and the development of cervical dysplasia [16]. Furthermore, ongoing risky sexual behaviors can perpetuate recurrent infections and pathologies. Early sexual activity can potentially increase susceptibility to HR-HPV infections due to underdeveloped immune responses in younger individuals [16]. It can also make the cervix more susceptible to persistent HPV infections and abnormalities due to its immaturity [16]. Additionally, women with multiple sexual partners are potentially exposed to a greater assortment of HR-HPV strains, thereby elevating the risk. Furthermore, the chances are amplified if the partner indulges in extramarital affairs due to increased exposure to various strains of HPV [16].
Overall, 91.3% of CC cases were HPV-positive and all identified HPVs were high-risk genotypes. These results agree with those found in several studies in Morocco [22, 23] and in other countries around the world [24–26].
In this study, our interest was focused on HR-HPV 16, 18, 31, and 33 and LR-HPV 6 and 11, as they are the main target genotypes in the 2 most used prophylactic vaccines (6/11 and/or 16/18) or covered by the vaccines by the cross-reactive protection (31 and 33) [17]. Results clearly showed a predominance of HPV 16, identified in 60.3% of cases, followed by HPV 18, 33, and 31 reported in 20.5%, 12.3%, and 6.8% of cases, respectively. The high prevalence of HPV 16 in CC cases and the presence of the other HR-HPV 18, 33, and 31 are widely reported and well documented [24–26].
The high prevalence of HPV 16 in CC cases is widely reported and it is considered as the most oncogenic genotype. Elasbali et al. [13] showed the presence of HPV 16 in 50% of Sudanese women with CC. The same result was reported by Amrani et al. [7], in Moroccan women with CC. In 2009, Khair et al. [8] reported that HPV 16, alone or in co-infection, was identified in more than 70% of CC cases.
HPV 18, also considered as a high oncogenic genotype, was reported in many studies of various populations around the world. In this field, HPV 18 was reported to be associated with 15% of CC cases in Spain and 27.9% in the Philippines [26]. In Morocco, HPV 18, alone or in co-infection, was reported in 22% of cases from Rabat [7] and in 46% of cases from Casablanca [8].
However, multiple studies have shown that positive surgical cervical margins and HR-HPV, especially HPV 16/18, persistence are the primary risk factors for the persistence and recurrence of cervical dysplasia, which is related to a significant risk of CC [27]. Indeed, after surgical therapy for cervical intraepithelial neoplasia (CIN) 2, CIN 3, or CC, the 5-year risk of recurrence ranges from 5% to 10% [28]. However, according to Bogani et al. [29], one of the key variables influencing the potential of a high-grade cervical dysplasia CIN 2+ recurrence is HPV persistence, and for a maximum of one year, the probability of CIN 2+ recurrence increased as HPV persistence increased. It does not seem that HPV persistence beyond the first year presents a risk factor.
Widely, HPV 33 and 31 are weakly identified in CC cases, and usually, the prevalence of infection with these low-risk genotypes doesn’t exceed 15%, which is in agreement with our findings. In this regard, the study conducted by Ahmed et al. [30] in Yemen and Kuguyo et al. [24] revealed a rate of HPV 31 infection of 6% and 6.6%, respectively, in women with CC. Moreover, Kuguyo et al. [24], have reported that 9.7% of CC cases from Zimbabwe were HPV 33 positive.
These differences in frequencies are mainly due to the study setting, the sensitivity of the molecular approach used for HPV detection and genotyping, as well as genuine variation across populations and disease subgroups [31].
Of interest, no double infection was identified in this study. Many studies conducted in Morocco and elsewhere have reported the presence of co-infection in some cancer cases especially in precancerous cervical lesions [16, 19]. Indeed, integration of viral DNA in the host genome is the key factor in cancer initiation and development and is more likely done with one genotype [20].
It is very crucial to highlight the significant effect of cervical diseases such as cervical incompetence and mainly cancer in our case study on obstetric outcomes. Cervical incompetence is considered a key cause of premature births and pregnancy loss and can be detected through transvaginal ultrasound [20]. Fertility issues arise mainly from CC, which also influences childbirth outcome; it is hence crucial to diagnose early. Early diagnosis aids in enhancing obstetrical performance while dealing with related clinical risks. CC screening programs, notably the Pap smear and HR-HPV testing for prevention and thereby optimizing maternal-fetal health [20], also for reducing incidence rates by enabling early detection of precancerous lesions for timely treatment [20]. These methods improved the detection of pre-cancerous changes, lowering CC rates. Adding to this is the preventive approach of HPV vaccination that aims to significantly decrease disease incidence by addressing its primary cause [20]. HPV vaccination reduces health disparities, particularly in underserved areas, by promoting prevention. The link between effective screening and HPV vaccines is key to curbing CC prevalence, underlining the role of thorough strategies in public health efforts [20].
This study has shown that HPV-DNA was prevalent in most examined CC tissues and that HPV 16, HPV 18, HPV 33, and HPV 31 were the most detected genotypes, at single infection, in all HPV-positive cases. These results will provide more detailed information about HPV, reinforce available data on HPV circulating genotypes as strong biomarkers of CC risk, and may contribute significantly to enhancing CC management through the implementation of HPV DNA-based screening tests and the use of available vaccines to limit HPV infection, viral dissemination, and CC development. It may lead to more effective treatments and personalized interventions, as well as potential modifications in patient management protocols. Our findings aim to raise awareness among healthcare professionals and patients about specific health issues, emphasizing the importance of prevention by increasing efforts to fight the vaccination issue depending on multiple HPV genotypes, facing many populations around the world, and to develop more adequate and pertinent screening tests in the future. It also adds to existing research reports on HPV genotypes and CC globally and strengthens our understanding of the prevalence of HPV in Moroccan CC patients.
The main limitations of the study are the number of cases studied, the non-representativeness of the case’s origins, as all cases were recruited at Mohammed IV Oncology Center from patients living mainly in Casablanca and neighboring cities, and the number of target HR-HPV assessed by type-specific genotyping protocol. Using this protocol for detecting HPV can indeed miss other HPV genotypes that are not specifically targeted by the primers used in the assay.
Further studies are necessary to detect a wide range of HPV genotypes, providing the use of broad-spectrum or consensus primer sets that can amplify a broader spectrum of HPV types. These primer sets are designed to target conserved regions within the HPV genome, allowing for the detection of a wider variety of HPV genotypes. However, even these broad-spectrum primers may not capture all known HPV genotypes, as new variants can emerge over time. Nevertheless, researchers often combine both strategies to get a comprehensive view of the HPV genotypes in a given sample.
Abbreviations
| CC: |
cervical cancer |
| CI: |
confidence interval |
| CIN: |
cervical intraepithelial neoplasia |
| FIGO: |
International Federation of Gynecology and Obstetrics |
| HPV: |
human papillomavirus |
| HR-HPV: |
high-risk human papillomavirus |
| LR-HPV: |
low-risk human papillomavirus |
| OR: |
odds ratio |
| SCC: |
squamous cell carcinoma |
Declarations
Acknowledgments
Special acknowledgment for Faculty of Sciences and Techniques of Mohammedia, University Hassan II of Casablanca and Ministry of Higher Education, Scientific Research and Innovation in Morocco.
Author contributions
KAT: Conceptualization, Methodology, Formal analysis, Investigation, Project administration, Writing—original draft, Writing—review & editing. MB and LB: Resources. AL: Writing—review & editing. EAB: Formal analysis. MEM: Validation, Formal analysis, Writing—review & editing. MME: Project administration, Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study protocol was approved by the Ethics Committee for Biomedical Research of the Faculty of Medicine and Pharmacy of Casablanca, Morocco (Reference 3/2018 on 30.04.2018).
Consent to participate
The informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed in the course of the present study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2024.