Abstract
Therapy of malignant tumors still represents a huge problem for healthcare, since these diseases lead to a high rate of disability and premature death of the population. The main problems of adoptive cell therapy for malignant tumors are a low rate of migration of effector lymphocytes into tumors, as well as their low activity in tumors due to suppressive tumor microenvironment. In addition, it should be noted that systemic intravenous administration of a large number of activated lymphocytes may be accompanied by a pronounced cytokine release syndrome, which leads to significant negative side effects, including high temperature, blood clotting disorders, aggression of immune cells against their own tissues, even neurotoxicity. Functional nanomaterials, such as magnetic nanoparticles with various surface modifications (PEG, PEI, DMSA, citrate, etc.) are highly promising agents for targeted delivery of different anti-tumor substances. Magnet-driven enrichment of effector anti-tumor lymphocytes in tumors would highly increase the effectiveness and enhance safety of adoptive lymphocyte therapy. However, different research groups obtained opposing data about the feasibility and efficiency of such approach. Thus, this review is focused on experimental details of the contradicting studies and aims to elucidate the possible reasons of these controversies and the best practices to efficiently target lymphocytes into tumors.
Keywords
Magnetic nanoparticles, lymphocytes, anti-cancer cell immunotherapy, magnetic targetingIntroduction
Nanoparticles (NPs) are one of the most promising types of nanomaterials. Due to their nanosize, NPs have a higher surface-to-volume ratio compared to bulk materials [1]. This is the reason for their unique structural, mechanical and functional properties. Pronounced magnetic properties also enlarge the scope of potential applications of NPs. Thus, magnetic NPs (MNPs) are a promising basis for the development of biomedical applications. According to research, MNPs are used in targeted drug delivery systems [2, 3], siRNA delivery for cancer treatment [4], regenerative medicine techniques [5], diagnostics and treatment [6]. Assembly of NPs acting in the superparamagnetic regime is characterized by magnetization relaxation due to thermal fluctuations, which is advantageous for their use in medical and biological contexts [7–9]. This relaxation of magnetic moments is beneficial to avoid sticking of MNPs inside vessels and tissues. However, the thermal fluctuations of the magnetic moments of the particles necessitate the application of substantial magnetic fields and magnetic field gradients to direct their movement effectively [10]. In addition, for very small particles, a significant volume of material is constituted by surface atoms, which exhibit disordered magnetic moments. From the point of view of magnetic properties, such a layer is “dead” and leads to a decrease in the saturation magnetization of MNPs compared to bulk material [11, 12]. So, NPs of ferromagnetic bulk materials can be superparamagnetic.
In order to achieve the required therapeutic effect, MNPs must possess a high level of biocompatibility with healthy cells and tissues. Oxide-based MNPs such as maghemite (γ-Fe2O3) and magnetite (Fe3O4) possess moderate toxicity and magnetic properties (relatively high value of saturation magnetization) [12–14]. In order to increase biocompatibility, MNP surface can be modified with dextran, polyethylene glycol (PEG), dimercaptosuccinic acid (DMSA), 3-aminopropyl-triethoxysilane (APTES), citrate, or poly(ethylenimine) (PEI), which increases chemical stability, reduces cytotoxic effects, increases the circulation time of MNPs in the bloodstream, and prevents opsonization processes [15–19].
Cell immunotherapy is widely explored as a highly promising way of cancer therapy. One of major limitations of cell anti-tumor immunotherapy is the poor migration of effector cells into tumors. MNPs were successfully employed in experimental studies to direct a variety of cell types including stem cells, macrophages or mesenchymal cells as well as dendritic cells (DCs) mainly for regenerative therapies and during autoimmune disorders [20–24]. The most potent immune cells that eradicate tumors are cytotoxic T cells, natural killer (NK) cells and chimeric-antigen receptor (CAR)-lymphocytes. Obviously, delivering these cell types into tumors is most desirable. However, there are not many studies resulting in successful magnetic targeting of lymphocytes into tumors with further tumor growth inhibition [25]. This review focuses on the reported studies in this field and discusses possible reasons of their different outcomes.
Current research in magnetically-driven migration of lymphocytes for antitumor adoptive cell therapy
Lymphocytes, such as T and NK cells are constantly circulating cells and have high motility in comparison with other immune cells. Moreover, they do not endocytose MNPs [25]. It is supposed that MNPs mainly bind to lymphocytic membranes via electrostatic interactions. Most of the studies use positively charged coatings for MNPs to increase their binding with negatively charged cellular membranes [25]. However, in the studies by Mühlberger M et al. [26, 27] negatively charged citrate-coated MNPs sufficiently associated with T cells and induced their retention by a magnetic field. Interestingly, it was shown in the recent study by the same scientific group [28] that citrate-coated MNPs can be internalized by T cells. Transmission electron microscopy of the unstimulated cells in their study revealed both the binding of the citrate-coated MNPs to the plasma membrane as well as internalization into vesicles. In the case of lower concentrations of the MNPs, most of the NPs were located intracellularly in vesicles. Only some particles were attached to the plasma membrane. With increasing concentrations, more NPs were associated with the plasma membrane. These NPs seemed to be tightly attached in a uniform layer at one side of the cell, invaginations of the cellular membrane were visible.
Thus, MNP interactions with lymphoid cells seem not to be solely dependent on charge and are much more complicated. Lymphocyte natural localization is determined by chemotactic signals, cellular adhesion molecules and hydrodynamic forces within blood and lymphatic vessels. Therefore, the localization of MNP-bound lymphocytes in an organism in the presence of external magnetic field (EMF) is not determined solely by the magnetic forces, it results from multiple counteracting chemical and physical factors. Several studies aimed in improving lymphocyte migration to the tumor and promoting their accumulation and infiltration in it by magnetic targeting. An excellent review by Sanz-Ortega L et al. [25] describes a number of studies demonstrating the low toxicity of MNPs in both human and murine T and NK cell lines and in primary T and NK cells. Moreover, MNPs do not cause significant changes in viability and functional activity of NK and T cells, as it was shown by numerous research teams (reviewed in [25]). Citrate-coated MNPs seem to be especially attractive for magnetic lymphocyte targeting as this type of coating is rather cheap, easy to manufacture, but enables significant magnetic labeling due to internalization of such MNPs. Moreover, citrate-coated MNPs do not spill to other cells [28]. Besides, loading with citrate coated MNPs did not impair the T cell proliferation, expression of activation markers, cytokine secretion, and tumor cell killing after antigen-specific activation mediated by the T-cell receptor (TCR) [29]. However, the task of magnetic directing anti-tumor lymphocytes proved to be rather complicated and it is still unresolved.
Experimental therapies based on magnetic targeting of lymphocytes promise higher efficiency in comparison with cancer-therapy techniques based on direct administration of anti-cancer NPs into blood stream. NPs introduced into the body encounter physical and biological barriers (diffusion, protein adsorption, aggregation, blood vessel flow, renal clearance) on their path targeting tumor cells [30]. According to literature [30], on average, only 0.7% of the administered NPs reaches tumor cells. On the other hand, NPs are able to accumulate in tumors via enhanced permeability and retention effect (EPR effect) [31, 32]. This effect is caused by abnormal proliferation of tumor blood vessels (the size of interendothelial pores in the walls of tumor blood vessels can reach sizes of up to 2,000 nm) and compression of lymphatic vessels. This leads to disruption of the lymphatic outflow of fluid (impaired lymphatic drainage) and limitation of the release of NPs from the tumor site [32]. The formation of reactive oxygen species (ROS) is one of the mechanisms of cytotoxicity of NPs, leading to oxidative stress with the subsequent damage of cell membranes, proteins and the genetic apparatus of the cell [33]. At the same time, the processes of biodegradation of the NPs, including the components of biocompatible coatings and functional groups should be studied due to the possible induction of cellular reactions with chemically degraded NPs [34]. NPs can actively interact with the components of biological fluids, in particular with proteins. As a result, a protein crown is formed on the surface of the NPs, which plays a key role in the interaction of the NPs with biological structures. The protein corona blocks NP functions, leads to an immune response and results in accelerated elimination of the NPs from the body [35]. After administration of NPs, they can spread throughout the body and accumulate in the bone marrow, liver, heart, spleen and other organs. In this process, the liver serves as a biological filtration system and is able to absorb from 30% to 99% of the introduced NPs [36]. This process leads to a decrease in the efficiency of the delivery of NPs to target tissue and potentially leads to the cytotoxic effects in the liver cells (hepatotoxicity) [35]. Otherwise, techniques based on magnetic targeting of lymphocytes create wide opportunities to prevent possible side effects of MNPs in case of their localization on outer lymphocyte’s cellular membranes or intracellular internalization. However possible shedding of MNPs from lymphocyte membranes should be taken into account. Up to date, a few approaches based on application of MNPs for magnetic targeting of anti-tumor lymphocytes have been reported. MNPs can be additionally armed to fight tumors or increase anti-cancer lymphocyte efficiency.
Jang ES et al. [37] successfully targeted human NK92-MI cells into xenograft GFP labeled RPMI8226 human B cell lymphoma in NSG immunodeficient mice model. They report that magnetic cell labelling did not negatively affect cell vitality or cytotoxicity. They also showed that fluorescently labeled NK cells with attached MNPs were rapidly (within 10 min) enriched into the tumor site by an EMF. However, NK92-MI cells did not remain in the tumors after the EMF removal, and in a few minutes disappeared from the tumor sites. The authors were not able to investigate a longer action of the EMF. Thus, in vivo anti-tumor action of the magnetically targeted NK cells in their study remained unclear. Moreover, they used immortalized tumor NK cells. Therefore, NK92-MI cells are tumor cells themselves and may develop tumors in immunodeficient mice hosts.
However, magnetic delivery of T-cells into tumors proved to be rather difficult [38]. Sanz-Ortega L et al. [19, 38, 39] presented very detailed research on MNP-coated T-cells and their behavior in mouse models in absence of EMF and under its action. They tried to direct the MNP-coated T-cells into mouse tumors via EMF application [38]. They used the most widely known murine tumor model to study specific antitumor T cell responses. This model is based on syngeneic mouse tumors expressing a specific ovalbumin (OVA)-derived antigenic peptide presented by H-2 Kb in combination with adoptive transfer of CD8+ T cells from OT-I mice. Namely, they performed their study on E.G7-OVA (derivative of EL4) tumors that can be targeted by OVA-peptide-specific T-cells from OT-I transgenic mice. OT-I CD8+ T cells were purified from spleen and lymph node-cell suspensions obtained from OT-I transgenic mice. These cell suspensions were cultured with the soluble OVA257–264 peptide (SIINFEKL) in order to activate the OT-I CD8+ T cells in the suspensions by antigen-presenting cells (APCs) that reside in spleen in lymph nodes (Figure 1A).
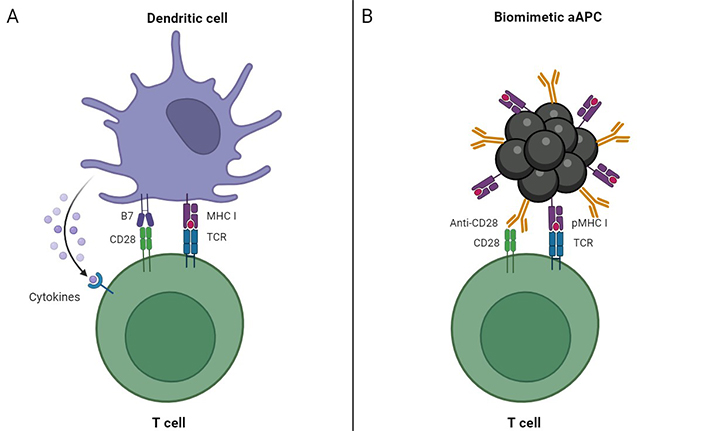
Different approaches to stimulate OT-I CD8+ T cells used in experimental models by Sanz-Ortega L et al. [38] (A) and Nie W et al. [42] (B). The study by Nie W et al. [42] resulted in successful enrichment of lymphocytes into tumors. Use of biomimetic magnetic aAPCs possibly was helpful in this process. Such aAPCs not only stimulate T cells but may also direct them in the presence of EMF. aAPCs: artificial antigen-presenting cells; EMF: external magnetic field; pMHC: peptide-major histocompatibility complex; TCR: T-cell receptor. Created by BioRender.com
They cultured the suspensions in such conditions for 2 days to remove cells, which do not respond to the OVA-peptide [38]. Afterwards, remaining populations of enriched OT-I CD8+ T cells were allowed to proliferate for 3 days more. The authors report that finally they obtained from 90% to 95% of OT-I CD8+ T cells. These enriched cell populations were loaded with different types of MNPs. The MNPs had an iron oxide core of 12.5 nm, which were subsequently coated, obtaining negatively charged DMSA-MNPs, positively charged APTES-MNPs, and non-charged dextran-MNPs (DEXT-MNPs). Further, they performed a variety of in vitro analysis of different functional aspects of CD8+ T cells associated with MNPs, such as their ability to conjugate with and lyse target cells, as well as their capacity to degranulate and produce IFN-γ. All the types of MNPs were proven not to influence significantly functional characteristics of the lymphocytes. The best binding capacities were shown for the positively charged MNPs. Then, they tested the retention efficacy of antigen-specific CD8+ T cells associated with MNPs in vivo using E.G7-OVA (derivative of EL4) tumor model. Mice were randomised into 4 groups, receiving an inoculation of phosphate buffer saline (PBS) as a control, OT-I CD8+ T cells, APTES-MNP loaded OT-I CD8+ T cells, or APTES-MNP-loaded OT-I CD8+ T cells together with the application of an EMF over the tumor for approximately 90 min. Surprisingly, placement of a magnet near the tumors during transfer of APTES-MNP-loaded CD8+ T cells did not increase tumor infiltration by these cells or decrease tumor volume compared to non-EMF-exposed tumors. Moreover, the tumor growth inhibition was even worse in the mice groups exposed to EMF compared to non-exposed ones. In hands of the authors, application of an EMF close to the tumor resulted in accumulation of CD8+ T cells not in tumors, but in tumor-draining lymph nodes [38].
Magnetic targeting of T cells was not an exact aim of the study by Zhang Q et al. reported in 2017 [40], but it turned out that their biomimetic magnetosomes could bind and lead lymphocytes in EMF besides their designated functions. Their purpose was to create an artificial APC (aAPC) to potentiate adoptive T-cell based cancer therapy. Magnetic nanoclusters (MNCs) with satisfactory superparamagnetism and magnetic response served as the base for their aAPCs. These MNCs consisted of magnetite Fe3O4-based building units of 10 nm coated by PEI [41]. The MNCs were about 50–100 nm large [40]. Afterwards, the research team covered those MNCs with leukocyte membrane fragments thus producing LMNCs. For their research, the authors also chose E.G7-OVA tumors that can be eliminated by the OVA-peptide (SIINFEKL)-specific T-cells from OT-I transgenic mice. So, they conjugated to the LMNCs SIINFEKL-loaded major histocompatibility complex class-I (MHC-I) and co-stimulatory ligand anti-CD28 (αCD28). The resultant biomimetic aAPC could not only efficiently expand and stimulate OT-I CD8+ T cells ex vivo but also direct the reinfused cytotoxic T-lymphocytes (CTLs) into tumors via magnetic control. As a result, the tumor growth in the murine tumor model was efficiently delayed.
Later on, this research team employed a similar approach to directly target effector T cells into tumors [42]. They used the same murine tumor model (E.G7-OVA). Splenocytes were obtained from the spleens of OT-I mice, and CD8+ T cells were isolated using a CD8+ no-touch isolation kit. For stimulation and expansion of OVA-specific CTLs, they used aAPC described earlier (Figure 1B) [40].
The expanded and activated CTLs were finally separated from the aAPCs through magnetic separation. After that, the same PEI-coated MNCs were armed with PD-1 antibody (aP) through pH-sensitive benzoic-imine bond and inverse electrondemand Diels-Alder cycloaddition [42]. The authors suppose that formed NC-aP could then bind to effector T cells due to their PD-1 expression. However, positively charged PEI-coating can facilitate lymphocyte surface binding as well, as membrane surfaces are predominantly charged negatively. After adoptive intravenous transfer into mice with E.G7-OVA-tumors, both the T cells and aP were shown to be magnetically enriched to enter solid tumors with guidance of EMF. The EMF was kept for 24 hours after intravenous infusion of the MNC-CTLs. During intratumoral infiltration, the slightly acidic extracellular microenvironment triggered hydrolysis of the benzoic-imine bond, leading to the release of aP for PD-1 blocking. As a result, the adoptive T cells and aP worked in a synergistic manner, and the growth of the tumors was significantly inhibited compared with experimental mice groups, which received only effector CTLs, CTLs with free aP, or aP-MNC-CTLs in the absence of EMF. Tumor accumulation of the aP-MNC-loaded lymphocytes was evidenced by in vivo fluorescence and magnetic resonance (MR) imaging, as well as by confocal laser scanning microscope (CLSM).
Therefore, this research team apparently successfully targeted T lymphocytes through EMF into tumors and that gave the expected enhancement in their antitumor effect in contrast to the study by Sanz-Ortega L et al. [38]. The reason for this discrepancy is not easy to find but most probably it lies in the basic properties of the magnetic systems employed in the studies (Figure 2).
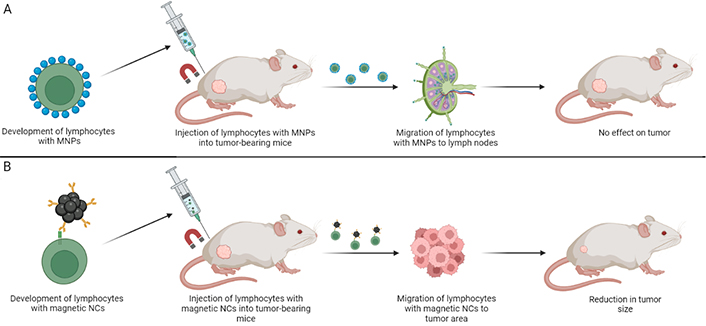
Schematic representation of different magnetic systems used in experimental models by Sanz-Ortega L et al. [38] (A) and Nie W et al. [42] (B) and the results of the studies. The most probable reason of successful migration in the study by Nie W et al. [42] is the higher magnetic load of lymphocytes. Magnetic NCs used in the work are bigger and seem to interact with T cells more strongly. MNPs: magnetic nanoparticles; NCs: nanoclusters. Created by BioRender.com
For magnetic targeting, Sanz-Ortega L et al. [38] produced MNPs with core size about 13 nm (Figure 2A), whereas Nie W et al. [42] used much bigger MNCs up to 100 nm in diameter, built from several 10 nm particles (Figure 2B). Zhang F et al. [41] discussed that these MNCs showed superparamagnetic behavior and no remanence even though their sizes exceeded the critical size for superparamagnetic-ferromagnetic transition (~24.8 nm). Sanz-Ortega L et al. [38] used iron oxide MNPs with diameters around 13 nm (optimal superparamagnetism). Such particles have enough saturation magnetization for MR imaging, but the low magnetization per unit complicates effective control of their movement with moderate magnetic fields [43]. Moreover, MNPs in the study by Sanz-Ortega L et al. [38] were linked with lymphocyte surfaces only via electrostatic interactions of positively charged APTES-coatings and negatively charged cellular membranes. Whereas, Nie W et al. [42] in addition employed more strong interactions between PD-1 and aP, as well as interactions between positively charged PEI and cell membrane. Thus, obviously more MNCs with stronger interactions bound CTLs in the study by Nie W et al. There were even more differences (summarized in Table 1) between the studies that supposedly resulted in their opposing outcomes.
| Experiment parameter | Sanz-Ortega L et al. [38] | Nie W et al. [42] |
|---|---|---|
| MNP types | Single MNPs | MNCs built from 10 nm units |
| Size of MNPs | 13 nm | Up to 100 nm |
| MNP-coating | APTES | PEI |
| EMF exposure time | 90 minutes | 24 hours |
| Method of OVA-peptide-specific CTL-activation | Conventional APCs | Biomimetic aAPC |
aAPC: artificial antigen-presenting cell; APTES: 3-aminopropyl-triethoxysilane; CTL: cytotoxic T-lymphocyte; EMF: external magnetic field; MNCs: magnetic nanoclusters; MNP: magnetic nanoparticle; OVA: ovalbumin; PEI: poly(ethylenimine)
According to the observations by Sanz-Ortega L et al. [38] MNP-loaded CTLs were accumulated within tumor-draining lymph nodes (Figure 2). It can’t be excluded that this preferential lymph node and not tumor homing resulted from their way of OVA-specific CTL-activation (Figure 1). The authors [38] noted that MNP-CTLs tended to localize to lymph nodes and remain there even in the absence of EMF due to a little lowered motility. It would be highly interesting to find out whether MNC-loaded CTLs in the study by Nie W et al. [42] had similar behavior. Jang ES et al. [37] showed that lymphocytes rapidly leave tumors in the absence of EMF. Therefore, prolonged EMF-exposure time is likely to be favorable for better anti-tumor effect (Table 1).
Luo Z et al. [44] also successfully directed CTLs into tumors and achieved enhancement of anti-tumor effects. They termed their nanoplatform dual-binding magnetic NPs (DBMN). These MNPs were based on magnetite 200 nm NPs modified with amino groups, PEG-maleimide (Mal), and hyaluronic acid (HA). HA- and PEG-Mal-coated MNPs were covalently anchored onto the cell membrane via reaction between the Mal and the sulfhydryl groups on the surface of T cells, generating magnetized T cells. Such big MNPs and their covalent linking to the cell surface obviously promoted tight magnetic control of the MNP-CTLs. Directed by EMF, these DBMN-T cells were magnetically recruited to solid tumors. Binding between HA and CD44 highly expressed in tumors provided tumor retention and facilitated recognition and killing of tumor cells by CTLs. Murine lymphoma E.G7-OVA model was used in this study too. OT-I CD8+ T cells were activated by the same as in the previous two studies OVA-peptide presented by bone-marrow derived DCs. Thus, cell activation system used by Luo Z et al. [44] resembles more the one by Sanz-Ortega L et al. [38], than aAPC in the study by Nie W et al. [42]. Therefore, analysis of successful magnetic tumor targeting studies indicates high magnetic properties of MNPs bound to lymphocyte surface as the most likely factor of strict magnetic control of the anti-tumor effector cell motion to the desirable tumor location. First, big MNCs possess more prominent magnetic properties, than small MNPs optimized for MR scanning. Second, strong adhesion between MNPs and cell membrane is also very important as it provides more super-paramagnetic particles stably bound to an effector anti-tumor lymphocyte.
The problem of toxicity of MNP-coatings is still under investigation. Our review does not intend to thoroughly cover this topic. However, we will provide some information about safety of the most common coatings used in MNPs for lymphocyte binding.
PEI-coatings for MNPs were used in the three studies discussed above [40–42]. PEI is a cationic polymer with repeating units composed of the amine group and two carbon aliphatic CH2CH2 spacers. Two commonly used polycations, PEI and poly(L-lysine) were demonstrated to induce apoptosis in a wide range of human cell lines [45]. PEI can both induce membrane damage and initiate apoptosis [46]. Moreover, it was observed that positively-charged PEI-coated gold NPs exhibited significant toxicity and teratogenicity, whereas PEG conjugated gold NPs did not [47]. PEI-coated MNPs also revealed significant toxicity both in vitro and in vivo [16]. PEI-coated MNPs exhibited dose-dependent lethal toxicity in BALB/c mice. Thus, PEI-coatings, despite their easy chemical modification and good adhesion properties with cell membranes, are potentially hazardous and should be systematically evaluated if intended for clinical practice.
Sanz-Ortega L et al. [19, 38, 39] chose APTES as an optimal coating of their MNPs. APTES is an aminosilane. It is also cationic due to amine groups and is highly hydrophobic. APTES is a toxic compound with health hazard score of 3, based on its material safety data sheet (MSDS). Its fumes are destructive to the mucous membranes and can damage the upper respiratory tract. The substance should be used in a fume hood with gloves. The target organs of APTES are nerves, liver and kidney. APTES-functionalized surfaces were shown to be nontoxic to embryonic rat cardiomyocytes in vitro [48]. However, when acute and chronic toxicity of APTES-coated silicon oxide (SiO2) nanostructures was investigated in Daphnia magna, its hazardous effects were revealed [49]. Exposure to APTES-modified surfaces induced alterations in the microvilli and mitochondria of the D. magna intestine. Certain damage to egg cells was also observed. Therefore, safety of APTES-coated MNPs should be intensively studied in acute and chronic toxicity assays.
PEG-coated MNPs were used in the most recently discussed studies [44]. PEG is a widely used polymer with well-established safety. It is a polyether compound derived from petroleum. It has a variety of applications, from industrial manufacturing to medicine. The structure of PEG is generally expressed as H-(O-CH2-CH2)n-OH [50]. PEG possesses very low oral toxicity; it becomes toxic only at doses about 10’s of grams per kg body weight [50]. PEG is considered biologically inert and safe by the U.S. Food and Drug Administration (FDA). Because of its low toxicity, the polymer is widely used in numerous commercial, chemical, biological and medical applications, including skin creams, toothpastes, and hydrogels for different purposes, etc. However, PEG-coating toxicity seems to depend on the nature of NPs covered by this polymer. PEG-coated MNPs were found to be rather safe and non-toxic both in vitro and in vivo [16]. PEG-coating was shown to reduce damage from intravenous injection of nanoscale graphene oxide (NGO) in mice [51]. The PEG coating effectively reduces the early weight loss caused by NGO and alleviates NGO-induced acute tissue injuries, which can include damage to the liver, lung, and kidney, and chronic hepatic and lung fibrosis. Probably this effect may be explained by the ability of PEG-coating to reduce the retention of NGO in the liver, lung, and spleen and to promote the clearance of NGO from these organs. However, PEG-coated gold NPs were shown to promote acute injuries to the liver, kidney, and spleen [52]. Long-term toxicity was also revealed. Importantly, the large percentage of the population has antibodies to PEG due to its presence in a multitude of products [53, 54]. Allergy to PEG is becoming an increasing concern [53, 54]. Therefore, safety of PEG-coated MNPs should be investigated in more detail. Allergy to PEG can limit administration of PEG-coated MNPs in some of patients.
Citrate-coated MNPs were shown to be non-toxic both in vitro and in vivo [55]. They did not affect the viability of different cell lines. Moreover, the in vivo acute dose assay showed no alterations in clinical parameters, relevant biochemical variables, or morphological aspects of vital organs (such as brain, liver, lung and kidney). Iron concentrations were slightly increased in the liver, but this finding was considered non-adverse, given the absence of adverse functional/clinical outcomes.
Conclusions
Therefore, the most successful attempts of magnetic-directed accumulation of anti-tumor lymphocytes within tumors were based on MNCs with marked magnetic properties and strong interactions with cell membranes [40–43]. Such MNCs provided lymphocytes with high load of superparamagnetic material that can be efficiently manipulated via EMF. However, more detailed studies by different research groups are highly desirable to evaluate the safety of such rather big MNCs. Possible shedding of big MNCs from lymphocyte surfaces and the risk of their agglomeration in different vessels of an organism are subjects of special concern. The safety of different biocompatible MNP coatings still needs more detailed investigation. Properties of citrate-coated MNPs are intriguing, as this type of coating promotes internalization of MNPs by lymphocytes, which is not very typical for this cell type [29]. Moreover, such coatings appear to be the safest based on the current research [55]. However, there are no available data on whether citrate-MNP-coated lymphocytes can be driven into tumors by EMF.
Up to date, magnetic directing of lymphocytes into tumors remains largely unexplored. We can only speculate why certain studies succeeded in contrast to the others. More studies in this field and direct comparison of the MNPs discussed above are required for better understanding of their functional characteristics and abilities to drive lymphocytes into tumors.
Abbreviations
| aAPC: | artificial antigen-presenting cell |
| aP: | PD-1 antibody |
| APTES: | 3-aminopropyl-triethoxysilane |
| CTLs: | cytotoxic T-lymphocytes |
| EMF: | external magnetic field |
| HA: | hyaluronic acid |
| Mal: | maleimide |
| MNCs: | magnetic nanoclusters |
| MNPs: | magnetic nanoparticles |
| MR: | magnetic resonance |
| NGO: | nanoscale graphene oxide |
| NK: | natural killer |
| NPs: | nanoparticles |
| OVA: | ovalbumin |
| PEG: | polyethylene glycol |
| PEI: | poly(ethylenimine) |
Declarations
Acknowledgments
The authors thank Dr. A. Omelyanchik from Research and Educational Centre “Smart Materials and Biomedical Applications”, Immanuel Kant Baltic Federal University for editing the text and fruitful discussion.
Author contributions
IC: Conceptualization, Writing—original draft, Writing—review & editing. PF, IS, and SP: Writing—original draft, Writing—review & editing. KL: Conceptualization, Writing—review & editing. VA and MK: Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.